Abstract
Background: The value of hepatic resection (HR) for huge hepatocellular carcinomas (HCC) (≥ 10 cm in diameter) remains controversial. The aim of this study is to evaluate the efficacy of hepatic resection (HR) for patients with huge HCC. Methods: A total of 739 patients with huge HCC (≥ 10 cm in diameter) (huge HCC group, n = 244) or small HCC (< 10 cm in diameter) (small HCC group, n = 495) who received initial HR were retrospectively analyzed. Overall survival (OS) and disease-free survival (DFS) were obtained using the Kaplan-Meier method and compared by Log-Rank test. Prognostic factors of huge HCC were identified based on Cox regression analyses. Results: The hospital mortality of these two groups were similar (P = 0.252). The 5-year OS of huge HCC group and small HCC group were 30.3% and 51.9%, respectively (P < 0.001). Uninodular huge HCC had a significant higher 5-year OS (50.6%) than mutinodular huge HCC (26.9%) (P = 0.016). Multivariate analysis revealed that uninodular huge HCC and absence of PVTT independently predicted better OS for huge HCC patients. Conclusion: HR is a safe and effective approach for the treatment of huge HCC, especially for the uninodular subtype.
Keywords: Hepatic resection, hepatocellular carcinoma, huge, uninodular, mortality, overall survival, disease-free survival
Introduction
Hepatocellular carcinoma (HCC) is one of the most common causes of cancer death worldwide [1]. Hepatic resection (HR), radiofrequency ablation (RFA), and percutaneous ethanol injection (PEI) are widely used for the treatment of small HCC (< 10 cm in diameter) [2]. Besides, the results of our previous study indicated that HR should be first-line treatment for early-stage large (> 5 cm) HCC [3]. However, treatments for huge HCC (≥ 10 cm) of which most are often considered to be at advanced stage at the time of diagnosis and unresectable are still controversial. Theoretically, transarterial chemoembolization (TACE) is an appropriate approach for the treatment of unresectable huge HCC [4,5]. But the 5-year survival rate of huge HCC patients after TACE treatment was less than 10% [6,7]. On the other hand, it is recommended by most published series that HR could provide acceptable long-term survival for huge HCC [8-24]. However, HR may be associated with increased morbidity and mortality because of the technical difficulties and possible postoperative hepatic decompensation, especially when HCC patients were with cirrhosis. Thus the efficacy of HR needs further investigation. Moreover, a specific subtype of HCC, uninodular huge HCC, was proposed by Yang LY et al. to have similar clinicopathologic features and prognosis after HR compared with small HCC [25]. However, the novel concept was rarely validated in other published series. The aim of the present study was to evaluate the efficacy of HR for patients with huge HCC. In addition, prognosis of subtypes of huge HCC was further investigated.
Materials and methods
Ethics statement
First, this study was conducted in accordance with the Declaration of Helsinki. Secondly, written informed consent was given by all participants for their clinical records to be used in this study. Lastly, it was approved by the Institutional Review Board of Affiliated Tumor Hospital of Guangxi Medical University.
Patients
From April 2007 to April 2011, a total of 1395 HCC patients with newly diagnosed HCC in the department of hepatobiliary surgery at our Hospital were enrolled and retrospectively analyzed (Figure 1). Of these, 362 were excluded because they had received initial HCC treatment at other centers. Among the remaining 1033 patients, 785 patients underwent curative HR and all patients had a confirmed histological diagnosis of HCC. Of these patients, 46 were excluded because of incomplete data. The remaining 739 patients were categorized into two groups: patients with tumors larger than 10 cm in diameter (huge HCC group, n = 244) and patients with tumors less than 10 cm (small HCC group, n = 495). The clinicopathological characteristics of the two groups were compared (Table 1).
Figure 1.
