Abstract
Hypoxia-inducible factor 1-alpha (HIF-1α) protects hypoxic cells from apoptosis or necrosis under ischemic and anoxic conditions. Allicin is characterized by the anti-cancer characteristics. This study aims to explore whether allicin is involved in renal clear cell carcinoma progression through HIF-1α. A total of 40 RCC tissues and 39 normal renal tissues were collected H&E and immunohistochemistry were applied to study morphology changes. MTT assay and flow cytometry (FCM) were used to analyze cell viability and apoptosis. In vitro colony formation assay and wound healing assay were conducted to explore cell migration. The protein levels of Bcl-2, VEGF and HIF-1α were increased in RCC tissues. More importantly, treatment with allicin significantly decreased HIF-1α protein level, thereby reducing Bcl-2 and VEGF expression. In addition, allicin also obviously enhanced apoptotic cells. And colony formation capacity and cell migration rate were reduced in RCC-9863 cells treated with allicin. Further study revealed that overexpression of HIF-1α could partially repress allicin-induced downstream effects. To conclude, allicin inhibits human renal clear cell carcinoma progression partially by suppressing HIF pathway.
Keywords: Allicin, RCC, HIF1α, apoptosis, migration
Introduction
Hypoxia pathway plays a key role in the pathologies of cancers [1,2]. It is reported to mainly maintain the appropriate level of oxygen concentration, thereby regulating the normal cellular metabolism [3]. In normal status, the level of the hypoxia-inducible factor-1α (HIF-1α) is regulated by the VHL tumor suppressor for ubiquitination and proteasomal degradation [4]. To respond to hypoxia, HIF-1α and HIF-2α can bind HIF-1β and initiate the expression of downstream genes thereby alleviating hypoxic stress [5]. HIF-1α induces the expression of VEGF, which regulate the expression of vascular development [6]. And HIF-1α can also interact with Bcl-2 and Bax, thereby regulating cell apoptosis.
Sporadic clear cell renal cell carcinoma (ccRCC) is the most common adult kidney cancer [7]. The underlying mechanism includes both environmental and genetic risks, such as smoking, obesity and acquired kidney disease [8-10]. Besides, renal cell carcinoma is also suggested to be characterized by oxidative stress [11,12]. However, the specific pathologic mechanism has far from been elucidated.
Garlic is widely used as food and remedy in China. It has been found that garlic is characterized by a variety of biological functions, such as anti-atherosclerotic, anti-hypertensive, anti-microbial, and anti-cancer roles [13]. Allicin is the major component of garlic, which is proved to inhibit cancer progression partially through relieving oxidative stress in gastric cancer, colon cancer, liver cancer and so on [14]. However, the mechanism by which allicin exerts its anti-oxidative function has not been fully elucidated. The present study offers new evidence showing that allicin suppresses RCC partially through inhibition of HIF-1α.
Materials and methods
Patients
A sample of 40 patients receiving curative radical or partial nephrectomy with a histological diagnose of RCC at West China Hospital of Sichuan University were collected between 2012 and 2014. None of these patients received adjuvant therapy. And the normal tissues were collected due to biopsy. Experiments involving human subjects are in accord with the Helsinki Declaration. The consent form was signed by all patients.
Histological examination
Samples obtained from patients were fixed in 10% formalin and routinely processed for paraffin embedding. Histologic sections of 4 μm were stained with hematoxylin and eosin. Two senior pathologists performed the evaluations of the specimens.
Cell culture
RCC-9863 Cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), streptomycin (100 mg/ml) and penicillin (100 IU/ml) at 37°C in a humidified atmosphere containing 5% CO2.
Effect of allicin on cell proliferation
RCC-9863 cells were cultured in six-cell chamber at 60-70% confluent. And different concentrations (0.016 mg/ml, 0.05 mg/ml, 0.1 mg/ml) of allicin were added to the cells for 48 hours. To determine the time-dependent effect, RCC-9863 cells were treated with 0.05 mg/ml allicin for 24 h, 48 h and 72 h, respectively. MTT assay was performed as previously described [15].
