Abstract
Objective: Cystatin C is a well established marker of kidney function. There is evidence that cystatin C concentrations are also associated with myocardial infarction. The purpose of the present study is to clarify the link between cystatin C with myocardial infarction using a meta-analysis approach. Methods: We searched articles indexed in the Pubmed and Sciencedirect published as of August 2015 that met our predefined criteria. A meta-analysis was used to pool estimates of the multivariate adjusted relative risk (RR) with 95% confidence interval (CI), of the association between cystatin C and subsequent risk of myocardial infarction. Results: Four eligible articles with 10491 subjects from 5 cohort studies were considered in the analysis. Overall, the random-effects meta-analysis results indicated that the highest cystatin C category versus lowest was associated with greater risk of myocardial infarction (RR, 1.78; 95% CI, 1.27 to 2.49; P=0.001). No evidence of publication bias was observed. Conclusions: This meta-analysis showed that cystatin C is strongly and independently associated with subsequent risk of myocardial infarction. Further investigation is warranted to clarify whether measurement of cystatin C can usefully reduce the myocardial infarction beyond established predictors already in clinical use.
Keywords: Cystatin C, risk factor, myocardial infarction, meta-analysis
Introduction
Chronic kidney disease, a worldwide health problem, is associated with a substantial risk for cardiovascular morbidity [1-3]. In clinical practice and in epidemiological research studies, glomerular filtration rate (GFR) is considered to be the best overall index of kidney function. However, the widely used creatinine-based modification of renal disease study formula for GFR still has substantial inaccuracy when applied to healthy persons and older individuals mass [4]. Recently, the use of serum creatinine-based formulas to assess renal function has been challenged by novel markers, particularly cystatin C, which may be a more reliable index of renal function [5,6].
Cystatin C, as an improved estimate of GFR, is a cysteine protease inhibitor produced by nearly all human cells and excreted into the bloodstream and do not depend on muscle mass. At a molecular weight of 13 kD, the protein is eliminated exclusively by glomerular filtration and tubular reabsorption with subsequent catabolism [7,8]. It has been reported that GFR equations that incorporate the cystatin C concentration may perform better in the normal GFR range than those relying on creatinine [9,10]. As a marker of renal function, cystatin C is a powerful tool for risk evaluation of cardiovascular disease above and beyond its role as a surrogate for GFR [11,12]. Observational studies suggest that cystatin C is strongly and independently associated with subsequent risk of myocardial infarction [13-16]. Shlipak et al. reported a statistically significant association between the incidence of myocardial infarction and the elevated cystatin C in 4637 patients [14]. Windhausen et al. demonstrated a similar result in 1128 patients [15]. Negrusz-Kawecka et al. suggested that the high cystatin C level plays a role in the pathogenesis of myocardial infarction in 63 patients [16]. However, no systematic review has been conducted to evaluate the totality of available evidence regarding a link between cystatin C and subsequent risk of myocardial infarction.
Meta-analysis is a well-established statistical tool that serves for integration of data from independent studies in order to formulate more general conclusions. We therefore undertook a meta-analysis of cohort studies to qualitatively and quantitatively assess the association between cystatin C and risk of myocardial infarction.
Materials and methods
Search strategy
We searched all English written articles indexed in Pubmed and Science direct published up to August 2015. literature searches were performed using medical subject heading (MeSH) or free text words. The searching keywords were: cystatin C AND myocardial infarction. Reference lists of all eligible studies were screened to identify potentially eligible studies. Emails were sent to the authors of identified studies for additional information if necessary.