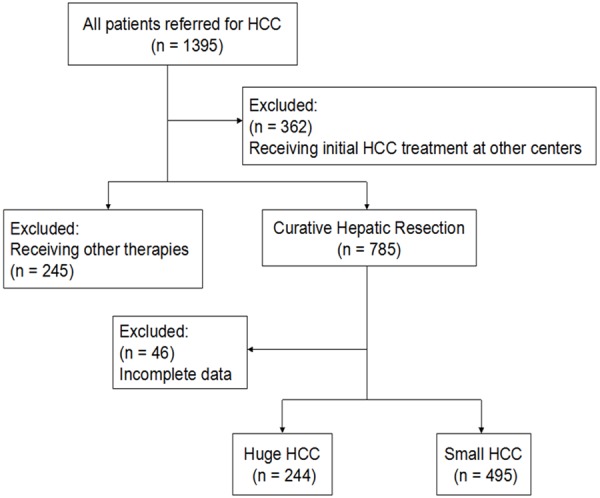
Study flowchart.
Table 1.
Comparison of clinicopathologic features between huge HCC patients and small HCC patients
| Variable | Huge HCC (n = 244) | Small HCC (n = 495) | P value |
|---|---|---|---|
| Gender (M/F) | 209/35 | 436/59 | 0.352 |
| Age (year) | 46.8 ± 11.3 | 50.3 ± 11.2 | < 0.001 |
| Child-Pugh class, n (%) | |||
| A | 210 (86) | 431 (87) | 0.705 |
| B | 34 (14) | 64 (13) | |
| HBsAg (+), n (%) | 209 (86) | 425 (86) | 0.991 |
| Platelet count (109/L) | 188.1 ± 60.5 | 169.5 ± 66.9 | 0.033 |
| ALT (U/L) | 42.4 ± 33.7 | 44.1 ± 47.5 | 0.835 |
| AST (U/L) | 52.1 ± 32.2 | 51.4 ± 25.8 | 0.892 |
| Total bilirubin (μmol/L) | 13.5 ± 7.4 | 21.2 ± 38.5 | 0.002 |
| Direct bilirubin (μmol/L) | 5.3 ± 3.9 | 5.4 ± 4.1 | 0.808 |
| Albumin (g/L) | 40.4 ± 5.3 | 40.5 ± 3.9 | 0.967 |
| Prothrombin time (s) | 14.8 ± 24.3 | 12.9 ± 1.7 | 0.078 |
| Prealbumin (mg/L) | 188.1 ± 60.5 | 214.1 ± 62.9 | 0.002 |
| AFP (ng/ml), n (%) | |||
| ≥ 400 | 99 (41) | 107 (22) | < 0.001 |
| < 400 | 145 (59) | 388 (78) | |
| Cirrhosis, n (%) | 67 (27) | 129 (26) | 0.685 |
| Tumor size (cm) | 12.0 ± 2.3 | 4.8 ± 2.3 | < 0.001 |
| Tumor number, n (%) | |||
| Uninodular | 42 (17) | 86 (17) | 0.957 |
| Multinodular | 202 (83) | 409 (83) | |
| PVTT, n (%) | |||
| Present | 104 (43) | 33 (7) | < 0.001 |
| Absent | 140 (57) | 462 (93) | |
| Encapsulation, n (%) | |||
| Present | 120 (51) | 240 (48) | 0.859 |
| Absent | 124 (49) | 255 (52) | |
| Edmondson-grade, n (%) | |||
| I-II | 127 (52) | 272 (55) | 0.457 |
| III-IV | 117 (48) | 223 (45) |
Values with “±” are written as mean ± SD. AFP, alpha-fetoprotein. ALT, alanine aminotransferase; AST, aspartate aminotransferase; PVTT, portal vein tumor thrombosis.
Hepatic resection
Indications for surgery were lack of ascites, hepatic encephalopathy, and hypersplenism, as well as the presence of appropriate residual liver volume, as determined by volumetric computed tomography [26]. The HR technique was performed as described [3,27,28]. The clinicopathological data for these patients are summarized in Table 1.