Western blotting analyses
15 mg of RCC tissues was collected in RIPA buffer (SolarBio, Beijing, China) containing 1% (v/v) PMSF (SolarBio), 0.3% (v/v) protease inhibitor (Sigma-Aldrich, St. Louis, MO, USA) and 0.1% (v/v) phosphorylated proteinase inhibitor (Sigma-Aldrich, St. Louis, MO, USA). After centrifugation, the supernatant was collected and the protein concentration was determined using a BCA protein assay kit (Millipore, Billerica, MA, USA). Then, proteins were separated on a 10% SDS-PAGE gel and transferred onto a PVDF membrane. The membrane was incubated with primary antibodies against HIF-1α, Bcl-2, Bax, VEGF and GAPDH (Cell signaling, Danvers, MA, USA; 1:1000) overnight at 4°C. GAPDH was used as the internal control.
Hoechst 33258 staining
RCC-9863 cells (1 × 105 cells per well) were cultured in six-well tissue culture plates. At 70-80% confluence, the cells were incubated for 16 h in serum-free RPMI-1640 medium. Then, 0.05 mg/ml allicin was added to the fresh medium and pre-incubated with the cells for 48 h. After drug treatment, the medium was removed, and the cells were fixed with 4% formaldehyde (Zhongshan Technology) in PBS for 20 min at room temperature. Then, the cells were washed three times with cold PBS and stained with Hoechst 33258 (10 μg/mL) (Sigma-Aldrich, St. Louis, MO, USA) for 5 min. After staining, the cells were further washed with cold PBS and examined under fluorescence microscope (Leica CM3000; Leica Microsystems GmbH).
Quantification of apoptotic cells
An Annexin-V FITC-PI Apoptosis Kit (Invitrogen, Carlsbad, CA) was applied to determine cell apoptosis. Briefly, cells were washed with PBS (5 min/time, 3 times) and suspended at 2-3 × 106 cells/ml in 1 × Annexin-V Binding Buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2). Annexin-V FITC and Propidium Iodide Buffer were added to the cells, respectively. Then, they were incubated at room temperature for 15 min in darkness. After incubation, the cells were filtered by a filter screen and the cells were analyzed by flow cytometry (Becton Dickinson, Franklin Lakes, NJ) within 1 h of staining.
In vitro colony formation assay
RCC-9863 cells that were treated with 0.05 mg/ml were seeded into 6-well plates at 60-70% confluent. The cells were cultured at 37°C for two weeks and then stained with 0.1% crystal violet for 10 minutes. After washing 3 times with PBS, the clones were counted, and the data represent the mean +/- standard deviation (SD) from experiments repeated three times.
Cell migration assay
The in vitro wound healing assay was performed according to a published protocol [16]. Briefly, RCC-9863 cells were seeded in 6-well plates to form a confluent monolayer. The monolayer was scratched with a sterile 10 μl pipette tip, and the floating cells were carefully removed by PBS. Then, the cells were cultured in RPMI-1640 medium without FBS at 37°C in a 5% CO2 atmosphere. The wound scratches were photographed at 0 hour and 12 hours after scraping.
Adenovirus vector construction
The adenovirus vector (Ad)-HIF-1α and Ad-con were purchased from the Chinese National Human Genome Center (Beijing, China).
Statistical analysis
The data were expressed as the mean ± SEM. The number of independent experiments was represented by “n”. Multiple comparisons were performed using one-way ANOVA followed by Tukey’s multiple-comparison test, where P<0.05 was considered significant.
Results
Increased HIF-1α protein level in RCC tissues
First of all, we explored the protein levels of HIF-1α in RCC tissues. As shown in Figure 1A, the protein level of HIF-1α was increased in RCC tissues compared with normal tissues. Immunohistology assay also showed extensive HIF-1α expression in RCC tissues.
Figure 1.
