Abstract
This study is to investigate the effects of propofol on primary hippocampal neurons under oxygen-glucose deprivation (OGD) condition and related mechanisms. The apoptotic process was detected with flow cytometry, and the cell viability was assessed with CCK-8 assay. The expression levels of microRNA (miRNA)-134 were detected with quantitative real-time PCR. Protein expression levels were detected by Western blot analysis. Dual-luciferase reporter assay was also performed to confirm the prediction of the target genes of miRNA-134. Our results from flow cytometry showed that the apoptosis rate was significantly increased in the primary hippocampal neurons under OGD condition. However, the treatments of propofol (25, 50, 100, and 150 µmol/L) suppressed the apoptotic process. Moreover, propofol restored the declined cell viability in the primary hippocampal neurons under OGD condition. In addition, compared with the OGD model group, the Bcl-2/Bax ratios were significantly elevated in the propofol-treated groups, indicating the protective effects of propofol against cellular apoptosis. Quantitative real-time PCR showed that propofol reduced the expression levels of miRNA-134 in the primary hippocampal neurons under OGD condition. Bioinformatics analysis revealed that BDNF might be a target of miRNA-134. The treatment of antago-miRNA-134 significantly down-regulated the expression level of BDNF. In line with this, dual-luciferase reporter assay suggested that miRNA-134 targeted BDNF in the 3’-TUR. Under OGD condition, propofol could down-regulate miRNA-134, and subsequently modulate the expression of BDNF, to exert neuroprotective effects.
Keywords: Propofol, neuroprotective effects, oxygen-glucose deprivation (OGD) condition, microRNA-134, BDNF
Introduction
Propofol is a commonly used intravenous anesthetic for the induction and/or maintenance of general anesthesia in clinic [1-3]. It is characterized by a rapid onset of action, with more complete consciousness and less side effects. In recent years, propofol has been shown to exert protective effects on multiple organs during surgery, especially for the brain tissues [4-8]. It has been shown that propofol decreases the intracranial pressure and venous oxygen content in cerebral hemorrhage patients, and contributes to the organ protection in ischemia/reperfusion-induced injuries [9,10]. Moreover, propofol can significantly inhibit the apoptotic processes in hippocampal neurons induced by hypoxia/reperfusion [11] and in primary glial cells under oxygen-glucose deprivation (OGD) condition [12].
MicroRNA (miRNA) is a class of endogenous, non-coding, single-stranded small RNA molecules, usually with 18-28 nucleotides. The miRNAs could bind to the 3’-UTR of the target gene, with fully or partially complementary sequences, to regulate the gene expression [13]. Studies have shown that the miRNA levels are significantly altered in several organs following ischemia/reperfusion [14]. miRNA-134 is specifically expressed in the brain tissues [15]. High expression of miRNA-134 could result in the apoptosis of neuronal cells, and the expression level of miRNA-134 has been shown to be significantly elevated in the rat brain after ischemia/reperfusion [16]. However, the role of miRNA-134 in the protective effects of propofol on the brain tissues has not yet been fully established.
In this study, the effects of propofol on the primary hippocampal neurons under OGD condition and the involvement of miRNA-134 were investigated. Our results showed that propofol could increase cell viability and suppress apoptosis in the primary hippocampal neurons under OGD condition, via down-regulating the expression level of miRNA-134, which targeted BDNF in the 3’-UTR.
Materials and methods
Materials and reagents
Trizol was purchased from Invitrogen, Carlsbad, CA, USA. Rabbit anti-human anti-BDNF polyclonal antibody and mouse anti-human anti-GAPDH monoclonal antibody were purchased from Bioworld, Atlanta, GA, USA. PrimeScript RT Regent Kit and SYBR PrimeScript RT-PCR Kit were purchased form Takara, Dalian, Liaoning, China. Dual-luciferase Reporter Assay System was purchased from Promega, Madison, WI, USA. ANXN V FITC Apoptosis Dtec Kit I was purchased from BD, Billerica, MA, USA. Antago-miRNA-134 and miRNA-134 mimics were purchased from Ruibio, Guanzhou, Guangdong, China.