Follow-up
After HR, all survival patients received liver function test, measurement of serum α-fetoprotein, abdominal ultrasonography, dynamic liver computer tomography (CT), magnetic resonance imagine (MRI), and chest radiography examination for at 3-month intervals for the first year, and then every 6 months. Recurrence of HCC was identified by new or growing lesions on imaging with appearances typical of HCC or a rising AFP. Lesions not typical of HCC were confirmed by biopsy. When recurrence was confirmed, secondary HR, RFA, or TACE was the treatments of choice. Hospital mortality was defined as death that occurred within 30 days of the operation. Overall survival (OS) was determined as from the day of surgery to the date of the last follow-up. Disease-free survival (DFS) was determined as from the date of surgery to the date when disease recurrence was confirmed with abdominal CT.
Statistical analyses
All statistical analyses were performed using the statistical software SPSS statistics 19.0 (IBM, USA). Continuous variables are expressed as mean ± standard deviation and analyzed using the independent-samples t test. Categorical variables were analyzed using the chi-square test or Fisher exact test. OS and DFS analyses were done using the Kaplan-Meier method, and the difference between the two groups was compared by Log-Rank test. Cox proportional hazard regression analysis was used to identify independent prognostic factors.
Results
Clinicopathologic characteristics
The clinicopatholgical characteristics of 739 HCC patients were shown in Table 1. There were 209 (85.7%) men and 35 (14.3%) women in huge HCC group and the mean age was 46.8 ± 11.3 year, which was significantly younger than that in small HCC group (50.3 ± 11.2 year) (P < 0.001). The proportion of positive for hepatitis B surface antigen (HBsAg) in both groups was more than 85%. Besides, huge HCC group had significant larger tumor size, higher AFP level and more presence of PVTT (All P < 0.001) than small HCC group (P < 0.05). There was no significant difference in other clinicopatholgical parameters such as levels of AFP; albumin; alanine aminotransferase (ALT); aspartate aminotransferase (AST) or prothrombin time (PT) between the two groups.
Surgical outcome
Surgical outcome in the huge HCC group and small HCC group were summarized in Table 2. Huge HCC group had a significantly lower rate of surgical margin > 1 cm (P < 0.001) and higher intrahepatic recurrence rate (P < 0.001) compared with small HCC group. Higher rate of postoperative complications was observed in huge HCC group (28.3% vs. 15.6% P < 0.001) and hydrothorax was the most common complications in both groups. The mortality rate in both groups were similar (3.7% vs. 2.2%, P = 0.252). There were no significance in terms of operative time, estimated blood loss and blood transfusion between the two groups.
Table 2.
Comparison of surgical outcomes between huge and small HCC patients treated by hepatic resection
| Variable | Huge HCC (n = 244) | Small HCC (n = 495) | P value |
|---|---|---|---|
| Operative time (min) | 210 ± 62.0 | 197.4 ± 71.8 | 0.435 |
| Estimated blood loss (ml) | 776.7 ± 1005.4 | 621.1 ± 364.1 | 0.510 |
| Blood transfusion, n (%) | 114 (46.7%) | 223 (45.1%) | 0.668 |
| Surgical margin > 1 cm, n (%) | 68 (27.9%) | 263 (53.1%) | < 0.001 |
| Postoperative TACE, n (%) | 77 (31.6%) | 144 (29.1%) | 0.491 |
| Recurrence, n (%) | 97 (39.8%) | 102 (20.6%) | < 0.001 |
| Intrahepatic recurrence, n (%) | 88 (36.1%) | 82 (16.6%) | < 0.001 |
| Extrahepatic recurrence, n (%) | 9 (3.7%) | 20 (4.0%) | 0.817 |
| Hospital mortality, n (%) | 9 (3.7%) | 11 (2.2%) | 0.252 |
| Complications, n (%) | 69 (28.3%) | 77 (15.6%) | < 0.001 |
Values with “±” are written as mean ± SD. TACE, transarterial embolization.