Selection criteria
The search was conducted by three authors independently. Titles and abstracts were screened for subject relevance. Studies that could not be definitely excluded based on abstract information were also selected for full text screening. Two authors independently selected eligible studies for inclusion possibility. Where there was a disagreement for study inclusion, a discussion was held to reach a consensus. Eligible studies had to meet the following criteria: (1) human study; (2) cohort study or clinical trial; (3) cystatin C measured at baseline; (4) reported myocardial infarction as outcomes; (5) reported quantitative estimates of the multivariate adjusted relative risk (RR) and 95% confidence interval (CI) for the outcomes associated with baseline cystatin C. Exclusion criteria included: (1) animal study, review or case report; (2) in vitro or laboratory study; (3) the majority of participants had end-stage renal disease (dialysis or GFR <15 mL/min/1.73 m2) or kidney transplant; (4) the study only reported unadjusted RR, or not reported 95% CI, or data were duplicative; (5) sample less than 20.
Data extraction and quality assessment
The following information was extracted from each included study: country, first author’s family name, year of publication, demography of subjects (number of subjects, sex and age), year of follow-up, cystatin C levels, adjusted RR and 95% confidence interval. Two authors independently extracted data using a standard form. Discrepancies were resolved by discussion with a third investigator and by referencing the original report. Our primary outcomes of interest were the association of myocardial infarction and cystatin C categorical level, based on the comparison of the highest category of cystatin C versus lowest.
Two authors assessed the quality independently. The qualities of all included studies were assessed using the Newcastle-Ottawa Scale (NOS). Quality assessment was conducted in three domains: subject selection, comparability of groups and the ascertainment of outcomes. The total NOS Star count ranges from zero to nine. A rating of 5 or more was considered to be indicative of a higher quality study.
Statistical analysis
Data analyses used the Multivariate-adjusted outcome data expressed as RRs and 95% CIs. We pooled risk estimates comparing the highest to the lowest category of cystatin C. Heterogeneity between studies was tested through the Chi-square and I-square tests. If the I2 value was greater than 50% and the p value was less than 0.10, the meta-analysis was considered as homogeneous. The RRs were calculated using either fixed-effects models or, in the presence of heterogeneity, random-effects models.
Subgroup analyses were used to identify the possible sources of heterogeneity. Subgroup analyses were conducted based on the following characteristics: location, mean age, follow-up duration, cutoff point of the lowest interval of cystatin C, and cutoff point of the highest interval of cystatin C. The stability of the study was also detected by sensitivity analysis, through re-meta-analysis with one involved study excluded each time. Publication bias was measured using Begg’s tests and visualization of funnel plots. All statistical analyses were performed with Stata version 11.0 (Stata Corp, College Station, TX, USA). A p value less than 0.05 was considered statistically significant.
Results
Literature search
The systematic literature review identified 7 full articles for detailed assessment, among which 3 were excluded for not reporting RR and 95% CI for the risk of myocardial infarction. Overall, 4 eligible articles with 10491 subjects from 5 cohort studies were considered in the analysis [13-16]. A flow diagram of the study selection process is presented in Figure 1.
Figure 1.
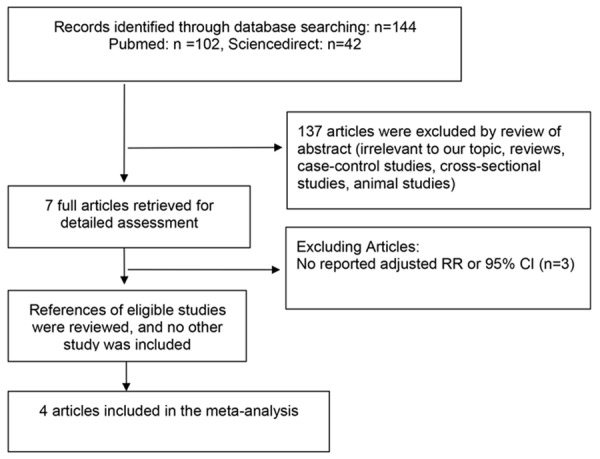
Flow diagram of screened and included papers.