Primary hippocampal neuron culture and modeling
Primary hippocampal neuron culture was performed according to a previously published protocol [17]. On days in vitro (DIV) 4-5, these primary neuronal cells were subjected to model establishment, and divided in to the following groups: (1), the normal control group of primary hippocampal neurons without any intervention; (2), the OGD model group, in which primary neuronal cells were cultured under OGD condition for 12 h, and then cultured with normal medium for 8 h; and (3), the treatment groups, in which OGD model neurons were established and treated with propofol (25, 50, 100, and 150 μmol/L, respectively) for 6 h.
Quantitative real-time PCR
Cells were collected, and total RNA was extracted with the Trizol reagent. 1 μg RNA was used for the reverse transcription reaction, and the cDNA was stored in -20°C. Quantitative real-time PCR reaction system consisted of 10 mL qRT-PCR-Mix, 0.5 mL forward primer, 0.5 μL universal primer, 2 mL cDNA template, and 7 mL ddH2O. The forward primer sequence was 5’-TGTGACTGGTTGACCAGAGGGG-3’. The real-time PCR condition was as follows: denaturation at 95°C for 10 min; 95°C for 1 min, and 60°C for 30 s, for totally 40 cycles.
Cell counting kit (CCK-8) assay
CCK-8 assay was performed according to the manufacturer’s instructions. After discarding the medium, cells were washed with PBS twice. Fresh medium containing 10% CCK-8 reaction solution was used to incubate the cells at 37°C for 1 h. Then the absorbance at 450 nm was detected with a microplate reader.
Flow cytometry
Cells were collected and subjected to the treatment with ANXN V FITC Apoptosis Dtec Kit I, according to the manufacturer’s instructions. Cellular apoptosis was detected with flow cytometry. Cells positive for ANNEXIN V alone were recognized as early apoptosis, cells positive for PI were recognized as necrosis, and cells positive for both ANNEXIN V and PI were recognized as late apoptosis.
Western blot analysis
Cells were collected and lysed with RIPA lysis buffer containing 1% PMSF. After centrifugation at 12000 g for 10 min, the supernatant was collected, and the protein concentration was measured with the BSA method. 20 μg protein was separated by SDS-PAGE, and then electronically transferred onto a PVDF membrane. The membrane was blocked with 5% non-fat milk for 1 h, and incubated with rabbit anti-human anti-BDNF antibody (1:1000 dilution) overnight. HRP-conjugated secondary antibody was used to incubate the membrane for 1 h. Protein bands were visualized with the ECL chemoluminescence method.
Dual-luciferase reporter assay
Dual-luciferase reporter assay was performed according to the manufacturer’s instructions. The fragment of the 3’-UTR of BDNF (wild-type or mutant, respectively) was amplified and cloned into the pMIR-REPORT luciferase vector. For the luciferase assay, the 293T cells were co-transfected with 100 nM miR-134 mimics and 0.8 µg pMIR-REPORT luciferase reporters (containing wild-type or mutant 3’-UTR of BDNF). After 24 h, cells were lysed, and the luciferase was detected with the GloMax 20/20 luminometer. The Renilla luciferase plasmid was used as the internal control.
Statistical analysis
Data were expressed as mean ± SD. SPSS 11.0 software was used for the statistical analysis. One-way analysis of variance was used for multiple comparisons, followed by the Bonferroni’s Student’s t-test for unpaired values. P < 0.05 was considered as statistically significant.
Results
Propofol suppresses apoptosis of primary hippocampal neurons under OGD condition
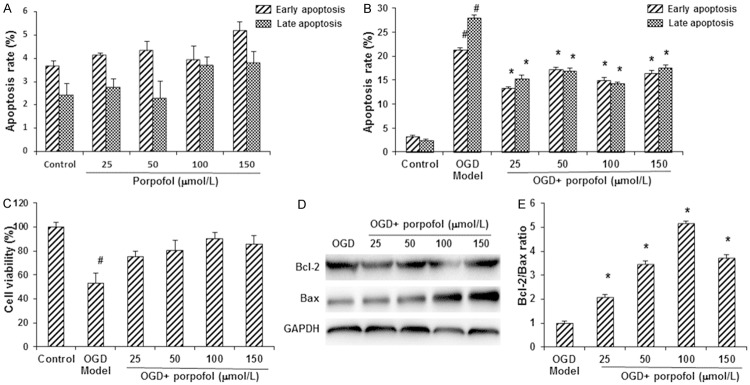
To investigate the effects of propofol on primary hippocampal neuronal cells, especially under OGD condition, the apoptotic process was detected by flow cytometry. These primary hippocampal neurons were first treated with propofol alone at increasing concentrations (i.e., 25, 50, 100, and 150 μmol/L, respectively). Flow cytometry did not reveal significant differences in the apoptotic process in these cells (P > 0.05) (Figure 1A). However, under OGD condition, our results showed that the early and late apoptosis rates were both significantly elevated, compared with the control group (P < 0.05). When treated with propofol, the apoptosis rates were dramatically declined in these hippocampal neurons under OGD condition (P < 0.05) (Figure 1B). Moreover, the cell viability was also evaluated with the CCK-8 assay. Our results showed that, compared with the control group, the cell viability was significantly decreased in the OGD model neuronal cells (P < 0.05), which could be restored by the treatments of propofol (P < 0.05) (Figure 1C).
