Abstract
Airway smooth muscle (ASM) cell proliferation and migration play important roles in airway remodeling in asthma. In vitro platelet-derived growth factor (PDGF) induced ASM cell proliferation and migration. Baicalin is one of flavonoid extracts from Scutellaria baicalensis, which has an anti-asthma effect. However, little is known about its role in PDGF-induced proliferation and migration in rat ASM (RASM) cells. In this study, we aimed to investigate the effects of baicalin on PDGF-induced RASM cell proliferation and migration. We also identified the signaling pathway by which baicalin influences RASM cell proliferation and migration. In the current study, we demonstrated that baicalin suppressed PDGF-induced RASM cell proliferation, arrested PDGF-induced cell-cycle progression. It also suppressed PDGF-induced RASM cell migration. Furthermore, baicalin suppressed PDGF-induced expression of phosphorylated p38, ERK1/2 and JNK in RASM cells. In summary, our study is the first to show that baicalin pretreatment can significantly inhibit PDGF-induced RASM cell proliferation and migration by suppressing the MAPK signaling pathway, and baicalin may be a useful chemotherapeutic agent for asthma.
Keywords: Baicalin, rat airway smooth muscle (RASM) cell, proliferation, migration
Introduction
Asthma is a chronic inflammatory disease of the lower airways, associated with various comorbidities and characterized by airflow obstruction, airway inflammation and airway remodeling [1]. It is believed that airway smooth muscle (ASM) cell proliferation and migration play important roles in airway remodeling [2]. Previous studies have shown that some inflammatory mediators, such as platelet-derived growth factor (PDGF) and transforming growth factor-β (TGF-β), are greatly elevated in the lung of asthmatic patients and are thought to promote proliferation and migration of ASM cells [3-5]. Therefore, inhibiting PDGF-induced proliferation and migration of ASM cells may be a therapeutic method for asthma.
Baicalin is one of the main bioactive flavone glucuronides derived as a medical herb from the dried roots of Scutellaria baicalensis Georgi [6]. Baicalin has been shown to possess anti-bacterial [7], anti-tumor [8] and anti-inflammatory [9] properties. It has also been shown to protect against ischemia-reperfusion injury in various organs, because of its anti-oxidative, anti-inflammatory and anti-apoptotic effects [10-13]. Recent studies showed that baicalin has an anti-asthma effect. Sun et al. reported that baicalin inhibited ovalbumin-induced asthmatic airway remodeling mice model by decreasing expression of transforming growth factor-β1, interleukin (IL)-13, and vascular endothelial growth factor [14]. Another study showed that baicalin inhibited ovalbumin-induced airway resistance and eosinophil count, as well as the levels of IL-4, IL-17A [15]. However, little is known about its role in PDGF-induced proliferation and migration in rat ASM (RASM) cells. We undertook the present study to determine the effect of baicalin on PDGF-induced RASM cell proliferation and migration. We also identified the signaling pathway by which baicalin influences RASM cell proliferation and migration.
Materials and methods
Cell culture
Primary cultures of rat airway smooth muscle (RASM) cells from 8-week-old SD rats were isolated and identified as previously described [16]. All animal experiments were approved by the Experimental Animal Ethics Committee of the second Hospital of Shanxi Medical University.
Cell proliferation assay
RASM cells were seeded in 96-well plates at 1 × 104 cells/well. Then, cells were treated with various concentrations of baicalin (10, 25 and 100 nM) for 1 h, followed by incubation with 10 ng/mL PDGF-BB for 24 h. Cell proliferation was assayed with the Cell Counting Kit-8 (CCK-8) (Dojindo, Kumamoto, Japan). The optical density value (OD) of each well was detected at a wave length of 570 nm by a spectrophotometer (Multiskan MK3, Thermo Labsystem, Waltham, MA).
Cell cycle assay
RASM cells were seeded in 6-well plates and treated with PDGF in the presence or absence of baicalin for 24 h. Cells were then harvested and fixed with ice-cold 70% (v/v) ethanol for 24 h. After an additional washing, cells were incubated with RNase A (20 µg/mL) at 37°C for 30 min, stained with propidium iodide (100 µg/mL; Sigma Aldrich) for 10 min, and analyzed with flow cytometry using a FACScan flowcytometer (Becton-Dickinson, San Jose, CA).