Survival analysis
The median follow-up of huge HCC group and small HCC group after HR were 29.4 and 35.2 months, respectively. The 1-year (66.0%), 3-year (40.6%), and 5-year (30.3%) OS in huge HCC group were significantly lower than that of small HCC group (1-year OS: 81.9%, 3-year OS: 60.9%, 5-year OS: 51.9%; P < 0.001, Figure 2A). The 1-, 3-, and 5-year DFS in huge HCC group (46.1%, 25.7%, and 19.4%, respectively) were significantly lower than that small HCC group (65.5%, 42.1%, and 33.6%, respectively; P < 0.001; Figure 2B).
Figure 2.

Overall survival and disease-free survival curves of patients with huge HCC and small HCC.
Prognostic factors for huge HCC
In the univariate analysis, HbsAg (-), AFP level < 400 ng/ml, uninodular huge HCC and absence of PVTT predicted better OS for huge HCC. The above predictive factors in univariate analysis were contained in the multivariate analysis. Uninodular huge HCC (HR = 1.834, 95% CI: 1.108-3.037, P = 0.018) and PVTT (HR = 1.656, 95% CI: 1.159-2.366, P = 0.006) still independently predicted better OS for huge HCC in multivariate analysis (Table 3).
Table 3.
Univariate and multivariate analysis of prognostic factors for the overall survival of huge HCC patients
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender (M/F) | 1.007 | 0.979-1.007 | 0.350 | |||
| HbsAg (+/-) | 1.750 | 1.042-2.941 | 0.034 | |||
| Child-Pugh grade (A/B) | 1.076 | 0.753-1.146 | 0.490 | |||
| AFP (≥ 400/< 400 ng/ml) | 1.400 | 1.014-1.933 | 0.041 | |||
| Tumor number (uninodular/multinodular) | 1.806 | 1.103-2.959 | 0.019 | 1.834 | 1.108-3.037 | 0.018 |
| Cirrhosis (present/absent) | 1.166 | 0.605-1.217 | 0.391 | |||
| Resection margin (> 1/≤ 1 cm) | 1.127 | 0.732-1.734 | 0.588 | |||
| Encapsulation (present/absent) | 1.264 | 0.916-1.745 | 0.153 | |||
| Edmondson-grade (I-II/ III-IV) | 1.385 | 0.283-1.842 | 0.496 | |||
| Preoperative TACE (yes/no) | 1.548 | 0.315-1.325 | 0.235 | |||
| Blood transfusion (yes/no) | 1.220 | 0.817-1.822 | 0.331 | |||
| Blood loss (≥ 1000/< 1000 ml) | 1.230 | 0.498-3.038 | 0.654 | |||
| PVTT (present/absent) | 1.588 | 1.119-2.253 | 0.010 | 1.656 | 1.159-2.366 | 0.006 |
HR, Hazard Ratio; 95% CI, 95% confidence interval; AFP, alpha-fetoprotein; TACE, transarterial embolization; PVTT, portal vein tumor thrombosis.
Subgroup analysis of huge HCC
The huge HCC group was categorized into the uninodular huge HCC subgroup (n = 42) and multinodular huge HCC subgroup (n = 202). The 1-year (73.2%), 3-year (50.6%), and 5-year (50.6%) OS in uninodular huge HCC subgroup were significantly higher than that in multinodular huge HCC subgroup (1-year OS: 61.3%, 3-year OS: 36.9%, 5-year OS: 26.9%; P = 0.016; Figure 3A) and similar to that in small HCC group (1-year OS: 81.9%, 3-year OS: 60.9%, 5-year OS: 51.9%; P = 0.598; Figure 4A). The 1-year (54.1%), 3-year (27.0%), and 5-year (27.0%) DFS in uninodular huge HCC subgroup were higher than that in multinodular huge HCC subgroup (1-year DFS: 42.3%, 3-year OS: 23.1%, 5-year OS: 17.2%), but the difference was not significant (P = 0.070; Figure 3B). The 1-, 3-, and 5-year DFS in uninodular huge HCC subgroup were similar to that in small HCC group (1-year OS: 65.5%, 3-year OS: 42.1%, 5-year OS: 33.6%; P = 0.166; Figure 4B).
