Abstract
Objective: To observe the role of ornithine decarboxylase (ODC)/polyamine pathway in focal cerebral ischemia-reperfusion injury and to explore its mechanism in rats. Methods: This study was randomly divided into 3 groups including sham-operation (sham) group, ischemia-reperfusion (I/R) group and α-difluoromethylornithine (DFMO) group (each group with 80 rats). In DFMO group, 300 mg/kg of DFMO was injected by tail vein 24 h before reperfusion. According to different time points (3 h, 12 h, 24 h, 48 h and 72 h) after reperfusion, each group was divided into 5 subgroups (each subgroup with 16 rats). Results: In I/R group, apoptosis began increasing 3 h after reperfusion, reached a peak 24 h after perfusion and began decreasing 48 h after perfusion. Compared with sham group, apoptosis significantly increased in I/R and DFMO groups (P<0.05). However, apoptosis was significantly lower in DFMO group than in I/R group at each time point (P<0.05). In I/R group, CHOP expression began increasing 3 h after reperfusion, reached a peak 24 h after perfusion and began decreasing 48 h after perfusion. CHOP expression was significantly lower in DFMO group than in I/R group at each time point (P<0.05). The level of polyamines was significantly higher in I/R and DFMO groups than in sham group, and in I/R group than in DFMO group 12 h, 24 h and 48 h, respectively (P<0.05). Conclusion: Down-regulation of ODC/polyamine pathway may inhibit CHOP-mediated apoptosis caused by endoplasmic reticulum stress and plays a protective role in cerebral I/R injury.
Keywords: Cerebral ischemia-reperfusion injury, endoplasmic reticulum stress, ornithine decarboxylase/polyamine pathway
Introduction
Cerebral ischemia-reperfusion (I/R) injury is common in thrombolytic therapy, craniocerebral trauma and operation. In most cases, injured structure may be restored after reperfusion of ischemic brain tissue. However, sometimes, rather than relieving cerebral tissue injury, reperfusion aggravates nerve cell damage. In cerebral I/R injury, elevation of reactive oxygen species, overload of Ca2+, enhancement of excitatory amino acid toxicity, changes in cell membrane permeability, leukocyte aggregation and reduction of ATP all may induce endoplasmic reticulum stress (ERS). Durative and severe ERS can activate CHOP and/or caspase-12 to induce apoptotic pathways, leading to neuron death [1]. CHOP/GADD153 is one of the classic markers of ERS [2]. In cerebral I/R injury, ERS usually occurs and CHOP expression increases, which activate a series of apoptotic pathways and induces nerve cell apoptosis [3,4]. It has been reported that in ischemic environment, polyamines and their metabolites can affect neurons [5]. Ornithine decarboxylase is a key enzyme for polyamine synthesis and its specific inhibitor, α-difluoromethylornithine (DFMO), can inhibit polyamine synthesis. In this study, we used DFMO to inhibit polyamine synthesis, and then observed the effect of down-regulation of ODC/polyamine pathway on cerebral I/R injury and explored its possible mechanism.
Materials and methods
All study methods were approved by ethics committee of the First Affiliated Hospital, Liaoning Medical University.
Animals and grouping
Two hundred and forty SD rats weighing between 180 g and 240 g were provided by the Experimental Animal Center, Liaoning Medical University (Jinzhou, China). DFMO (70052-12-9) and dansyl chloride were purchased from Sigma (Silicon Valley, USA). TUNEL kit was purchased from Promega (Madison, State of Wisconsin, USA). Rabbit anti rat GADD153/CHOP kit was purchased from Boosen biological engineering company (Beijing, China). Hydral was purchased from Chemical Reagent Factory (Shanghai, China). According to random digits table, 240 rats were divided into sham-operation (sham) group, ischemia-reperfusion (I/R) group and α-difluoromethylornithine (DFMO) group (each group with 80 rats). According to different time points (3 h, 12 h, 24 h, 48 h and 72 h) after reperfusion, each group was divided into 5 subgroups (each subgroup with 16 rats). In each subgroup, samples of the right cerebral hemisphere between optic chiasma and stalk hypophysial from 8 rats were used for TUNEL and immunohistochemistry, and from other 8 rats were used for high performance liquid chromatography (HPLC) and Western-blot.