Transwell assay
RASM cell migration was measured with the transwell migration assay. In brief, RASM cells were digested and resuspended gently in DMEM/F-12 medium containing 2% FBS with or without baicalin and inoculated into the inner chambers at a concentration of 5 × 104 cells/well. Six hundred microliter of DMEM/F-12 medium containing 10% FBS with or without PDGF was pipetted into each outer chamber. After incubation in a humidified atmosphere of 5% CO2/95% air at 37°C for 24 h, the membrane with cells was fixed. Non-migrated cells were removed from the upper chamber using a cotton bud, whereas the migrated cells were stained with Wright’s stain. The number of cells per four high power fields was counted under a microscope. Cells counted in each field were averaged and normalized to a non-stimulated control. Each treatment was performed in triplicate.
Real-time PCR
Total RNA was extracted from ASMCs by Trizol reagents (Invitrogen) and reverse transcribed to obtain single-strand cDNA using a Reverse Transcription System (Promega) in accordance with the manufacturer’s protocol. Quantitative real-time PCR was performed on ABI Step One (Applied Biosystems, Foster City, CA, USA). The primer sequences used in this study were as follows: matrix metalloproteinase (MMP)-9 (primers, sense 5’-AAGGATGGTCTACTGGCAC-3’; antisense 5’-AGAGATTCTCACTGGGGC-3’), β-actin (primers, sense 5’-TCATGAAGTGTGACGTTGAC-3’; antisense 5’-CCTAGAAGCATTTGCGGTGC-3’). The reactions were initially heated at 94°C for 4 min; then at 94°C for 40 s, 60°C for 40 s and 72°C for 50 s, totally 40 cycles; finally stopped at 72°C for 7 min. Relative gene expression was determined by the ΔΔCT method using the β-actin as an internal control.
Western blot
Cells were homogenized and the total proteins were extracted by RIPA lysis buffer (Beyotime Biotech. CO., China). Equal amounts of protein (40 μg/lane) were resolved by 12% SDS-PAGE and transferred to a nitrocellulose membrane (Amersham Pharmacia, Germany). Then, the membrane was blocked by incubating with 5% nonfat milk in Tris-buffered-saline with Tween (TBST; 10 mM Tris-HCl, pH of 7.5, 150 mM NaCl, and 0.05% Tween-20) at room temperature for 1 h. Immunodetection of target proteins (PCNA, cyclinD1, MMP-9, p-ERK1/2 and total ERK/2, p-JNK and total JNK, p-p38 MAPK and total p38 MAPK) and β-actin was performed using rabbit monoclonal antibody (1:1500, Santa Cruz), and anti-β-actin (Santa Cruz), respectively. After washing with PBST, the membranes were incubated with the secondary antibody (Goat Anti Rabbit IgG/HRP (1:10,000)) at room temperature for 1 h. The immunoreactive protein bands were visualized using an enhanced chemiluminescence detection system (Amersham, Little Chalfont, UK).
Statistical analysis
Experimental results were presented as mean ± SD. Statistical significance was assessed by one-way ANOVA followed by Bofferoni’s test. Statistical significance was determined when P<0.05.
Results
Baicalin inhibited PDGF-induced RASM cell proliferation
To explore the effect of baicalin on RASM cell proliferation, we adopted a CKK-8 assay. As shown in Figure 1A, we found that PDGF obviously promoted RASM cell proliferation as compared to the control group, while pretreatment with baicalin significantly prevented PDGF-induced RASM cell proliferation in a concentration-dependent manner. Moreover, we evaluated the effect of baicalin on PCNA expression in PDGF-induced RASM cells. As shown in Figure 1B, PDGF significantly up-regulated the expression of PCNA as compared to the control group, while baicalin pretreatment significantly attenuated PDGF-induced up-regulation of PCNA protein. These results demonstrated that baicalin pretreatment inhibited PDGF-induced RASM cell proliferation.
Figure 1.