Figure 3.

Overall survival and disease-free curves of patients with uninodular huge HCC and multinodular huge HCC.
Figure 4.
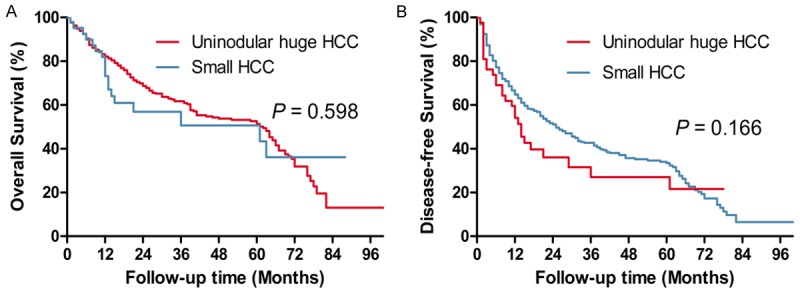
Overall survival and disease-free curves of patients with uninodular huge HCC and small HCC.
Discussion
Huge HCC is common in clinical practice and most huge HCC tumors are often considered to be at advanced stage at the time of diagnosis and unresectable. A larger number of huge HCC patients accepted TACE but the 5-year survival is less than 10% [6,7]. The published literatures have suggested that HR is still an important treatment approach for huge HCC [8-24]. But the value of HR for huge HCC remains controversial [8] because it may be associated with the increased morbidity and mortality due to the technical difficulties and possible postoperative hepatic decompensation, especially when HCC patients were with cirrhosis. The efficacy of HR for the treatment of huge HCC needs further investigation.
In recent years, the surgical technique has been refined gradually. The morbidity ranged from 0 to 8.0% and the mortality ranged from 10.9 to 42.0% [8-24]. In the present study, the morbidity and the mortality in huge HCC group were 28.3% and 3.7%, respectively, which were comparable to those reported in previous studies. The low mortality rate may be explained by skillful surgical techniques and the perfect indications of HCC patients. Moreover, the 5-year OS in huge HCC group was 30.3% in our study, which was comparable to those reported in previous studies ranging from 16.8 to 54.0% [8-24]. These findings suggested that HR is a safe and effective approach for the treatment of huge HCC.
The results of Choi GH et al. have suggested that huge HCC exhibits a more aggressive clinical behavior and poor prognosis after resection than small HCC [14]. Similarly in the present study, huge HCC group showed significantly higher rate of PVTT (43 vs. 7%, P < 0.001), higher rate of AFP level ≥ 400 ng/ml (41 vs. 22%, P < 0.001) and more intrahepatic recurrence (36.1 vs. 16.6%, P < 0.001) than small HCC group. Furthermore, the 5-year OS and DFS in huge HCC group were significantly worse than that in small HCC group (All P < 0.001). Notably, a specific subtype of HCC, uninodular huge HCC, was proposed by Yang LY et al. This subtype of HCC has just a solitary node and is large in size but exhibits a low invasive and metastatic potential and a good outcome after HR [25]. The novel concept was rarely validated in other published series. In the present study, the specific subtype of HCC was found independently predicted better OS in huge HCC group. And the huge HCC group was further categorized into the uninodular huge HCC subgroup (n = 42) and multinodular huge HCC subgroup (n = 202). We found uninodular huge HCC subgroup showed better prognosis than multinodular huge HCC subgroup (5-year OS: 50.6 vs. 26.9%, P = 0.016; 5-year DFS: 27.0 vs. 17.2%, P = 0.070). Moreover, the prognosis of uninodular huge HCC subgroup was also compared with that of the small HCC group and it can be observed that that 5-year OS (P = 0.598) and DFS (P = 0.166) in uninodular huge HCC subgroup were similar to that in small HCC group. Our results support the previous findings [11,25]. Besides the low invasive and metastatic potential and good outcome in this subgroup, we also found only 4 of those 42 patients had cirrhosis. Thus, HR may be an optimal approach for this subtype of HCC.