Figure 1.
Propofol increased cell viability and suppressed apoptosis in primary hippocampal neurons under OGD condition. A, B. The apoptotic process in primary hippocampal neuronal cells was detected by flow cytometry. A. Apoptosis rates of primary hippocampal neurons treated with propofol alone at 25, 50, 100, and 150 μmol/L. B. Apoptosis rates of primary hippocampal neurons under OGD condition. C. The cell viability was evaluated with the CCK-8 assay. D. The expression levels of apoptosis-related proteins, Bcl-2 and Bax, were detected with Western blot analysis. E. The Bcl-2/Bax ratios in the primary hippocampal neurons. Compared with the control group, #P < 0.05; compared with the OGD model group, *P < 0.05.
To further confirm the effects of propofol on apoptosis of hippocampal neurons under OGD condition, the expression levels of apoptosis-related proteins, Bcl-2 and Bax, were detected with Western blot analysis. Our results showed that, compared with the OGD model group, the expression levels of Bcl-2 were significantly elevated by the treatments of propofol, while the expression levels of Bax were significantly decreased by propofol treatments (P < 0.05) (Figure 1D). Therefore, compared with the OGD model group, the Bcl-2/Bax ratios were significantly elevated in the propofol-treated groups (P < 0.05) (Figure 1E). These results suggest that propofol could protect the primary hippocampal neurons from the apoptotic process induced by the OGD condition.
Propofol reduces miRNA-134 expression levels in primary hippocampal neurons under OGD condition
The involvement of miRNA1-34 has been reported in the apoptotic process [16]. We next investigated the effects of propofol on the expression levels of miRNA-134 in primary hippocampal neurons under OGD condition. Our results from quantitative real-time PCR showed that, propofol alone did not influence the expression level of miRNA-134. Under OGD condition, the expression level of miRNA-134 was significantly increased in these neuronal cells (P < 0.05) (Figure 2). However, the treatments of propofol (25, 50, 100, and 150 µmol/L) significantly declined the expression levels of miRNA-134 in these OGD model neurons (P < 0.05) (Figure 2). These results suggest that miRNA-134 might be implied in the protective effects of propofol against apoptosis in primary hippocampal neurons under OGD condition.
Figure 2.
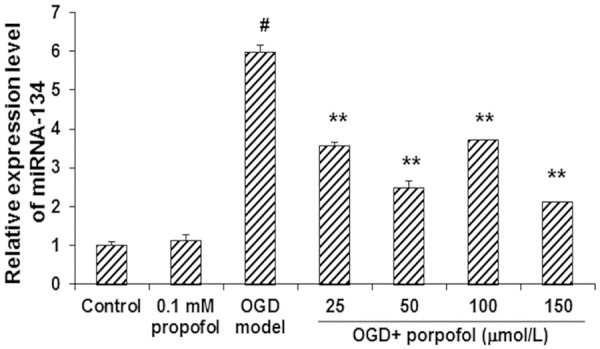
Propofol reduced the expression levels of miRNA-134 in primary hippocampal neurons under OGD condition. The expression levels of miRNA-134 were investigated with the quantitative real-time PCR. Compared with the control group, #P < 0.05; compared with the OGD model group, *P < 0.05.
The miRNA-134 targets brain-derived neurotrophic factor (BDNF) in the 3’-UTR
The target genes of miRNA-134 in the primary hippocampal neurons were next predicted and investigated. Targetscan software was used to predict the target genes of miRNA-134. Analyses of KEGG and GO terms revealed 17 binding sites for miRNA-134 in the 3’-UTR of BDNF, indicating that BDNF might be a target of miRNA-134. BDNF participates in the regulation of a variety of neurological signaling pathways, which are important in the growth and repairing of neurons.