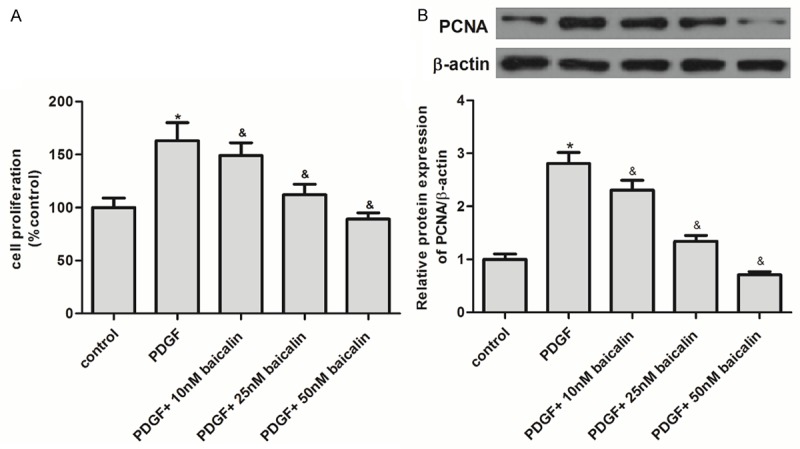
Baicalin inhibited PDGF-induced RASM cell proliferation. A: RASMCs were pre-treated with baicalin for 1 h before stimulation with 10 ng/mL PDGF for 24 h. Cell proliferation was determined by CCK-8 test. B: Representative western blot analysis for PCNA protein. All experiments were repeated three times (n = 3). Data is shown as the mean ± SD; *P<0.05 vs. control group; &P<0.05 vs. PDGF group.
Baicalin inhibited PDGF-induced RASM cell cycle
To further interpret the effect of baicalin on PDGF-induced RASM cell proliferation, we investigated the effect of baicalin on cell cycle in PDGF-induced RASMCs. As shown in Figure 2A, PDGF induced the entry of RASM cells to synthesis phase (S-phase) to undergo proliferation, with 63.1 ± 2.1% cells in G0/G1 phase (P<0.05 vs. 76.2 ± 3.7% in the control) and 31.4 ± 2.6% in S-phase (P<0.05 vs. 19.3 ± 1.5% in the control), while pretreatment with baicalin (50 nM) significantly arrested the cell cycle at G2/M phase (28.1 ± 3.1% vs. 4.5 ± 1.2% in the PDGF group, P<0.05). In addition, we tested the effect of baicalin on cell cycle related protein, cyclin D1. As shown in Figure 2B, PDGF significantly up-regulated the expression of cyclin D1 as compared to the control group, while baicalin pretreatment significantly attenuated PDGF-induced up-regulation of cyclin D1 protein in RASM cells.
Figure 2.

Baicalin inhibited PDGF-induced RASM cell cycle. A: RASMCs were pre-treated with baicalin for 1 h before stimulation with 10 ng/ml PDGF for 24 h. Cell cycle analysis was performed using FACScan flow cytometer. Cell cycle distributions were expressed as the percentage of total cells. B: Representative western blot analysis for cyclin D1 protein. All experiments were repeated three times (n = 3). Data is shown as the mean ± SD; *P<0.05 vs. control group; &P<0.05 vs. PDGF group.
Baicalin inhibited PDGF-induced RASM cell migration
Next, we evaluated the effect of baicalin on PDGF-induced RASM cell migration by transwell assay. As shown in Figure 3A, PDGF significantly increased the number of cells that migrated through the membrane as compared to the control, while pretreatment with baicalin significantly suppressed PDGF-induced RASMC migration compared to the PDGF group. In addition, we tested the effect of baicalin on MMP-9 expression. As shown in Figure 3B and 3C, PDGF obviously up-regulated the expression of MMP-9 as compared to the control group, however, baicalin pretreatment significantly attenuated PDGF-induced MMP-9 expression in RASM cells.
Figure 3.