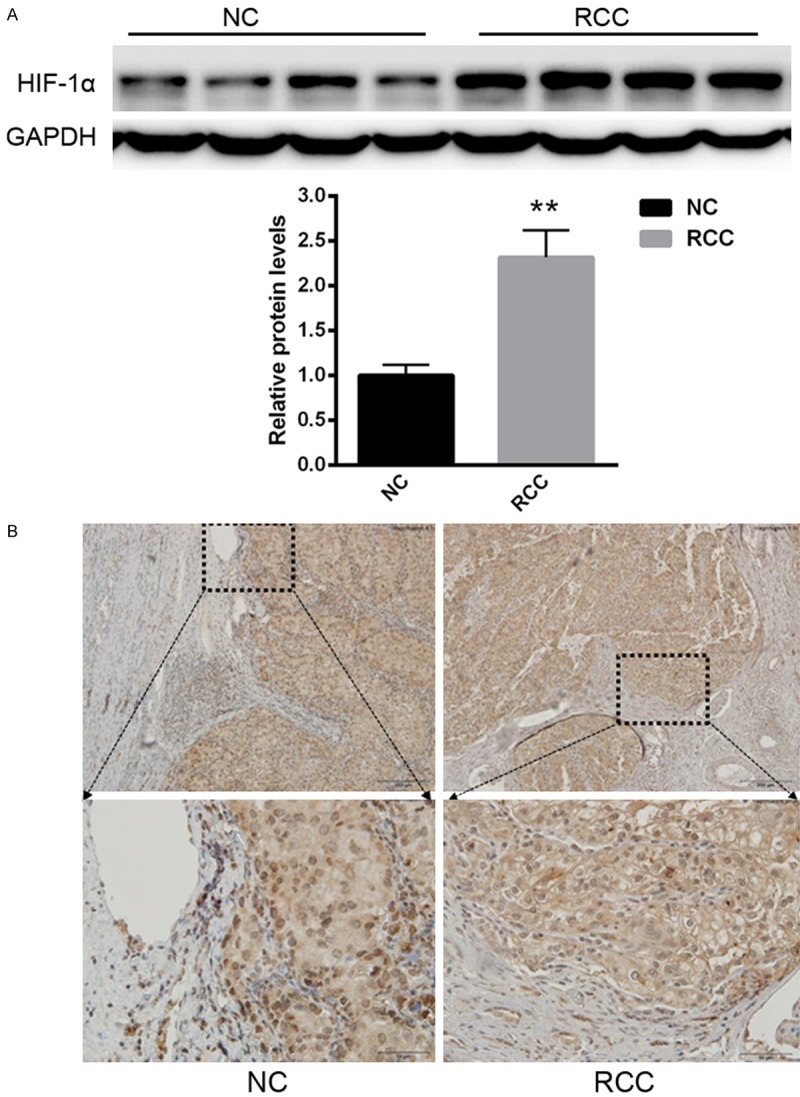
The protein level of HIF-1α was increased in RCC tissues compared with normal tissues. A. Western blot analysis of HIF-1α expression. B. IHC staining of HIF-1α expression. **P<0.01 versus control.
RCC-9863 cell viability was affected by allicin in a dose- and time-dependent manner
To investigate the impacts of allicin on cell viability, RCC-9863 cells were treated with allicin at the concentration of 0.016 mg/ml, 0.05 mg/ml, 0.1 mg/ml for 48 h. Then, cell viability was analyzed by MTT assay. As shown in Figure 2A, cell viability of RCC-9863 cells was significantly decreased by about 35% when the concentration of allicin was 0.1 mg/ml. Furthermore, RCC-9863 cells were also treated with 0.05 mg/ml allicin for 24 hours, 48 hours and 72 hours, the corresponding cell viability was determined by MTT. According to the statistics, RCC-9863 cell viability was reduced by about 16% and 24% at 48 hours and 72 hours respectively (Figure 1B). These data suggested that RCC-9863 cell viability was significantly reduced by allicin in a dose- and time-dependent manner.
Figure 2.