The current study had some limitations. Firstly, it was a single-center study performed in the Asia-Pacific region with significantly higher prevalence of hepatitis B virus infection (> 80%) than most western countries. Thus, the results may not be representative of all HCC patients. Secondly, the retrospective nature made this study vulnerable to potential bias. Lastly, presence of cirrhosis was not a risk factor for the OS in huge HCC group. This may be explained by the relatively low rate of cirrhosis compared with those reported by previous series which were summarized in Table 4. Therefore, more prospective and randomized control trials should be performed for further research to revalidate these findings.
Table 4.
Published efficacy of hepatic resection for huge HCC from 2004 to 2014
| Authors | Year | Country | Number | Morbidity (%) | Mortality (%) | Cirrhosis (%) | 5-Year OS (%) |
|---|---|---|---|---|---|---|---|
| Min et al. [8] | 2014 | Korea | 84 | NA | 2.4 | NA | 39.8 |
| Ariizumi et al. [9] | 2013 | Japan | 177 | NA | 5.6 | 4.0 | 42.0 |
| Allemann et al. [10] | 2013 | Switzerland | 22 | 23.0 | 0 | 41.0 | 21.0 |
| Yang et al. [11] | 2013 | China | 258 | 10.9 | 0.78 | 66.1 | 33.0 |
| Shrager et al. [12] | 2013 | United States | 130 | 21.5 | 6.9 | 39.8 | 18.8 |
| Yamashita et al. [13] | 2011 | Japan | 53 | 24.5 | 3.8 | NA | 35.0 |
| Choi et al. [14] | 2009 | Korea | 50 | 24.0 | 0 | 26.0 | 40.2 |
| Taniai et al. [15] | 2008 | Japan | 29 | 27.6 | 6.9 | 41.4 | 33.6 |
| Shimada et al. [16] | 2008 | Japan | 85 | NA | NA | 11.0 | 32.2 |
| Shah et al. [17] | 2007 | Canada | 24 | 42.0 | 8.0 | NA | 54.0 |
| Pandey et al. [18] | 2007 | Singapore | 166 | NA | 3.0 | 48.2 | 28.6 |
| Lee et al. [19] | 2007 | Korea | 100 | NA | NA | NA | 31.0 |
| Chen et al. [20] | 2006 | China | 780 | 26.8 | 2.2 | 86.3 | 18.2 |
| Nagano et al. [21] | 2005 | Japan | 26 | 30.8 | 3.8 | 19.2 | 29.3 |
| Liau et al. [22] | 2005 | United States | 82 | 28.0 | 2.0 | 10.0 | 33.0 |
| Pawlik T et al. [23] | 2005 | International | 300 | NA | 5.0 | 26.0 | 27.0 |
| Chen et al. [24] | 2004 | China | 525 | NA | 2.7 | 91.4 | 16.8 |
HCC, hepatocellular carcinoma; NA not available; OS, overall survival.
In conclusion, our findings suggested that HR is a safe and effective approach for patients with huge HCC, especially for those with uninodular huge HCC. Uninodular huge HCC showed significant better prognosis than multinodular huge HCC.
Acknowledgements
National Science and Technology Major Special Project, No. 2012ZX10002010001009; Self-Raised Scientific Research Fund of the Ministry of Health of Guangxi Province, No. Z2015621 and No. Z2014241; and Guangxi University of Science and Technology Research Fund, No. KY2015LX056.