Modeling
Rat models of the right middle cerebral artery occlusion (MCAO) were prepared with thread occlusion method [6,7]. The blood supply was restored 1.5 h after ischemia. In sham group, the thread was not inserted into the right middle cerebral artery, but other procedures were the same as that in rat MCAO models. Successful models were that rats exhibited adduction and inflection of the left forelimb in tail suspension, and left tumble or counterclockwise circling in crawl. In DFMO group, 300 mg/kg of DFMO was injected by tail vein 24 h before reperfusion.
Nerve cell apoptosis detected by TUNEL method
After brain tissue from the right cerebral hemisphere between optic chiasma and stalk hypophysial was subjected to paraffin embedding and slicing, nerve cell apoptosis was detected according to the instructions of TUNEL kit. Nuclei of apoptotic cells were brown. Eight sections were randomly selected from each rat, and 8 high-power fields (×400) were selected in each section to count the number of apoptotic cells.
CHOP-positive cells detected by immunohistochemistry
Rats were fixed after they were anesthetized by intraperitoneal injection of 10% chloral hydrate (350 ml/kg). A cut was made on the neck to expose the right common carotid artery followed by perfusion of 50 ml physiological saline and 4% paraformaldehyde at 4°C, respectively. The brain was taken after decapitation. The brain tissue from the right cerebral hemisphere between optic chiasma and pituitary stalk was fixed in 4% paraformaldehyde for 24 h, and then were subjected to gradient dehydration, transparency and paraffin embedding. Samples were sectioned at a thickness of 5 μm for future use. SABC method was performed according to the instructions of immunohistochemistry kit. In negative control group, PBS was used instead of primary antibody. One section was taken from each rat, and then 8 high-power fields (×400) in hippocampal CA1 region were selected to count the number of positive cells. CHOP protein expression was calculated by the following formula: CHOP protein expression = number of positive cells/total number of cells ×100.
CHOP protein expression detected by Western-blot
Rat brain was taken after rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (350 ml/kg). Brain tissue was made into pieces which were placed in lysate followed by centrifuged at 12000 r at 5°C for one hour. Supernatant fluid was taken to determined protein concentration. Samples were placed in boiling water for 5 min, underwent electrophoresis, and then were transferred on nitrocellulose filter. The membrane was washed with TTBS balanced solution containing 5% milk powder, and then primary antibody (goat anti rabbit 1:500) was added at 4°C overnight. After washing the membrane, horseradish peroxidase link-coupled secondary antibody (rabbit anti rat 1:400) was added at room temperature for one hour. After washing the membrane, film exposure was performed by enhanced chemiluminescence followed by analysis of CHOP protein. The relative expression of sample was obtained by the ratio of optical density of sample to β-actin.
Levels of polyamines determined by HPLC
Levels of polyamines in brain tissue were determined according to the method reported by Kabra et al. [8]. Chromatographic conditions were as follows: mobile phase consisting of methanol and double distilled water, gradient elution, column temperature at 40°C, 1 ml/min of flow rate, fluorescence detection including 330 nm of excitation wave length and 510 nm of emission wave length.
Statistical analysis
Data were expressed as mean ± standard deviation. One-factor analysis of variance was performed using SPSS17.0 software. Statistical significance was established at P<0.05.
Results
Nerve cell apoptosis
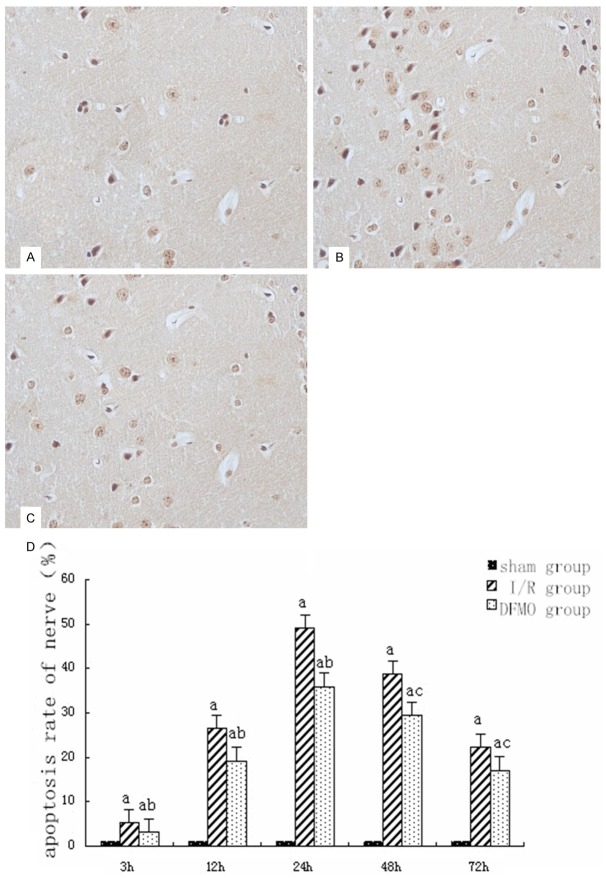
In sham group, less apoptosis was found at each time point. In I/R group, apoptosis began increasing 3 h after reperfusion, reached a peak 24 h after perfusion and began decreasing 48 h after perfusion. Compared with sham group, apoptosis significantly increased in I/R and DFMO groups (P<0.05). However, apoptosis was significantly lower in DFMO group than in I/R group at each time point (P<0.05 or P<0.01) (Figure 1).