Study characteristics and quality assessment
The detailed characteristics of the included studies and the results of the quality assessment were summarized in Table 1. By geographic location, studies were conducted in 3 different countries (USA, Poland, Netherlands). The earliest study was published in 2005, and the latest in 2014. The mean age of population ranged from 62 to 75 years. All study included both men and women. The number of subjects in each study ranged from 63 to 4637. The follow-up duration ranged from 0.5 to 9.3 years. The RR is adjusted for other risk factors, such as age, sex, diabetes, myocardial infarction, heart failure, blood pressure, high-density lipoprotein cholesterol level, total cholesterol or low-density lipoprotein cholesterol level, smoking, body-mass index and C-reactive protein. The overall study quality was good (averaged 6; range 5-7) on a scale of 0 to 9 point.
Table 1.
Characteristics of subjects in eligible studies
| Studies | Country | Population | Participant No. (Women, %) | Mean Age, y | Follow-Up, year | Comparison Cystatin C Level, mg/L | Adjusted risk factors | Study quality |
|---|---|---|---|---|---|---|---|---|
| Shlipak 2005 | USA | Elderly ages ≥65 y | 4637 (58) | 75 | 7.4 | Highest quintile (>1.29) vs lowest (<0.89) | age, sex, diabetes, left ventricular hypertrophy, fibrinogen level, C-reactive protein , hemoglobin level, myocardial infarction, stroke, and heart failure | 6 |
| Shlipak 2006-1 | USA | Elderly ages ≥65 y | 3659 (NA) | >65 | 9.3 | Highest quintile (≥1 mg/L) vs lowest (<1 mg/L) | age, sex, race, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, hypertension, diabetes, current smoking, C-reactive protein level, prevalent heart failure, coronary artery disease, stroke, height, weight, and physical activity. Cystatin C and creatinine concentrations | 5 |
| Shlipak 2006-2 | USA | Elderly ages ≥65 y | 1004 (NA) | >65 | 9.3 | Highest quintile (≥1 mg/L) vs lowest (<1 mg/L) | age, sex, race, low-density lipoprotein cholesterol level, high-density lipoprotein cholesterol level, hypertension, diabetes, current smoking, C-reactive protein level, prevalent heart failure, coronary artery disease, stroke, height, weight, and physical activity. Cystatin C and creatinine concentrations | 5 |
| Windhausen 2009 | Netherlands | Acute coronary heart disease | 1128 (27) | 62 | 3 | Highest quintile (>1.01) vs lowest (<0.86) | age, sex, diabetes, coronary heart disease, hypertension, myocardial infarction, hypercholesterolemia, tobacco use, drug therapy | 7 |
| Negrusz-Kawecka 2014 | Poland | coronary artery disease | 63 (39) | 62.7 | 0.5 | Highest quintile (≥0.915 mg/L) vs lowest (<0.915 mg/L) | gender, age, diabetes mellitus, arterial hypertension, CKD, nicotine abuse and obesity | 7 |
NA, not available.
Cystatin C level and myocardial infarction
The random-effects meta-analysis results indicated that the highest cystatin C category versus lowest was associated with greater risk of myocardial infarction (RR, 1.78; 95% CI, 1.27 to 2.49; P=0.001) (Figure 2). The 5 sets of results showed significant amount of heterogeneity (I2=72.4%, P=0.006) (Figure 2).
Figure 2.
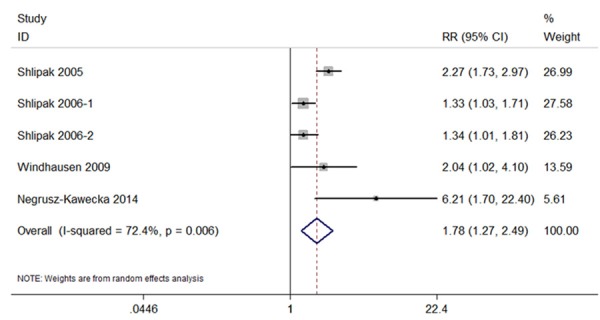
Forest plot of studies in the association between cystatin C with myocardial infarction.