Disclosure of conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010;52:762–773. doi: 10.1002/hep.23725. [DOI] [PubMed] [Google Scholar]
- 3.Zhu SL, Ke Y, Peng YC, Ma L, Li H, Li LQ, Zhong JH. Comparison of Long-Term Survival of Patients with Solitary Large Hepatocellular Carcinoma of BCLC Stage A after Liver Resection or Transarterial Chemoembolization: A Propensity Score Analysis. PLoS One. 2014;9:e115834. doi: 10.1371/journal.pone.0115834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004;127:S179–S188. doi: 10.1053/j.gastro.2004.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Poon RT, Ngan H, Lo CM, Liu CL, Fan ST, Wong J. Transarterial chemoembolization for inoperable hepatocellular carcinoma and postresection intrahepatic recurrence. J Surg Oncol. 2000;73:109–114. doi: 10.1002/(sici)1096-9098(200002)73:2<109::aid-jso10>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 7.Huang YH, Wu JC, Chen SC, Chen CH, Chiang JH, Huo TI, Lee PC, Chang FY, Lee SD. Survival benefit of transcatheter arterial chemoembolization in patients with hepatocellular carcinoma larger than 10 cm in diameter. Aliment Pharmacol Ther. 2006;23:129–135. doi: 10.1111/j.1365-2036.2006.02704.x. [DOI] [PubMed] [Google Scholar]
- 8.Min YW, Lee JH, Gwak GY, Paik YH, Lee JH, Rhee PL, Koh KC, Paik SW, Yoo BC, Choi MS. Long-term survival after surgical resection for huge hepatocellular carcinoma: Comparison with transarterial chemoembolization after propensity score matching. J Gastroenterol Hepatol. 2014;29:1043–1048. doi: 10.1111/jgh.12504. [DOI] [PubMed] [Google Scholar]
- 9.Ariizumi SI, Kotera Y, Takahashi Y, Katagiri S, Yamamoto M. Impact of hepatectomy for huge solitary hepatocellular carcinoma. J Surg Oncol. 2013;107:408–413. doi: 10.1002/jso.23226. [DOI] [PubMed] [Google Scholar]
- 10.Allemann P, Demartines N, Bouzourene H, Tempia A, Halkic N. Long-term outcome after liver resection for hepatocellular carcinoma larger than 10 cm. World J Surg. 2013;37:452–458. doi: 10.1007/s00268-012-1840-5. [DOI] [PubMed] [Google Scholar]
- 11.Yang L, Xu J, Ou D, Wu W, Zeng Z. Hepatectomy for huge hepatocellular carcinoma: single institute’s experience. World J Surg. 2013;37:2189–2196. doi: 10.1007/s00268-013-2095-5. [DOI] [PubMed] [Google Scholar]
- 12.Shrager B, Jibara GA, Tabrizian P, Schwartz ME, Labow DM, Hiotis S. Resection of large hepatocellular carcinoma (≥ 10 cm): A unique western perspective. J Surg Oncol. 2013;107:111–117. doi: 10.1002/jso.23246. [DOI] [PubMed] [Google Scholar]
- 13.Yamashita YI, Taketomi A, Shirabe K, Aishima S, Tsuijita E, Morita K, Kayashima H, Maehara Y. Outcomes of hepatic resection for huge hepatocellular carcinoma (≥ 10 cm in diameter) J Surg Oncol. 2011;104:292–298. doi: 10.1002/jso.21931. [DOI] [PubMed] [Google Scholar]
- 14.Choi GH, Han DH, Kim DH, Choi SB, Kang CM, Kim KS, Choi JS, Park YN, Park JY, Kim do Y, Han KH, Chon CY, Lee WJ. Outcome after curative resection for a huge (≥ 10 cm) hepatocellular carcinoma and prognostic significance of gross tumor classification. Am J Surg. 2009;198:693–701. doi: 10.1016/j.amjsurg.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Taniai N, Yoshida H, Tajiri T. Adaptation of hepatectomy for huge hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:410–416. doi: 10.1007/s00534-007-1317-3. [DOI] [PubMed] [Google Scholar]