Figure 1.
Nerve cell apoptosis detected by TUNEL. A. In sham group 24 h after reperfusion ×400; B. In I/R group 24 h after reperfusion ×400; C. In DFMO group 24 h after reperfusion ×400; D. Statistical analysis of nerve cell apoptosis in each group at different time points. Notes: sham group: sham-operation group; I/R group: ischemia-reperfusion group; DFMO group: α-difluoromethylornithine group. aindicates P<0.05 as compared to sham group at the same time point. band cindicate P<0.05 or P<0.01 as compared to I/R group at the same time point.
CHOP protein expression in hippocampal CA1 region detected by immunohistochemistry
In CHOP-positive cells, cytoplasm was brown and nucleus was occasionally light brown. Most CHOP-positive cells had nuclear shrinkage. In sham group, there were a few of CHOP-positive cells, and there was not significant difference in CHOP-positive cells between each time points (P>0.05). In I/R group, CHOP-positive cells began increasing 3 h after reperfusion, reached a peak 24 h after perfusion and began decreasing 48 h after perfusion. CHOP-positive cells in DFMO group were significantly decreased as compared to I/R group at each time point (P<0.05) (Figure 2).
Figure 2.
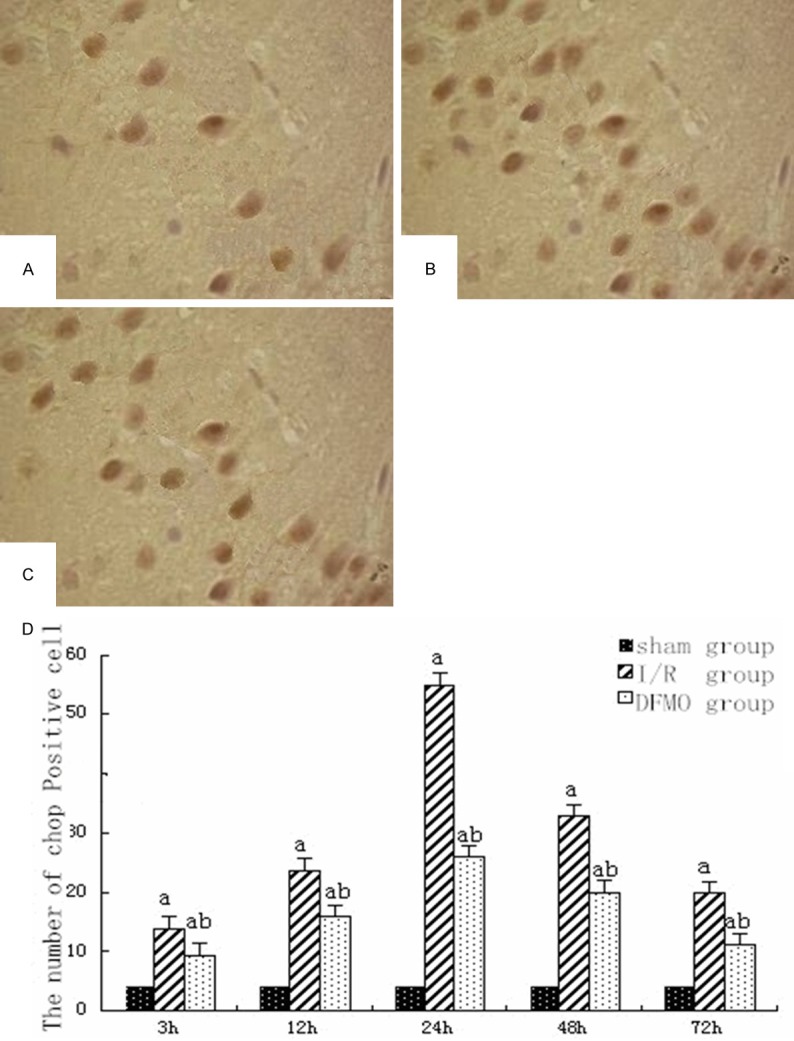
CHOP-positive cells in each group. A. In sham group 24 h after reperfusion ×400; B. In I/R group 24 h after reperfusion ×400; C. In DFMO group 24 h after reperfusion ×400; D. Statistical analysis of CHOP-positive cells in each group at different time points. Notes: sham group: sham-operation group; I/R group: ischemia-reperfusion group; DFMO group: α-difluoromethylornithine group. aindicates P<0.05 as compared to sham group at the same time point. bindicates P<0.05 as compared to I/R group at the same time point.