Potential sources of heterogeneity in the strength of the association between cystatin C (highest category versus lowest) and subsequent risk of myocardial infarction were examined by conducting subgroup analyses (Table 2). Significant heterogeneity between pooled analyses were noted for study location (USA versus Europe, I2=66.6%, P=0.084), mean age (<65 years versus ≥65 years, I2=66.6%, P=0.084), follow-up duration (≤5 years versus >5 years, I2=66.6%, P=0.084). There was no evidence that the strength of the association differed according to cutoff point of the lowest interval of cystatin C (<0.90 mg/L versus ≥0.90 mg/L, I2=27.2%, P=0.241), and cutoff point of the highest interval of cystatin C (>1 mg/L versus ≤1 mg/L, I2=27.2%, p=0.241).
Table 2.
Differences between studies by subgroup analysis
| Subgroups | Number of studies | RR (95% CI) | Heterogeneity within subgroups | Heterogeneity among subgroups |
|---|---|---|---|---|
| Location | ||||
| USA | 3 | 1.594 (1.125, 2.259) | I2=79.8%, P=0.075 | I2=27.2%, P=0.241 |
| Europe | 2 | 3.101 (1.078, 8.924) | I2=54.9%, P=0.340 | |
| Duration of follow-up | ||||
| ≤ 5 years | 3 | 1.594 (1.125, 2.259) | I2=79.8%, P=0.075 | I2=27.2%, P=0.241 |
| > 5 years | 2 | 3.101 (1.078, 8.924) | I2=54.9%, P=0.340 | |
| Cutoff point of the highest interval of cystatin C | ||||
| >1 mg/L | 2 | 2.238 (1.740, 2.880) | I2=0%, p=0.741 | I2=66.6%, P=0.084 |
| ≤1 mg/L | 3 | 1.499 (1.027, 2.187) | I2=62.6%, P=0.061 | |
| Mean age | ||||
| ≥ 65 years | 3 | 1.594 (1.125, 2.259) | I2=79.8%, P=0.075 | I2=27.2%, P=0.241 |
| < 65 years | 2 | 3.101 (1.078, 8.924) | I2=54.9%, P=0.340 | |
| Cutoff point of the lowest interval of cystatin C | ||||
| <0.90 mg/L | 2 | 2.238 (1.740, 2.880) | I2=0%, p=0.741 | I2=66.6%, P=0.084 |
| ≥0.90 mg/L | 3 | 1.499 (1.027, 2.187) | I2=62.6%, P=0.061 |
Sensitivity analysis and publication bias
Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially (Figure 3). Publication bias was determined by Begg’s test and visualization of funnel plot. There was no evidence of publication bias (P=0.436) (Figure 4).
Figure 3.
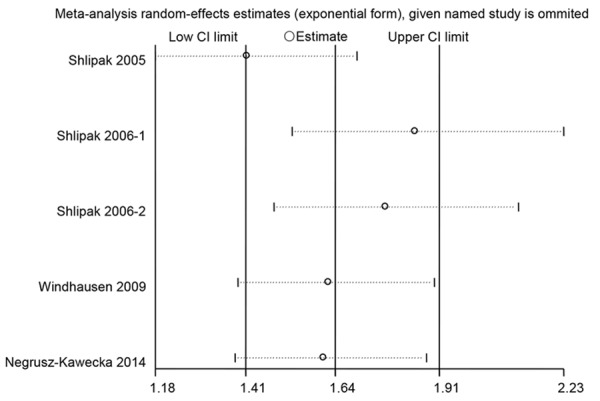
The stability of the study was detected through sensitivity analysis.
Figure 4.