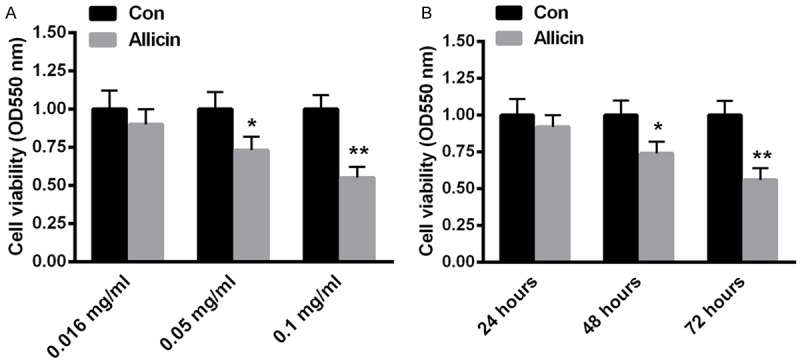
RCC-9863 cell viability was affected by allicin in a dose- and time-dependent manner. RCC-9863 cells were exposed to allicin at the concentration of 0.016 mg/mL, 0.05 mg/mL, and 0.1 mg/mL for 48 h (A). (B) RCC-9863 cells were treated with 0.05 mg/mL allicin for 24 hours, 48 hours and 72 hours. Cell viability was determined by MTT assay using the spectrophotometer at 550 nm. Data represent the means ± SEM, n=6 independent experiments. *P<0.05, **P<0.01 versus control.
Allicin enhanced RCC-9863 cells apoptosis and decreased colony formation, cell migration
We further treated RCC-9863 cells with 0.05 mg/ml allicin for 48 h. Hoechst staining showed obvious apoptotic cell morphology (Figure 3A). Besides, flow cytometry assay was also conducted to determine cell apoptosis rate. As shown in Figure 3B, allicin enhanced cell apoptosis by over ~1.5 fold. In vitro colony formation assay demonstrated that the number of colonies was significantly decreased in RCC-9863 cells with 0.05 mg/ml allicin (Figure 3C). Moreover, the in vitro wound healing assay indicated that allicin could significantly decrease cell migration (Figure 3D). These data suggested the anti-tumor function of allicin in renal cancer cells.
Figure 3.
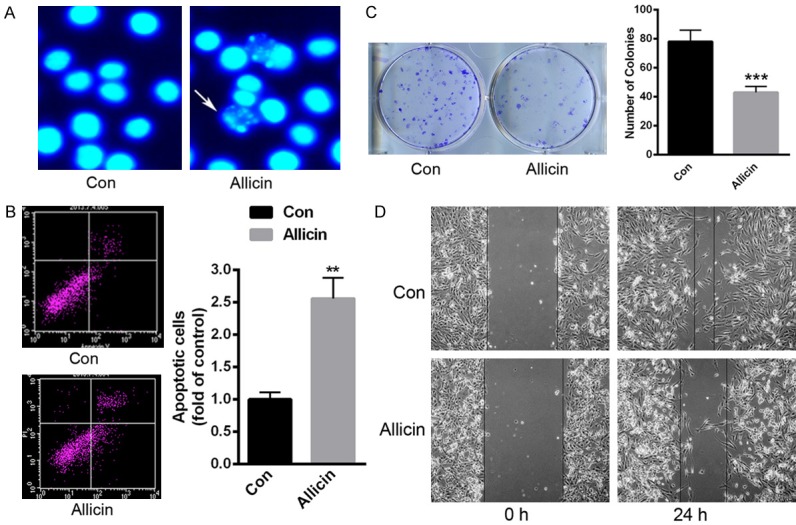
RCC-9863 cell apoptosis was enhanced, while colony formation and cell migration was decreased when these cells were incubated with 0.05 mg/ml allicin for 48 h. A. Hoechst staining demonstrated the enhanced cell apoptosis. B. An annexin V and PI kit was applied to detect cell apoptosis. C. In vitro colony formation assay was performed to determine the number of colonies in RCC-9863 cells with 0.05 mg/ml allicin. D. The in vitro wound healing assay was conducted to explore cell migration in RCC-9863 cells with 0.05 mg/ml allicin. *P<0.05, **P<0.01 versus control.