Level of CHOP protein determined by Western-blot
Western-blot showed no CHOP protein expression in sham group. In I/R group, CHOP protein expression began increasing 3 h after reperfusion, reached a peak 24 h after perfusion and began decreasing 48 h after perfusion. CHOP protein expression was significantly higher in I/R and DFMO groups than in sham group at each time point (P<0.05). CHOP protein expression in DFMO group was significantly decreased as compared to I/R group at each time point (P<0.05) (Figure 3).
Figure 3.
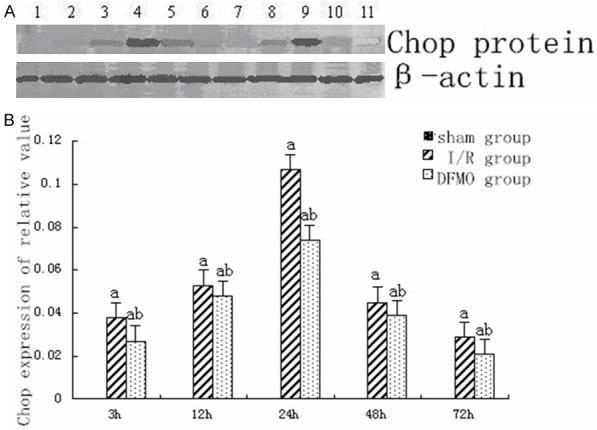
CHOP protein expression in each group at different time points. A. CHOP protein expression determined by Western-blot. 1: sham group; 2: I/R group 3 h after reperfusion; 3: I/R group 12 h after reperfusion; 4: I/R group 24 h after reperfusion; 5: I/R group 48 h after reperfusion; 6: I/R group 72 h after reperfusion; 7: DFMO group 3 h after reperfusion; 8: DFMO group 12 h after reperfusion; 9: DFMO group 24 h after reperfusion; 10: DFMO group 48 h after reperfusion; 11: DFMO group 72 h after reperfusion. B. Statistical analysis of CHOP protein expression. Notes: sham group: sham-operation group; I/R group: ischemia-reperfusion group; DFMO group: α-difluoromethylornithine group. aindicates P<0.05 as compared to sham group at the same time point. bindicates P<0.05 as compared to I/R group at the same time point.
Level of polyamines in each group at different time points
The peak of polyamines including putrescine, spermine and spermidine occurred 24 h after reperfusion in I/R and DFMO groups. The level of polyamines was significantly higher in I/R and DFMO groups than in sham group 12 h, 24 h and 48 h after reperfusion, respectively (P<0.05). Compared to I/R group, the level of polyamines significantly decreased in DFMO group 12 h, 24 h and 48 h after reperfusion, respectively (P<0.05) (Figure 4).
Figure 4.
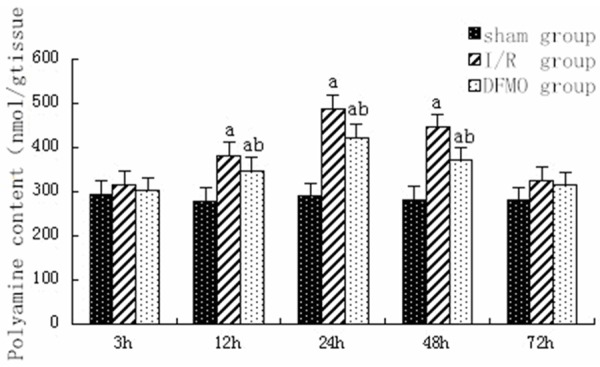
Level of polyamine in each group at different time points. Notes: sham group: sham-operation group; I/R group: ischemia-reperfusion group; DFMO group: α-difluoromethylornithine group. aindicates P<0.05 as compared to sham group at the same time point. bindicates P<0.05 as compared to I/R group at the same time point.