Baicalin inhibited PDGF-induced RASM cell migration. A: RASMCs were pre-treated with baicalin for 1 h before stimulation with 10 ng/ml PDGF for 24 h. RASM migration increased 2-fold in PDGF-BB-induced vs. vehicle-treated cells. Baicalin inhibited PDGF-induced RASM cell migration in a dose-dependent manner. B: Real-time PCR was performed to analyze the mRNA level of MMP-9. C: Representative western blot analysis for MMP-9 protein. All experiments were repeated three times (n = 3). Data is shown as the mean ± SD; *P<0.05 vs. control group; &P<0.05 vs. PDGF group.
Effect of baicalin on MAPK expression in PDGF-induced RASM cells
To further elucidate the mechanism of baicalin-inhibited RASMCs proliferation and migration induced by PDGF, we investigated the effect of baicalin on MAPK expression. As shown in Figure 4, PDGF significantly increased the expression levels of phosphorylated p38, ERK1/2 and JNK in RASM cells as compared to the control, while pretreatment with baicalin significantly suppressed PDGF-induced expression of phosphorylated p38, ERK1/2 and JNK in RASM cells.
Figure 4.
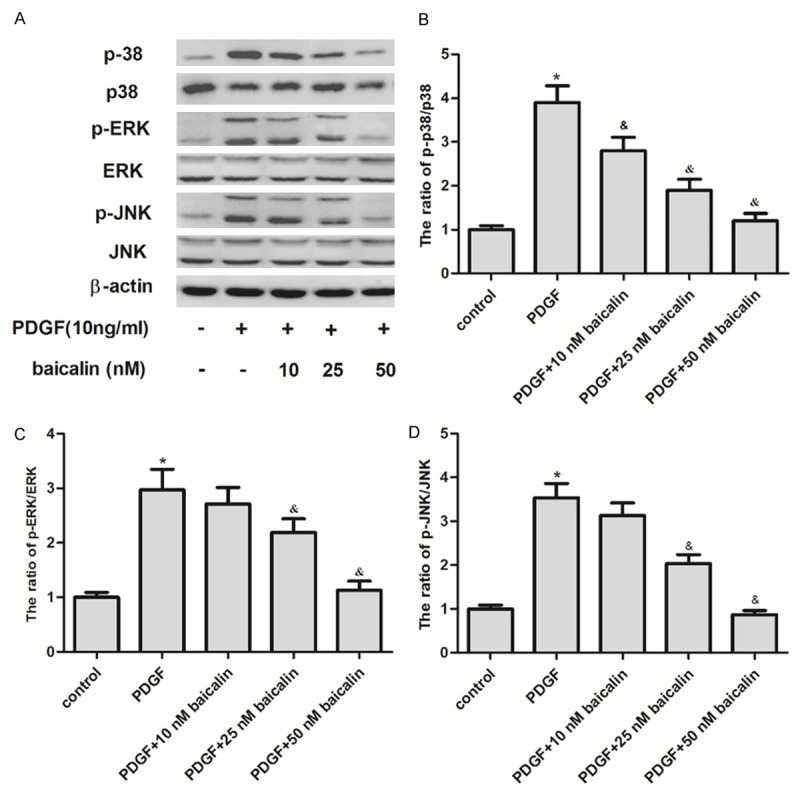
Effect of baicalin on MAPK expression in PDGF-induced RASM cells. A: RASM cells were growth-arrested and pretreated with baicalin for 24 h, followed by PDGF stimulation for 24 h. Detection of total and phosphorylated protein levels of ERK1/2, JNK or p38 MAPK by western blot analysis. B-D: The phosphorylated ERK1/2, JNK or p38 MAPK bands were quantified by densitometry and expressed as a fold change. All experiments were repeated three times (n = 3). Data are showed as the mean ± SD; *P<0.05 vs. control group; &P<0.05 vs. PDGF group.
Discussion
In the current study, we demonstrated that baicalin suppressed PDGF-induced RASM cell proliferation, arrested PDGF-induced cell-cycle progression. It also suppressed PDGF-induced RASM cell migration. Furthermore, baicalin suppressed PDGF-induced expression of phosphorylated p38, ERK1/2 and JNK in RASM cells.