To investigate whether BDNF is the target of miRNA-134, neuronal cells were traeted with antago-miRNA-134 for 48 h, and then the expreesion levels of BDNF were detected with Western blot analysis. Our results showed that, compared with the control cells, the expression level of BDNF was significantly declined by the antago-miRNA-134 transfection (P < 0.05) (Figure 3). To further confirm the interactoin between miRNA-134 and BDNF, the dual-luciferase reporter assay was performed. Our resutls showed that, compared with the miRNA-NC group, the fluorescence in 293T cells co-transfected with miRNA-134 mimics and pMIR-REPORT luciferase reporter plasmid containing the wild-type 3’-UTR of BDNF was significantly decreased (P < 0.05) (Figure 4). However, in the group tansfected with pMIR-REPORT luciferase reporter plasmid containing the mutant 3’-UTR of BDNF, no significant diffeneces were observed in the fluorescence (P > 0.05) (Figure 4). Taken together, these results suggest that miRNA-134 targets BNDF in the 3’-UTR, which might contribute to the protective effects of propofol in primary hippocampal neurons under OGD condition.
Figure 3.
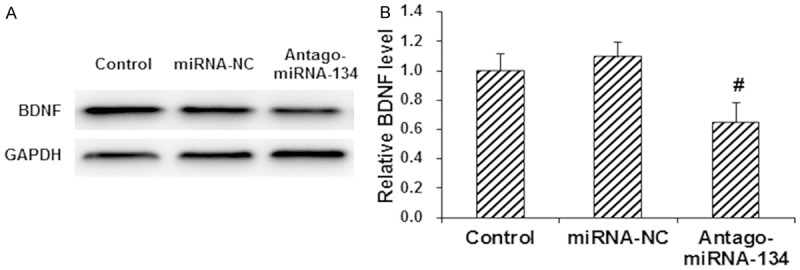
miRNA-134 targeted BDNF in the primary hippocampal neurons. Primary hippocampal neurons were transfected with miRNA-NC and antago-miRNA-134, respetively, for 48 h. Then the expression levels of BDNF were detected by Western blot analysis. A. Representative Western blot results. B. Quantiative Western blot results. Compared with the control group, #P < 0.05; compared with the OGD model group, *P < 0.05.
Figure 4.
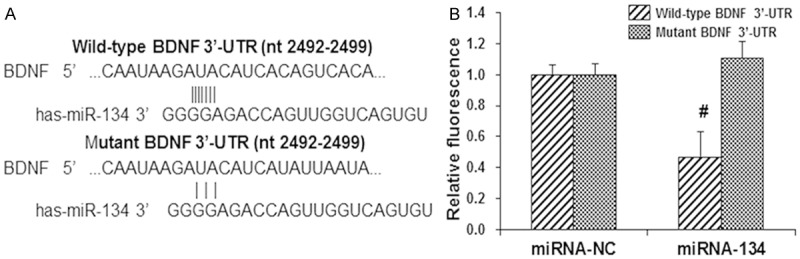
Dual-luciferase reporter assay. A. The 293T cells were co-transfected with 100 nM miRNA-134 mimics and 0.8 µg pMIR-REPORT luciferase reporters (containing wild-type or mutant 3’-UTR of BDNF). B. Statistical analysis of the luciferase results. Compared with the corresponding miRNA-NC group, *P < 0.05.
Discussion
In clinical practice of cardiovascular surgery, hypoxia due to ischemia represents a common situation, the most effective treatment for which might be restoring the blood supply. However, the impairments induced by blood reperfusion might greatly interfere with the postoperative recovery process. Propofol is a common intravenous anesthetic, which is widely used in clinical anesthesia and sedation. In recent years, increasing evidence reveals that propofol exerts protective effects on multiple organs under pathophysiological condition, which has attracted increasing attention. It has been shown that propofol could protect cells from oxidative stress [18]. Moreover, propofol has also been found to be associated with calcium overload [19]. However, these are few reports concerning the relationship between miRNAs and the protective effects of propofol in ischemia/reperfusion injuries. In the present study, primary hippocampal neurons were cultured under OGD condition, and the protective effects of propofol and the involvement of miRNA-134 were investigated.