Allicin suppressed RCC progression partially through HIF-1α
We further explored the specific molecular mechanism of allicin in RCC progression. As shown in Figure 4A, allicin treatment significantly decreased the protein level of HIF-1α, which is an important regulator in tumors. Moreover, the downstream signaling effectors, such as VEGF and Bcl-2, were increased, while the protein level of Bax was enhanced. Besides, an adenovirus vector overexpressing HIF-1α was introduced into RCC-9863 cells. No significant difference was identified in cells transfected with ad-con or ad- HIF-1α (Figure 4B). More importantly, we found that over expression of HIF-1α significantly enhanced the expression of VEGF and Bcl-2, thereby prompting cell proliferation. In comparison, allicin treatment could significantly reverse HIF-1α overexpression-induced VEGF and Bcl-2 upregulation (Figure 4C). These data indicated that allicin suppressed RCC progression partially through HIF-1α.
Figure 4.
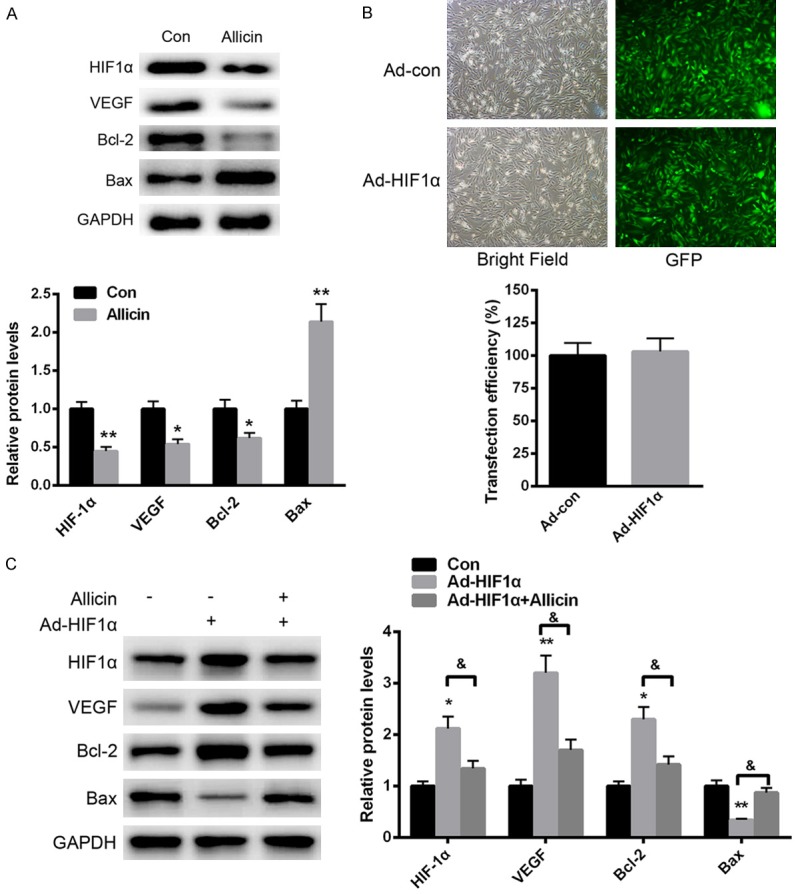
Allicin suppressed RCC progression partially through HIF-1α. A. Western analysis of HIF-1α expression after allicin treatment. B. Transfection efficiency was determined in RCC-9863 cells transfected with ad-con or ad-HIF-1α. C. Allicin treatment could significantly reverse HIF-1α overexpression-induced VEGF and Bcl-2 upregulation. Data represent the means ± SEM, n=3 independent experiments. *P<0.05, **P<0.01 versus control.
Discussion
Due to the high rate of recurrence and distant metastases in RCC, although the clinical treatment strategies and health surveillance have been greatly improved, the RCC prognosis remains unsatisfactory [17]. In the present study, we explored the protein level of HIF-1α in RCC tissues. Compared with normal tissue, we found HIF-1α expression was significantly enhanced. As the bulb of Allium, allicin is suggested to provide a new therapeutic method due to its potential anti-carcinoma characteristics [18]. Recent study suggests that allicin induces cancer cell apoptosis through the activation of Caspase-3, Caspase-8 and caspase-9 [19]. In addition, allicin is also characterized by anti-inflammatory and anti-oxidant functions [20,21]. However, the correlation between allicin and HIF-1α has never been explored.