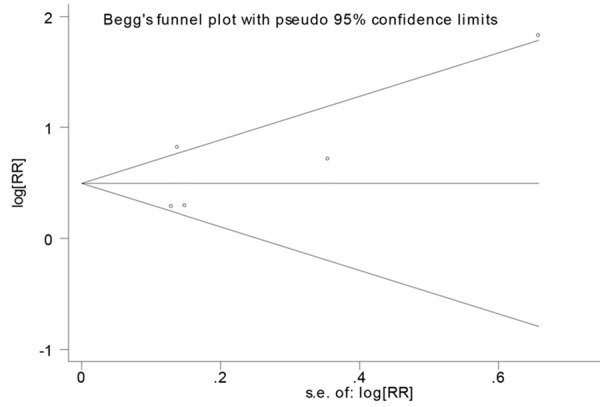
Funnel plot for studies in the association between cystatin C with myocardial infarction.
Discussion
Chronic kidney disease is a growing problem in everyday clinical practice, and is proven as a significant risk factor for cardiovascular disorders. It has been reported for many years that patients with end-stage renal disease have a high prevalence of cardiovascular disease [17]. Previous studies also find that mild to moderate renal impairment has also been shown to be strongly associated with cardiovascular events in patients in the general population [18,19]. Cystatin C has been shown to be a better endogenous marker of GFR than creatinine in chronic kidney disease [20]. With the introduction of rapid, sensitive, and precise immunoassays, it is now possible to use cystatin C as a marker of renal function routinely in the clinic. Recently, a clinic study of 9988 participants, which investigated cystatin C to predict coronary heart disease and heart failure, found that higher cystatin C concentrations were associated strongly with cardiovascular disorders and more predictive than GFR [21]. Thus, as a marker of renal function, cystatin C is a powerful tool for risk evaluation of cardiovascular disease above and beyond its role as a surrogate for GFR. The strong association between renal function and cardiovascular disorders probably is explained by several cooperating mechanisms. One reason is that renal dysfunction indicates a generalized atherosclerosis and vascular damage [22]. In addition, being a marker of increased risk, renal dysfunction may directly promote atherosclerosis by causing changes in parameters such as blood pressure, lipids, lipoproteins, homocysteine, and CRP [23]. Cystatin C which is produced and secreted by cardio myocytes, its synthesis is elevated when the heart is subjected to ischemia, and its accumulation can lead to adverse effects, such as a build-up of amyloid deposits in vascular walls [16,24].
In the present study, Our meta-analysis of over 10000 participants from 4 articles with 5 prospective studies showed that, compared with individuals with the lowest baseline cystatin C levels, those with the highest levels have a 78% increase in the risk of myocardial infarction. All studies included in our meta-analysis reported a multivariate-adjusted relative risk, mitigating the possibility of known confounding influencing our results. Cystatin C is not only a filtration marker, but also more important a tool for predicting the risk of myocardial infarction. This may be a result of extra renal effects of molecules previously considered exclusively indicating GFR [25]. For instance, cystatin C is affected by smoking, obesity, and inflammation, which themselves may be associated with increased risk [26-28]. Therefore, we performed the meta-analysis using multivariate-adjusted relative risk (RR) and 95% confidence interval (CI), and adjusted our analyses for these and conventional risk factors of myocardial infarction. Importantly, we found significantly increased probability of myocardial infarction across all the included studies, suggesting the possibility that cystatin C has an active role in biological processes leading to myocardial infarction.
As far as we know, this is the most comprehensive meta-analysis to estimate the association between cystatin C with myocardial infarction. We made sure to minimize the bias by means of study procedure. Not only did we search Pubmed and Science direct to identify potential studies, but also we manually examined all reference lists from relevant studies. The inclusion of only longitudinal studies strengthened the robustness of our findings because issues of selection bias, recall bias, and reverse causality were minimized. Sensitivity analysis showed that excluding any one study from the pooled analysis did not vary the results substantially. Publication bias was also absent, as determined by visualization of funnel plot and Begg’s test. However, the possible limitations of our study must be considered. First, the studies varied with respect to the characteristics of participants and follow-up duration, and indeed heterogeneity of the studies was found by formal analysis. To explore the potential heterogeneity, we performed subgroup analyses and identified study location, mean age and follow-up duration as sources of the variation. Significant heterogeneity between pooled analyses were noted for study location (USA versus Europe, I2=66.6%, P=0.084), mean age (<65 years versus ≥65 years, I2=66.6%, P=0.084), follow-up duration (≤5 years versus >5 years, I2=66.6%, P=0.084). Second, each included study had its own adjustment for variables. However, it is impossible for us to apply a uniform adjustment for variables to all studies, because this study was not an individual-level meta-analysis. Third, cystatin C is a novel biomarker, and the best cutoff point is still under debate. Our current meta-analysis does not solve this issue. Despite these limitations, our findings point out new direction for future research. We suggest that further investigation should be performed to clarify whether measurement of cystatin C can usefully reduce the myocardial infarction beyond established predictors already in clinical use.