Our results showed that, under normal conditions, propofol caused no apparent toxic effects on primary hippocampal neurons. However, under OGD condition, the apoptosis was enhanced in these neuronal cells, which could be alleviated by the treatments of propofol. These results were in line with the Western blot analysis detecting the expression levels of Bcl-2 and Bax. The Bcl-2/Bax ratios were significantly elevated by propofol treatment in the primary hippocampal neurons under OGD condition, indicating the protective effects of propofol. The expression levels of miRNA-134 were also investigated. Quantitative real-time PCR showed that, propofol did not influence the expression of miRNA-134 in the control group, while the miRNA-134 expression level was significantly elevated in the OGD model group. The treatments of propofol could dramatically down-regulated the expression levels of miRNA-134 in these neuron cells under OGD condition, which might contribute to the protective effects of propofol.
The targets of miRNA-134 were then investigated. Bioinformatics analysis revealed that BDNF might be the target gene of miRNA-134. BDNF is one of the most important neurotrophic factors, widely expressed in the brain and nerve tissues. BDNF can activate a variety of signaling pathways, regulating the neuronal differentiation, and participate in the growth processes and repairing of nerve cells [20,21]. Antago-miRNA-134 was transfected into these primary hippocampal neurons, which significantly declined the expression level of BDNF. Dual-luciferase reporter assay was performed to further confirm the interaction between miRNA-134 and BDNF, and the results showed that miRNA-134 could bind to BDNF in the 3’-UTR. Based on these results, we proposed that, under OGD condition, the expression level of miRNA-134 was elevated, which lead to the down-regulation of BNDF, further influencing the downstream signaling pathways and resulting in neuronal cell damage. The treatment of propofol could down-regulate the miRNA-134 expression to exert neuroprotective effects.
In conclusion, our results showed that propofol could suppress apoptosis and restore cell viability in primary hippocampal neurons under OGD condition. Moreover, propofol reduces the expression levels of miRNA-134 in the OGD neuronal cells. Furthermore, our results showed that miRNA-134 targeted BDNF in the 3’-TUR. Taken together, under OGD condition, propofol could down-regulate miRNA-134, and subsequently modulate the expression of BDNF, to exert neuroprotective effects.
Acknowledgements
We thank President Changqin Li, Director Weifu Lei, Prof. Xiaomei Li, and Dr. Renjie Xie for their guidance and support for this work, and assistance in the manuscript preparation.
Disclosure of conflict of interest
None.
References
- 1.Habre C, Tramèr MR, Pöpping DM, Elia N. Ability of a meta-analysis to prevent redundant research: systematic review of studies on pain from propofol injection. BMJ. 2014;348:g5219. doi: 10.1136/bmj.g5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar G, Stendall C, Mistry R, Gurusamy K, Walker D. A comparison of total intravenous anaesthesia using propofol with sevoflurane or desflurane in ambulatory surgery: systematic review and meta-analysis. Anaesthesia. 2014;69:1138–50. doi: 10.1111/anae.12713. [DOI] [PubMed] [Google Scholar]
- 3.Halladin NL, Zahle FV, Rosenberg J, Gögenur I. Interventions to reduce tourniquet-related ischaemic damage in orthopaedic surgery: a qualitative systematic review of randomised trials. Anaesthesia. 2014;69:1033–50. doi: 10.1111/anae.12664. [DOI] [PubMed] [Google Scholar]