It has been reported that pretreatment with baicalin has a dose-dependent inhibitory effect on PDGF-BB-stimulated vascular smooth muscle cell (VSMC) proliferation, accompanied with the reduction of PCNA expression [17]. Baicalin also attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation [18]. Consistent with these results, here, we found that pretreatment with baicalin significantly prevented PDGF-induced RASM cell proliferation and attenuated PDGF-induced up-regulation of PCNA protein in a concentration-dependent manner. These results suggested that baicalin pretreatment inhibited PDGF-induced RASMCs proliferation.
In cell cycle progression, we found that pretreatment with baicalin significantly blocked the G2/M phase transition by PDGF. Cyclin D1 is a cyclin-dependent subunits and important for cell cycle progression in airway smooth muscle cells [19]. Results of the present study show that baicalin pretreatment significantly attenuated PDGF-induced up-regulation of Cyclin D1 protein in RASMCs. Song et al. reported that baicalin inhibited cell proliferation via down-regulation of cyclin D1 expression in insulinoma cells [20]. These results suggested that baicalin pretreatment inhibited PDGF-induced RASMCs proliferation by inhibiting cell cycle progression.
Migration of ASM cells is not only essential for development of hollow airways and the respiratory system but also important for airway remodeling in asthma [2,21]. Elevated levels of PDGF have been observed in asthma, and PDGF can induce ASM cell migration in vitro [22]. Furthermore, it has been shown that treatment of baicalin inhibited the migration of endothelial cells and the differentiation of endothelial cells into branching networks of tubular structures in vitro, as well as the MMP-2 activity [23]. Results of the present study show that pretreatment with baicalin significantly prevented PDGF induced RASM cell migration.
Mitogen-activated protein kinase (MAPK) signaling pathway plays an important role in air remodeling [24]. Among the best-characterized mammalian MAPKs are 1) the 42- and 44-kDa extracellular signal-regulated kinases (ERKs) ERK2 and ERK1; 2) the c-Jun amino-terminal kinase (JNK); and 3) p38 MAPK [25]. The activation of ERK by various substances, such as TGF-β [26], PDGF [27], beta-hexosaminidase A [28] and endothelin [29] increased ASM cell proliferation and migration. Consistent with these results, we show that PDGF significantly increased the expression levels of phosphorylated p38, ERK1/2 and JNK in RASM cells. Previous studies have indicated that baicalin could notably down-regulate the phosphorylation of proteins in MAPK signaling pathway such as p-MRK1/2, p-ERK and p-p38 in oxygen-glucose deprivation (OGD)-injured brain microvascular endothelial cells (BMECs) [30]. In this study, western blot analyses revealed that baicalin drastically reduced PDGF-induced expression of phosphorylated p38, ERK1/2 and JNK in RASM cells. These results suggest that baicalin inhibited RASM cell proliferation and migration induced by PDGF through suppressing the MAPK signaling pathway.
Collectively, our study is the first to show that baicalin pretreatment can significantly inhibit PDGF-induced RASM cell proliferation and migration by suppressing the MAPK signaling pathway, and baicalin may be a useful chemotherapeutic agent for asthma.
Disclosure of conflict of interest
None.