Conclusion
This meta-analysis showed that cystatin C is strongly and independently associated with subsequent risk of myocardial infarction. Further investigation is warranted to clarify whether measurement of cystatin C can usefully reduce the myocardial infarction beyond established predictors already in clinical use.
Disclosure of conflict of interest
None.
References
- 1.Suzuki H, Kobayashi K, Ishida Y, Kikuta T, Inoue T, Hamada U, Okada H. Patients with biopsy-proven nephrosclerosis and moderately impaired renal function have a higher risk for cardiovascular disease: 15 years’ experience in a single, kidney disease center. Ther Adv Cardiovasc Dis. 2015;9:77–86. doi: 10.1177/1753944715578596. [DOI] [PubMed] [Google Scholar]
- 2.Katsiki N, Elisaf M. Multifactorial treatment for improvement of renal function and cardiovascular risk: an ATTEMPT for patients with metabolic syndrome and chronic kidney disease. Curr Med Res Opin. 2011;27:1669–1672. doi: 10.1185/03007995.2011.596410. [DOI] [PubMed] [Google Scholar]
- 3.Facila L, Bertomeu V, Bertomeu-Gonzalez V, Morillas P, Mazon P, Gonzalez-Juanatey JR VIIDA study. Association between renal function and cardiovascular disease in patients with left ventricular hypertrophy. VIIDA study. J Clin Hypertens (Greenwich) 2009;11:303–308. doi: 10.1111/j.1751-7176.2009.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrone RD, Madias NE, Levey AS. Serum creatinine as an index of renal function: new insights into old concepts. Clin Chem. 1992;38:1933–1953. [PubMed] [Google Scholar]
- 5.Grubb A, Bell M. Cystatin C: More than a renal function marker. Rev Clin Esp. 2015;215:102–103. doi: 10.1016/j.rce.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Woo KS, Choi JL, Kim BR, Kim JE, Han JY. Clinical usefulness of serum cystatin C as a marker of renal function. Diabetes Metab J. 2014;38:278–284. doi: 10.4093/dmj.2014.38.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coll E, Botey A, Alvarez L, Poch E, Quinto L, Saurina A, Vera M, Piera C, Darnell A. Serum cystatin C as a new marker for noninvasive estimation of glomerular filtration rate and as a marker for early renal impairment. Am J Kidney Dis. 2000;36:29–34. doi: 10.1053/ajkd.2000.8237. [DOI] [PubMed] [Google Scholar]
- 8.Fliser D, Ritz E. Serum cystatin C concentration as a marker of renal dysfunction in the elderly. Am J Kidney Dis. 2001;37:79–83. doi: 10.1053/ajkd.2001.20628. [DOI] [PubMed] [Google Scholar]
- 9.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20:2214–2222. doi: 10.1681/ASN.2008090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herget-Rosenthal S, Trabold S, Pietruck F, Holtmann M, Philipp T, Kribben A. Cystatin C: efficacy as screening test for reduced glomerular filtration rate. Am J Nephrol. 2000;20:97–102. doi: 10.1159/000013564. [DOI] [PubMed] [Google Scholar]
- 11.Muslimovic A, Tulumovic D, Hasanspahic S, Hamzic-Mehmedbasic A, Temimovi R. Serum cystatin C - marker of inflammation and cardiovascular morbidity in chronic kidney disease stages 1-4. Mater Sociomed. 2015;27:75–78. doi: 10.5455/msm.2015.27.75-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arpegard J, Viktorin A, Chang Z, de Faire U, Magnusson PK, Svensson P. Comparison of heritability of Cystatin C- and creatinine-based estimates of kidney function and their relation to heritability of cardiovascular disease. J Am Heart Assoc. 2015;4:e001467. doi: 10.1161/JAHA.114.001467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 14.