- 4.Schifilliti D, Grasso G, Conti A, Fodale V. Anaesthetic-related neuroprotection: intravenous or inhalational agents? CNS Drugs. 2010;24:893–907. doi: 10.2165/11584760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14:95–106. doi: 10.1111/j.1527-3458.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yao W, Luo G, Zhu G, Chi X, Zhang A, Xia Z, Hei Z. Propofol activation of the Nrf2 pathway is associated with amelioration of acute lung injury in a rat liver transplantation model. Oxid Med Cell Longev. 2014;2014:258567. doi: 10.1155/2014/258567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin C, Sui H, Gu J, Yang X, Deng L, Li W, Ding W, Li D, Yang Y. Effect and mechanism of propofol on myocardial ischemia reperfusion injury in type 2 diabetic rats. Microvasc Res. 2013;90:162–8. doi: 10.1016/j.mvr.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen RM, Tai YT, Chen TG, Lin TH, Chang HC, Chen TL, Wu GJ. Propofol protects against nitrosative stress-induced apoptotic insults to cerebrovascular endothelial cells via an intrinsic mitochondrial mechanism. Surgery. 2013;154:58–68. doi: 10.1016/j.surg.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Mayette M, Gonda J, Hsu JL, Mihm FG. Propofol infusion syndrome resuscitation with extracorporeal life support: a case report and review of the literature. Ann Intensive Care. 2013;3:32. doi: 10.1186/2110-5820-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kenyon CA, Flick R, Moir C, Ackerman MJ, Pabelick CM. Anesthesia for videoscopic left cardiac sympathetic denervation in children with congenital long QT syndrome and catecholaminergic polymorphic ventricular tachycardia--a case series. Paediatr Anaesth. 2010;20:465–70. doi: 10.1111/j.1460-9592.2010.03293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Zhang X, Zhou Q, Wang Y, Jiang Y, Cao J. Propofol’s effect on the sciatic nerve: Harmful or protective? Neural Regen Res. 2013;8:2520–30. doi: 10.3969/j.issn.1673-5374.2013.27.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Velly LJ, Rey MF, Bruder NJ, Gouvitsos FA, Witjas T, Regis JM, Peragut JC, Gouin FM. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–12. doi: 10.1097/01.anes.0000270734.99298.b4. [DOI] [PubMed] [Google Scholar]
- 13.Kalozoumi G, Yacoub M, Sanoudou D. MicroRNAs in heart failure: Small molecules with major impact. Glob Cardiol Sci Pract. 2014;2014:79–102. doi: 10.5339/gcsp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kukreja RC, Yin C, Salloum FN. MicroRNAs: new players in cardiac injury and protection. Mol Pharmacol. 2011;80:558–64. doi: 10.1124/mol.111.073528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bicker S, Lackinger M, Weiß K, Schratt G. MicroRNA-132, -134, and -138: a microRNA troika rules in neuronal dendrites. Cell Mol Life Sci. 2014;71:3987–4005. doi: 10.1007/s00018-014-1671-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W, Liu X, Cao J, Meng F, Li M, Chen B, Zhang J. miR-134 Regulates Ischemia/Reperfusion Injury-Induced Neuronal Cell Death by Regulating CREB Signaling. J Mol Neurosci. 2015;55:821–9. doi: 10.1007/s12031-014-0434-0. [DOI] [PubMed] [Google Scholar]
- 17.Turlova E, Bae CY, Deurloo M, Chen W, Barszczyk A, Horgen FD, Fleig A, Feng ZP, Sun HS. TRPM7 Regulates Axonal Outgrowth and Maturation of Primary Hippocampal Neurons. Mol Neurobiol. 2016;53:595–610. doi: 10.1007/s12035-014-9032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo C, Yuan D, Li X, Yao W, Luo G, Chi X, Li H, Irwin MG, Xia Z, Hei Z. Propofol attenuated acute kidney injury after orthotopic liver transplantation via inhibiting gap junction composed of connexin 32. Anesthesiology. 2015;122:72–86. doi: 10.1097/ALN.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 19.Lawton BK, Brown NJ, Reilly CS, Brookes ZL. Role of L-type calcium channels in altered microvascular responses to propofol inhypertension. Br J Anaesth. 2012;108:929–35. doi: 10.1093/bja/aes069. [DOI] [PubMed] [Google Scholar]
- 20.O’Donnell KJ, Glover V, Holbrook JD, O’Connor TG. Maternal prenatal anxiety and child brain-derived neurotrophic factor (BDNF) genotype: Effects on internalizing symptoms from 4 to 15 years of age. Dev Psychopathol. 2014;26:1255–66. doi: 10.1017/S095457941400100X. [DOI] [PubMed] [Google Scholar]
- 21.Xu Q, Ji XF, Chi TY, Liu P, Jin G, Gu SL, Zou LB. Sigma 1 receptor activation regulates brain-derived neurotrophic factor through NR2A-CaMKIV-TORC1 pathway to rescue the impairment of learning and memory induced by brain ischaemia/reperfusion. Psychopharmacology (Berl) 2014;232:1779–91. doi: 10.1007/s00213-014-3809-6. [DOI] [PubMed] [Google Scholar]