- 16.Shimada K, Sakamoto Y, Esaki M, Kosuge T. Role of a hepatectomy for the treatment of large hepatocellular carcinomas measuring 10 cm or larger in diameter. Langenbecks Arch Surg. 2008;393:521–526. doi: 10.1007/s00423-007-0264-4. [DOI] [PubMed] [Google Scholar]
- 17.Shah SA, Wei AC, Cleary SP, Yang I, McGilvray ID, Gallinger S, Grant DR, Greig PD. Prognosis and results after resection of very large (> or = 10 cm) hepatocellular carcinoma. J Gastrointest Surg. 2007;11:589–595. doi: 10.1007/s11605-007-0154-7. [DOI] [PubMed] [Google Scholar]
- 18.Pandey D, Lee K, Wai C, Wagholikar G, Tan K. Long term outcome and prognostic factors for large hepatocellular carcinoma (10 cm or more) after surgical resection. Ann Surg Oncol. 2007;14:2817–2823. doi: 10.1245/s10434-007-9518-1. [DOI] [PubMed] [Google Scholar]
- 19.Lee SG, Hwang S, Jung JP, Lee YJ, Kim KH, Ahn CS. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Brit J Surg. 2007;94:320–326. doi: 10.1002/bjs.5622. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Qiu F, Wu Z, Zhang B. Hepatectomy for huge hepatocellular carcinoma in 634 cases. World J Gastroenterol. 2006;12:4652–5. doi: 10.3748/wjg.v12.i29.4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagano Y, Tanaka K, Togo S, Matsuo K, Kunisaki C, Sugita M, Morioka D, Miura Y, Kubota T, Endo I, Sekido H, Shimada H. Efficacy of hepatic resection for hepatocellular carcinomas larger than 10 cm. World J Surg. 2005;29:66–71. doi: 10.1007/s00268-004-7509-y. [DOI] [PubMed] [Google Scholar]
- 22.Liau KH, Ruo L, Shia J, Padela A, Gonen M, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, DeMatteo RP. Outcome of partial hepatectomy for large (> 10 cm) hepatocellular carcinoma. Cancer. 2005;104:1948–1955. doi: 10.1002/cncr.21415. [DOI] [PubMed] [Google Scholar]
- 23.Pawlik TM, Poon RT, Abdalla EK, Zorzi D, Ikai I, Curley SA, Nagorney DM, Belghiti J, Ng IO, Yamaoka Y, Lauwers GY, Vauthey JN. Critical appraisal of the clinical and pathologic predictors of survival after resection of large hepatocellular carcinoma. Arch Surg-Chicago. 2005;140:450–458. doi: 10.1001/archsurg.140.5.450. [DOI] [PubMed] [Google Scholar]
- 24.Chen XP, Qiu FZ, Wu ZD, Zhang BX. Chinese experience with hepatectomy for huge hepatocellular carcinoma. Brit J Surg. 2004;91:322–326. doi: 10.1002/bjs.4413. [DOI] [PubMed] [Google Scholar]
- 25.Yang L, Fang F, Ou D, Wu W, Zeng ZJ, Wu F. Solitary large hepatocellular carcinoma: a specific subtype of hepatocellular carcinoma with good outcome after hepatic resection. Ann Surg. 2009;249:118–123. doi: 10.1097/SLA.0b013e3181904988. [DOI] [PubMed] [Google Scholar]
- 26.Hsieh CB, Yu CY, Tzao C, Chu HC, Chen TW, Hsieh HF, Liu YC, Yu JC. Prediction of the risk of hepatic failure in patients with portal vein invasion hepatoma after hepatic resection. Eur J Surg Oncol. 2006;32:72–76. doi: 10.1016/j.ejso.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 27.Zhong JH, Xiang BD, Gong WF, Ke Y, Mo QG, Ma L, Liu X, Li LQ. Comparison of long-term survival of patients with BCLC stage B hepatocellular carcinoma after liver resection or transarterial chemoembolization. PLoS One. 2013;8:e68193. doi: 10.1371/journal.pone.0068193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong JH, Ke Y, Gong WF, Xiang BD, Ma L, Ye XP, Peng T, Xie GS, Li LQ. Hepatic resection associated with good survival for selected patients with intermediate and advanced-stage hepatocellular carcinoma. Ann Surg. 2014;260:329–340. doi: 10.1097/SLA.0000000000000236. [DOI] [PubMed] [Google Scholar]