As an oxygen-sensitive transcriptional factor, HIF mainly acts as a heterodimer including HIF-1α and HIF-1β [22]. Under hypoxia, HIF-1α was obviously increased and it was easily decreased under normoxia [23]. HIF-1α significantly prompts the downstream gene expression, such as VEGF and EPO [24]. These factors play key roles in the maintenance of cell survival. In this study, we found that the expression of HIF-1α was significantly increased in RCC tissues. More importantly, we found that allicin treatment significantly decreased HIF-1α expression in RCC-9863 cells. Meanwhile, the downstream effectors, VEGF and Bcl-2 were also decreased. Besides, allicin treatment could partially reverse HIF-1α overexpression induced effects, indicating the protective role of allicin.
MTT assay demonstrated that allicin reduced RCC-9863 cell viability in a time- and dose-dependent manner. Hoechst staining and flow cytometry analysis indicated that allicin induced RCC cell apoptosis, which suggested that allicin acts as an anti-tumor drug. We also demonstrated that allicin reduced the level of HIF-1α, which is a biomarker for cancers due to the relative hypoxia environment. Further analysis revealed that allicin treatment also reduced the level of Bcl-2, an anti-apoptotic member. By controlling mitochondrial membrane channels, the decrease of Bcl-2 and the enhancement of Bax significantly lead to cancer cell apoptosis. Therefore, the ability of allicin to enhance the ratio of Bax/Bcl-2 suggests a potential pro-apoptotic function in RCC cancer cells.
To conclude, our results suggest that HIF-1α expression was significantly increased in RCC tissues, while allicin leads to the reduction of HIF-1α thereby inhibiting RCC cancer progression.
Disclosure of conflict of interest
None.
References
- 1.Eskandani M, Abdolalizadeh J, Hamishehkar H, Nazemiyeh H, Barar J. Galbanic acid inhibits HIF-1α expression via EGFR/HIF-1α pathway in cancer cells. Fitoterapia. 2015;101:1–11. doi: 10.1016/j.fitote.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Hsia TC, Liu WH, Qiu WW, Luo J, Yin MC. Maslinic acid induces mitochondrial apoptosis and suppresses HIF-1α expression in A549 lung cancer cells under normoxic and hypoxic conditions. Molecules. 2014;19:19892–906. doi: 10.3390/molecules191219892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan G, Zhang Y, Lu Y, Liu L, Shi D, Wen Y, Yang L, Ma Q, Liu T, Zhu X, Qiu X, Zhou Y. The HIF-1α/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells. Cancer Lett. 2015;357:254–64. doi: 10.1016/j.canlet.2014.11.034. [DOI] [PubMed] [Google Scholar]
- 4.Lessi F, Mazzanti CM, Tomei S, Di Cristofano C, Minervini A, Menicagli M, Apollo A, Masieri L, Collecchi P, Minervini R, Carini M, Bevilacqua G. VHL and HIF-1α: gene variations and prognosis in early-stage clear cell renal cell carcinoma. Med Oncol. 2014;31:840. doi: 10.1007/s12032-014-0840-8. [DOI] [PubMed] [Google Scholar]
- 5.Tian W, Wang Y, Xu Y, Guo X, Wang B, Sun L, Liu L, Cui F, Zhuang Q, Bao X, Schley G, Chung TL, Laslett AL, Willam C, Qin B, Maxwell PH, Esteban MA. The hypoxia-inducible factor renders cancer cells more sensitive to vitamin C-induced toxicity. J Biol Chem. 2014;289:3339–3351. doi: 10.1074/jbc.M113.538157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 7.Leibovich BC, Lohse CM, Crispen PL, Boorjian SA, Thompson RH, Blute ML, Cheville JC. Histological subtype is an independent predictor of outcome for patients with renal cell carcinoma. J Urol. 2010;183:1309–1315. doi: 10.1016/j.juro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 8.Naito S, Tomita Y, Rha SY, Uemura H, Oya M, Song HZ, Zhong LH, Wahid MI. Kidney Cancer Working Group report. Jpn J Clin Oncol. 2010;40(Suppl 1):i51–56. doi: 10.1093/jjco/hyq127. [DOI] [PubMed] [Google Scholar]
