Abstract
Lycium ruthenicum Murr. is commonly used in traditional Tibetan medicine, and the fruits of Lycium ruthenicum Murr. contain an immunologically active pectin which improves immune function against chronic diseases. The present study was performed to evaluate the immunomodulatory effects of Lycium ruthenicum Murr. polysaccharide 3 (LRGP3) in cyclophosphamide (Cy)-induced immunosuppressed mice. Mice were injected intraperitoneally once daily with low-dose (25 mg/kg), intermediate-dose (50 mg/kg), high-dose (100 mg/kg) of LRGP3 for 10 consecutive days, respectively. Compared with Cy group, LRGP3 accelerated recovery of spleen and thymus indices, enhanced T cell and B cell proliferation responses, as well as peritoneal macrophage phagocytosis. In addition, LRGP3 treatment restored the levels of interleukin-2 (IL-2), IL-6 and tumor necrosis factor-α (TNF-α) in the serum of Cy-treated mice. These results indicate that LRGP3 plays an important role in the protection against immunosuppression in Cy-treated mice and could be a potential immunomodulatory agent.
Keywords: Lycium ruthenicum Murr., polysaccharide, cyclophosphamide, immunomodulation
Introduction
Immunosuppression is a state of temporary or permanent immunity dysfunction and can make organism more sensitive to pathogens due to the damage of immune system. Immunomodulatory agents used with chemotherapy drugs appear to enhance immune response. Therefore, to study and develop new immunomodulatory agents is one of the most effective methods for prevention and treatment of the immunosuppressive diseases.
Polysaccharide is a potential immunomodulator because it has no significant side effects, which is a major problem associated with immunomodulatory bacterial polysaccharides and synthetic compounds, and it can enhance immunity by inducing lymphocyte proliferation, cytokine production and so on [1]. Therefore, in recent years, a great deal of experiments involved in immunomodulation of polysaccharides isolated from traditional medical plants was carried out. Shuai et al. reported that administration of Sophora subprosrate polysaccharide by intraperitoneal injection significantly raised spleen index, glutathione level, glutathione peroxidase activity and lysozyme activity in the immunosuppressed mice [2]. Chen et al. reported that Schisandra chinensis Baill polysaccharide exhibited potent immunomodulating properties, such as improving the weight of immune organs, enhancing the phagocytic activity of peritoneal macrophages and increasing the levels of cytokines in serum [3].
Lycium ruthenicum Murr., which belongs to the genus Lycium of the family Solanaceae, is a kind of wild resource plant distributed mainly in Qinghai and Xinjiang Provinces of China [4]. Its fruit has been used as traditional Tibetan medicine for thousands of years. Recently, some investigations indicated that Lycium ruthenicum Murr. exhibits a wide range of pharmacological properties, such as anti-oxidation, anti-fatigue and hypoglycemic activity [5-7]. Polysaccharides are a structurally diverse class of macromolecules, and this structural variability can affect their cell-type specificity and their biological activity in T lymphocytes, B lymphocytes and macrophages [8,9]. It has been reported that Lycium ruthenicum Murr. polysaccharide inhibits the lipopolysaccharide-induced nitric oxide (NO) production and the mRNA expression of inducible nitric oxide synthase (iNOS), and suppresses the levels of pro-inflammatory cytokines in lipopolysaccharide-stimulated macrophages [10]. However, the in vivo immunomodulatory efficacy of Lycium ruthenicum Murr. polysaccharide is poorly understood. The present study was to elucidate the effects of LRGP3 in immunosuppressed mice induced by Cy treatment.
Materials and methods
Materials
The fruit of Lycium ruthenicum Murr. was purchased from Jiahe Biological Engineering Co. (Qinghai, China). Other reagents and chemicals used were of analytical grade.
Isolation and purification of LRGP3
The crude polysaccharide was extracted from Lycium ruthenicum Murr., and then the ion-exchange and gel-permeation chromatography was used to isolate and purify the crude polysaccharides and a polysaccharide LRGP3 was obtained [11].