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145:237–246. doi: 10.7326/0003-4819-145-4-200608150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Windhausen F, Hirsch A, Fischer J, van der Zee PM, Sanders GT, van Straalen JP, Cornel JH, Tijssen JG, Verheugt FW, de Winter RJ Invasive versus Conservative Treatment in Unstable Coronary Syndromes (ICTUS) Investigators. Cystatin C for enhancement of risk stratification in non-ST elevation acute coronary syndrome patients with an increased troponin T. Clin Chem. 2009;55:1118–1125. doi: 10.1373/clinchem.2008.119669. [DOI] [PubMed] [Google Scholar]
- 16.Negrusz-Kawecka M, Poreba R, Hulok A, Sciborski K, Marczak J, Bankowski T. Evaluation of the significance of cystatin C levels in patients suffering from coronary artery disease. Adv Clin Exp Med. 2014;23:551–558. doi: 10.17219/acem/37222. [DOI] [PubMed] [Google Scholar]
- 17.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32(Suppl 3):S112–119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 18.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 19.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 20.Laterza OF, Price CP, Scott MG. Cystatin C: an improved estimator of glomerular filtration rate? Clin Chem. 2002;48:699–707. [PubMed] [Google Scholar]
- 21.Astor BC, Shafi T, Hoogeveen RC, Matsushita K, Ballantyne CM, Inker LA, Coresh J. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis. 2012;59:653–662. doi: 10.1053/j.ajkd.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerne D, Kaplan-Pavlovcic S, Kranjec I, Jurgens G. Mildly elevated serum creatinine concentration correlates with the extent of coronary atherosclerosis. Ren Fail. 2000;22:799–808. doi: 10.1081/jdi-100101965. [DOI] [PubMed] [Google Scholar]
- 23.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–152. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 24.Tousoulis D, Michalea S, Siasos G, Oikonomou E, Athanasiou D, Tourikis P, Kokkou E, Mazaris S, Konsola T, Papageorgiou N, Stefanadis C. Cystatin-C serum levels and vascular function in heart failure. Int J Cardiol. 2014;173:542–544. doi: 10.1016/j.ijcard.2014.03.083. [DOI] [PubMed] [Google Scholar]
- 25.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njolstad I, Solbu MD, Toft I, Eriksen BO. Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol. 2011;22:927–937. doi: 10.1681/ASN.2010050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Segarra A, de la Torre J, Ramos N, Quiroz A, Garjau M, Torres I, Azancot MA, Lopez M, Sobrado A. Assessing glomerular filtration rate in hospitalized patients: a comparison between CKD-EPI and four cystatin C-based equations. Clin J Am Soc Nephrol. 2011;6:2411–2420. doi: 10.2215/CJN.01150211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stevens LA, Schmid CH, Greene T, Li L, Beck GJ, Joffe MM, Froissart M, Kusek JW, Zhang YL, Coresh J, Levey AS. Factors other than glomerular filtration rate affect serum cystatin C levels. Kidney Int. 2009;75:652–660. doi: 10.1038/ki.2008.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White CA, Akbari A, Doucette S, Fergusson D, Ramsay T, Hussain N, Dinh L, Filler G, Lepage N, Knoll GA. Effect of clinical variables and immunosuppression on serum cystatin C and beta-trace protein in kidney transplant recipients. Am J Kidney Dis. 2009;54:922–930. doi: 10.1053/j.ajkd.2009.06.003. [DOI] [PubMed] [Google Scholar]