- 9.Lindblad P. Epidemiology of renal cell carcinoma. Scand J Surg. 2004;93:88–96. doi: 10.1177/145749690409300202. [DOI] [PubMed] [Google Scholar]
- 10.Murai M, Oya M. Renal cell carcinoma: etiology, incidence and epidemiology. Curr Opin Urol. 2004;14:229–233. doi: 10.1097/01.mou.0000135078.04721.f5. [DOI] [PubMed] [Google Scholar]
- 11.Pirincci N, Kaba M, Gecit I, Gunes M, Yuksel MB, Tanik S, Arslan A, Demir H. Serum prolidase activity, oxidative stress, and antioxidant enzyme levels in patients with renal cell carcinoma. Toxicol Ind Health. 2013 doi: 10.1177/0748233713498924. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Verheul HM, van Erp K, Homs MY, Yoon GS, van der Groep P, Rogers C, Hansel DE, Netto GJ, Pili R. The relationship of vascular endothelial growth factor and coagulation factor (fibrin and fibrinogen) expression in clear cell renal cell carcinoma. Urology. 2010;75:608–614. doi: 10.1016/j.urology.2009.05.075. [DOI] [PubMed] [Google Scholar]
- 13.Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y. Intake of garlic and its bioactive components. J Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch K, Danilenko M, Giat J, Miron T, Rabinkov A, Wilchek M, Mirelman D, Levy J, Sharoni Y. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer. 2000;38:245–254. doi: 10.1207/S15327914NC382_14. [DOI] [PubMed] [Google Scholar]
- 15.Yang F, Wang H, Jiang Z, Hu A, Chu L, Sun Y, Han J. MicroRNA‑19a mediates gastric carcinoma cell proliferation through the activation of nuclear factor‑κB. Mol Med Rep. 2015;12:5780–6. doi: 10.3892/mmr.2015.4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 17.Kim SP, Alt AL, Weight CJ, Costello BA, Cheville JC, Lohse C, Allmer C, Leibovich BC. Independent validation of the 2010 American Joint Committee on Cancer TNM classification for renal cell carcinoma: results from a large, single institution cohort. J Urol. 2011;185:2035–2039. doi: 10.1016/j.juro.2011.02.059. [DOI] [PubMed] [Google Scholar]
- 18.Andualem B. Combined antibacterial activity of stingless bee (Apis mellipodae) honey and garlic (Allium sativum) extracts against standard and clinical pathogenic bacteria. Asian Pac J Trop Biomed. 2013;3:725–731. doi: 10.1016/S2221-1691(13)60146-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mozaffari-Khosravi H, Hesabgar HA, Owlia MB, Hadinedoushan H, Barzegar K, Fllahzadeh MH. The effect of garlic tablet on pro-inflammatory cytokines in postmenopausal osteoporotic women: a randomized controlled clinical trial. J Diet Suppl. 2012;9:262–271. doi: 10.3109/19390211.2012.726703. [DOI] [PubMed] [Google Scholar]
- 20.Li L, Sun T, Tian J, Yang K, Yi K, Zhang P. Garlic in clinical practice: an evidence-based overview. Crit Rev Food Sci Nutr. 2013;53:670–681. doi: 10.1080/10408398.2010.537000. [DOI] [PubMed] [Google Scholar]
- 21.Pittler MH, Ernst E. Clinical effectiveness of garlic (Allium sativum) Mol Nutr Food Res. 2007;51:1382–1385. doi: 10.1002/mnfr.200700073. [DOI] [PubMed] [Google Scholar]
- 22.Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Structural and functional analysis of hypoxiainducible factor 1. Kidney Int. 1997;51:553–555. doi: 10.1038/ki.1997.77. [DOI] [PubMed] [Google Scholar]
- 23.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 24.Pfander D, Cramer T, Swoboda B. Hypoxia and HIF-1alpha in osteoarthritis. Int Orthop. 2005;29:6–9. doi: 10.1007/s00264-004-0618-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