References
- 1.Borish L, Culp JA. Asthma: a syndrome composed of heterogeneous disease. Ann Allergy Asthma Immunol. 2008;101:1–8. doi: 10.1016/S1081-1206(10)60826-5. [DOI] [PubMed] [Google Scholar]
- 2.Madison JM. Migration of airway smooth muscle cells. Am J Respir Cell Mol Biol. 2003;29:8–11. doi: 10.1165/rcmb.F272. [DOI] [PubMed] [Google Scholar]
- 3.Seidel P, Goulet S, Hostettler K, Tamm M, Roth M. DMF inhibits PDGF-BB induced airway smooth muscle cell proliferation through induction of heme-oxygenase-1. Respir Res. 2010;11:145. doi: 10.1186/1465-9921-11-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Khalil N. TGF-β1 increases proliferation of airway smooth muscle cells by phosphorylation of map kinases. Respir Res. 2006;7:2. doi: 10.1186/1465-9921-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makinde T, Murphy RF, Agrawal DK. The regulatory role of TGF-β in airway remodeling in asthma. Immunol Cell Biol. 2007;85:348–356. doi: 10.1038/sj.icb.7100044. [DOI] [PubMed] [Google Scholar]
- 6.Zhao L, Wei Y, Huang Y, He B, Zhou Y, Fu J. Nanoemulsion improves the oral bioavailability of baicalin in rats: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:3769–3779. doi: 10.2147/IJN.S51578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiu J, Niu X, Dong J, Wang D, Wang J, Li H, Luo M, Li S, Feng H, Deng X. Baicalin protects mice from Staphylococcus aureus pneumonia via inhibiting the cytolytic activity of α-hemolysin. J Infect Dis. 2012;206:292–301. doi: 10.1093/infdis/jis336. [DOI] [PubMed] [Google Scholar]
- 8.Chen WC, Kuo TH, Tzeng YS, Tsai YC. Baicalin induces apoptosis in SW620 human colorectal carcinoma cells in vitro and suppresses tumor growth in vivo. Molecules. 2012;17:3844–3857. doi: 10.3390/molecules17043844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li L, Bao H, Wu J, Duan X, Liu B, Sun J, Gong W, Lv Y, Zhang H, Luo Q. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int Immunopharmacol. 2012;13:15–22. doi: 10.1016/j.intimp.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Cao Y, Mao X, Sun C, Zheng P, Gao J, Wang X, Min D, Sun H, Xie N, Cai J. Baicalin attenuates global cerebral ischemia/reperfusion injury in gerbils via anti-oxidative and anti-apoptotic pathways. Brain Res Bull. 2011;85:396–402. doi: 10.1016/j.brainresbull.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhou QB, Duan CZ, Jia Q, Liu P, Li LY. Baicalin attenuates focal cerebral ischemic reperfusion injury by inhibition of protease-activated receptor-1 and apoptosis. Chin J Integr Med. 2014;20:116–122. doi: 10.1007/s11655-013-1441-7. [DOI] [PubMed] [Google Scholar]
- 12.Tu XK, Yang WZ, Shi SS, Wang CH, Chen CM. Neuroprotective effect of baicalin in a rat model of permanent focal cerebral ischemia. Neurochem Res. 2009;34:1626–1634. doi: 10.1007/s11064-009-9953-4. [DOI] [PubMed] [Google Scholar]
- 13.Kim SJ, Moon YJ, Lee SM. Protective effects of baicalin against ischemia/reperfusion injury in rat liver. J Nat Prod. 2010;73:2003–2008. doi: 10.1021/np100389z. [DOI] [PubMed] [Google Scholar]
- 14.Sun J, Li L, Wu J, Liu B, Gong W, Lv Y, Luo Q, Duan X, Dong J. Effects of baicalin on airway remodeling in asthmatic mice. Planta Med. 2013;79:199–206. doi: 10.1055/s-0032-1328197. [DOI] [PubMed] [Google Scholar]
- 15.Ma C, Ma Z, Fu Q, Ma S. Anti-asthmatic Effects of Baicalin in a Mouse Model of Allergic Asthma. Phytother Res. 2014;28:231–237. doi: 10.1002/ptr.4983. [DOI] [PubMed] [Google Scholar]