Discussion
Cerebral I/R injury can induce ERS which leads to unfolded protein response (UPR). In mammals, UPR regulates transcription and translation through IRE1-XBP1, ATF-6 and PERK pathways. ERS inhibits protein translation mostly through PERK protein activation and elf2 protein kinase phosphorylation, which leads to accumulation of peroxides in cells and promotes apoptosis [9,10]. CHOP is a classic marker of ERS and CHOP-induced apoptotic pathway is one of the ERS-apoptotic signal pathways.
Polyamines include putrescine, spermidine and spermine. Ornithine decarboxylase (ODC) is a key enzyme in polyamine synthesis and DFMO, an inhibitor of ODC, may reduce the production of polyamines. Polyamines have toxic effect on neurons in cerebral I/R because ① they can interfere with permeability of blood brain barrier, promote vasogenic edema and increase cellular sensitivity to N-methyl-D-aspartic acid receptor (NMDA)-mediated signal response, leading to neuronal damage [11,12]; ② they can inhibit the activity of nitric oxide synthase which is a protective agent in ischemic condition [13]. Furthermore, in cerebral I/R, elevation of activity and content of ODC also aggravate neuronal damage [14].
In this study, we detected apoptosis in rats at different time points after cerebral I/R, and found that apoptotic cells occurred 3 h after reperfusion and reached a peak 24 h after reperfusion in hippocampal CA1 region. In I/R group, most apoptotic cells were located in ischemic area and were brown. Apoptotic cells significantly increased in I/R group as compared to sham group; but they significantly decreased after DFMO treatment. This suggests that DFMO has inhibitory effect on cerebral I/R-induced apoptosis, but the mechanism has not been completely clear. Therefore, we observed CHOP expression to explore the mechanism that DFMO inhibits apoptosis.
In this study, we also found that CHOP-positive cells began increasing 3 h after reperfusion, reached a peak 24 h after reperfusion and began decreasing 48 h after reperfusion. CHOP-positive cells were significantly higher in I/R group than in sham and DFMO groups. At the same time, CHOP protein expression detected by Western-blot was consistent with CHOP-positive cells determined by immunohistochemistry. This demonstrated that ERS occurrence and CHOP elevation after cerebral I/R trigger CHOP-induced apoptotic pathway [15].
In this study, compared to sham group, level of polyamines and apoptotic cells were significantly increased in I/R group, demonstrating that polyamines have toxic effect on neurons in cerebral I/R. DFMO, a specific inhibitor of ODC, significantly decreased level of polyamines and apoptotic cells, suggesting that down-regulation of polyamine pathway has a protective role in cerebral I/R injury. This conclusion is consistent with the report [16]. At the same time, we also found that DFMO also decreased CHOP protein expression and CHOP-positive cells, suggesting that ODC/polyamine pathway is related to CHOP-induced apoptotic pathway in cerebral I/R injury. It has been reported that over-expression of ODC can inhibit CHOP expression through depression of PERK phosphorylation and caspase-4 activation [17].
In summary, down-regulation of ODC/polyamine pathway plays a protective role in cerebral I/R injury through inhibiting CHOP-induced apoptotic pathway. This study provides a new idea for the association between ODC/polyamine pathway and ERS in cerebral I/R injury.
Acknowledgements
This study was supported by Technology Department of Liaoning Province of China (2014022006).
Disclosure of conflict of interest
None.