- 16.Ning Y, Sun Q, Dong Y, Xu W, Zhang W, Huang H, Li Q. Slit2-N inhibits PDGF-induced migration in rat airway smooth muscle cells: WASP and Arp2/3 involved. Toxicology. 2011;283:32–40. doi: 10.1016/j.tox.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 17.Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ, Sun RH, Wu Y, Han M. Baicalin inhibits PDGFBB-stimulated vascular smooth muscle cell proliferation through suppressing PDGFRβ-ERK signaling and increase in p27 accumulation and prevents injury-induced neointimal hyperplasia. Cell Res. 2010;20:1252–1262. doi: 10.1038/cr.2010.111. [DOI] [PubMed] [Google Scholar]
- 18.Huang S, Chen P, Shui X, He Y, Wang H, Zheng J, Zhang L, Li J, Xue Y, Chen C. Baicalin attenuates transforming growth factor-β1-induced human pulmonary artery smooth muscle cell proliferation and phenotypic switch by inhibiting hypoxia inducible factor-1α and aryl hydrocarbon receptor expression. J Pharm Pharmacol. 2014;66:1469–1477. doi: 10.1111/jphp.12273. [DOI] [PubMed] [Google Scholar]
- 19.Page K, Li J, Wang Y, Kartha S, Pestell RG, Hershenson MB. Regulation of cyclin D1 expression and DNA synthesis by phosphatidylinositol 3-kinase in airway smooth muscle cells. Am J Respir Cell Mol Biol. 2000;23:436–443. doi: 10.1165/ajrcmb.23.4.3953. [DOI] [PubMed] [Google Scholar]
- 20.Song J, Sun J, Jia Y, Yang Y. Effect of baicalin on the proliferation of insulinoma cell line. J Cent South Univ. 2005;30:145–148. [PubMed] [Google Scholar]
- 21.Goncharova EA, Goncharov DA, Krymskaya VP. Assays for in vitro monitoring of human airway smooth muscle (ASM) and human pulmonary arterial vascular smooth muscle (VSM) cell migration. Nat Protoc. 2006;1:2933–2939. doi: 10.1038/nprot.2006.434. [DOI] [PubMed] [Google Scholar]
- 22.Dekkers B, Prins A, Oldenbeuving G, Pool K, Elzinga C, Meurs H, Schmidt M, Roscioni S. Epac And PKA Inhibit PDGF-induced airway smooth muscle phenotype modulation. Am J Respir Crit Care Med. 2010;181:A2142. [Google Scholar]
- 23.Liu JJ, Huang TS, Cheng WF, Lu FJ. Baicalein and baicalin are potent inhibitors of angiogenesis: inhibition of endothelial cell proliferation, migration and differentiation. Int J Cancer. 2003;106:559–565. doi: 10.1002/ijc.11267. [DOI] [PubMed] [Google Scholar]
- 24.Zhai W, Eynott PR, Oltmanns U, Leung SY, Chung KF. Mitogen-activated protein kinase signalling pathways in IL-1β-dependent rat airway smooth muscle proliferation. Brit J Pharmacol. 2004;143:1042–1049. doi: 10.1038/sj.bjp.0705971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerthoffer WT, Singer CA. MAPK regulation of gene expression in airway smooth muscle. Respir Physiol Neurobiol. 2003;137:237–250. doi: 10.1016/s1569-9048(03)00150-2. [DOI] [PubMed] [Google Scholar]
- 26.Stouffer G, Owens G. TGF-beta promotes proliferation of cultured SMC via both PDGFAA-dependent and PDGF-AA-independent mechanisms. J Clin Invest. 1994;93:2048–2055. doi: 10.1172/JCI117199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page K, Li J, Hershenson MB. Platelet-derived growth factor stimulation of mitogen-activated protein kinases and cyclin D1 promoter activity in cultured airway smooth-muscle cells. Role of Ras. Am J Respir Cell Mol Biol. 1999;20:1294–1302. doi: 10.1165/ajrcmb.20.6.3597. [DOI] [PubMed] [Google Scholar]
- 28.Lew D, Brown E, Dempsey B, Wright H, Malik K. Contribution of PKC to beta-hexosaminidase-induced airway smooth muscle proliferation. Am J Physiol. 1997;272:L639–L643. doi: 10.1152/ajplung.1997.272.4.L639. [DOI] [PubMed] [Google Scholar]
- 29.Whelchel A, Evans J, Posada J. Inhibition of ERK activation attenuates endothelin-stimulated airway smooth muscle cell proliferation. Am J Respir Cell Mol Biol. 1997;16:589–596. doi: 10.1165/ajrcmb.16.5.9160841. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Hou J, Fu J, Li D, Zhang C, Liu J. Baicalin protects rat brain microvascular endothelial cells injured by oxygen-glucose deprivation via anti-inflammation. Brain Res Bull. 2013;97:8–15. doi: 10.1016/j.brainresbull.2013.05.005. [DOI] [PubMed] [Google Scholar]