References
- 1.Rasheva VI, Domingos PM. Celluer responses to endoplasmic reticulum stress and apoptosis. Apoptosis. 2009;14:996–1007. doi: 10.1007/s10495-009-0341-y. [DOI] [PubMed] [Google Scholar]
- 2.Mozzini C, Fratta Pasini A, Garbin U, Stranieri C, Pasini A, Vallerio P, Cominacini L. Increased Endoplasmic Reticulum Stress and Nrf2 repression in peripheral blood mononuclear cells of patients with stable coronary artery disease. Free Radical Bio Med. 2014;68:178–185. doi: 10.1016/j.freeradbiomed.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 3.Paschen W, Gissel C, Linden T, Althausen S, Doutheil J. 3 Activati on of gadd153 exression through transient cerebral ischemia: evidence that ischemia causes endoplasmic reticulum function. Brain Res Mol Brain Res. 1998;60:115–22. doi: 10.1016/s0169-328x(98)00180-6. [DOI] [PubMed] [Google Scholar]
- 4.Yu L, Lu M, Wang P, Chen X. Trichostatin A Ameliorates Myocardial Ischemia/reperfusion Injury Through Inhibition of Endoplasmic Reticulum Stress-induced Apoptosis. Arch Med Res. 2012;43:190–196. doi: 10.1016/j.arcmed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Pietilä M, Dhungana H, Uimari A, Sironen R, Alhonen L. Systemic overexpression of antizyme 1 in mouse reduces ornithine decarboxylase activity without major changes in tissue polyamine homeostasis. Transgenic Res. 2014;23:153–163. doi: 10.1007/s11248-013-9763-y. [DOI] [PubMed] [Google Scholar]
- 6.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 7.Cao YJ, Cheng YB. Improvement for rat model of focal cerebral ischemia/reperfusion prepared by thread occlusion. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2001;17:198–200. [PubMed] [Google Scholar]
- 8.Kabra PM, Lee HK, Lubich WP, Marton LJ. Solid-Phase extraction and determination of dansyl derivatives of unconjugated and acetylated polyamines by reversed-phase liquid chromatography: improved separation systems for polyamines in cerebrospinal fluid, urine and tissue. J Chromatogr. 1986;380:19–32. doi: 10.1016/s0378-4347(00)83621-x. [DOI] [PubMed] [Google Scholar]
- 9.Spitler Kathryn M, Webb RC. Endoplasmic Reticulum Stress Contributes to Aortic Stiffening via Proapoptotic and Fibrotic Signaling Mechanisms. Hypertension. 2014;63:e40–5. doi: 10.1161/HYPERTENSIONAHA.113.02558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li YP, Zhu W, Tao JP. Fasudil Protects the Heart against Ischemia-Reperfusion Injury by Attenuating Endoplasmic Reticulum Stress and Modulating SERCA Activity: The Differential Role forPI3K/Akt and JAK2/STAT3 Signaling Pathways. PLoS One. 2012;7:e48115. doi: 10.1371/journal.pone.0048115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feith David J, Pegg Anthony E, Fong Louise Y. Targeted expression of Ornithine Decarboxylase antizyme prevents upper aerodigestive tract carcinogenesis in p53-deficient mice. Carcinogenesis. 2013;34:570–576. doi: 10.1093/carcin/bgs377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Y, Liu JC, Zhang XJ, Li GW, Wang LN, Xi YH, Li HZ. Downregulation of the Ornithine Decarboxylase/polyamine System Inhibits Angiotensin-induced Hypertrophy of Cardiomyocytes Through the NO/cGMP-dependent Protein Kinase Type-I Pathway. Cell Physiol Biochem. 2010;25:443–450. doi: 10.1159/000303049. [DOI] [PubMed] [Google Scholar]
- 13.Soda K. The mechanisms by which polyamines accelerate tumor spread. J Exp Clin Cancer Res. 2011;30:95. doi: 10.1186/1756-9966-30-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muth A, Madan M, Archer JJ, Ocampo N, Rodriguez L, Phanstiel O 4th. Polyamine transport inhibitors: design, synthesis, and combination therapies with difluoromethylornithine. J Med Chem. 2014;57:348–63. doi: 10.1021/jm401174a. [DOI] [PubMed] [Google Scholar]
- 15.DeGracia DJ, Montie HL. Cerebral ischemia and the unfolded protein response. J Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmitz MP, Combs DJ, Dempsey RJ. Difluoromethylornithine decreases postischemic brain edema and blood-brain barrier breakdown. Neurosurgery. 1993;33:882–7. doi: 10.1227/00006123-199311000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh WC, Hsu PC, Liao YF, Young ST, Wang ZW, Lin CL. Overexpression of ornithine decarboxylase suppresses thapsigargin-induced apoptosis. Mol Cells. 2010;30:311–318. doi: 10.1007/s10059-010-0120-1. [DOI] [PubMed] [Google Scholar]