Experimental animal
Kunming female mice of clean grade (8-weeks old; body weight 20.0±2.0 g) were purchased from the Animal Center of School of Medicine, Shanghai Jiaotong University. The animals were maintained on a 12-h-dark/12-h-light cycle at 20°C. They were allowed free access to standard diet and sterile water throughout the experiments. All experimental procedures were approved by the Animal Care and Use Committee of School of Medicine, Shanghai Jiaotong University, China. Adequate measures were taken to minimize pain of experimental animals.
Experimental design
Mice were randomly divided into five groups (10 in each group) and were allowed to acclimatize for 1 week before the experiment. Animals received Cy subcutaneously for five days at a dose of 80 mg/kg body weight to establish the immunosuppressive animal model. Different groups were treated with LRGP3 at 25, 50 and 100 mg/kg, whereas control group and model group were given the same volume of physiological saline via intraperitoneally injection. Animals were sacrificed after 10 days of treatment. Twenty-four hours after the last drug administration, the mice were weighed and killed by decapitation. The blood was collected to separate serum. Moreover, the spleen and thymus were excised from the sacrificed animals and weighed immediately. Thymus and spleen indices were calculated according to the following formula: thymus or spleen index (mg/g) = (weight of thymus or spleen/body weight).
Assay of splenocyte proliferation
Cell proliferation was assessed using MTT method [12]. Splenocytes were obtained by gently placing the organ in RPMI-1640 medium (Sigma, Saint Louis, MO, USA) under aseptic conditions, followed by centrifugation (1500 rpm, for 10 min) at room temperature. The erythrocytes were removed by hemolytic Gey’s solution, while the remaining cells were centrifuged at 200 × g for 10 min. After two washes, the splenocytes were resuspended in RMPI-1640 medium containing 5% fetal bovine serum (FBS), and adjusted to 5 × 106 cell/mL.
100 μL of splenocyte suspension (5 × 106 cell/mL) in a 96-well culture plate were preincubated in RPMI1640 medium for 24 h before the addition of mitogens (LPS and lectin at 5.0 µg/mL) (Sigma, Saint Louis, MO, USA). After incubation for 72 h at 37°C in a humidified 5% CO2 incubator, the number of proliferating cells was determined by MTT assay at a wavelength of 540 nm. The percentage of proliferation was calculated by the following equation: Proliferation (%) = ODtest/ODcontrol × 100%.
Phagocytosis of peritoneal macrophages
Macrophage phagocytic function was assessed as previously described [13]. In brief, 24 h after the last drug administration, mice were injected via a lateral tail vein with ink (10 mL/kg body weight). Blood sample were collected from the orbital sinus/retro-orbit venous plexus at 2 min (t1) and 10 min (t2) after the injection. And then the two samples were mixed rapidly with 2 mL of 0.1% Na2CO3 in a tube and optical density (OD1 for t1; OD2 for t2) was measured by spectrophotometry at 600 nm. The mice were killed, and each liver and spleen was removed and weighted. The rate of carbon clearance (K value) and phagocytic index (a value) were calculated by the formulas: K = (lg OD1-lg OD2)/(t2-t1); a = A(K1/3)/(B + C). A is the average body weight, B is the average liver weight and C is the average spleen weight for a particular group.
Serum hemolysin formation test
Serum hemolysin formation was assessed as previously described [14]. The mice were immunized by injection with 0.2 mL of 5% chicken red blood cell (CRBC) (Baiji Biological Engineering Co., Zhengzhou, China) on the fifth day. 1 h after the last drug administration, the peripheral blood samples were collected and centrifuged at 2000 rpm/min for 10 min, and 20 mL of supernatant serum was diluted to 500 times with normal saline. 0.5 mL of 5% sheep red blood cell (SRBC) (Baiji Biological Engineering Co., Zhengzhou, China) and 0.5 mL of fresh guinea pig serum (1:10 dilution) was added to the reaction tubes filled with diluted serum samples. After incubation for 1 h at 37°C, the reaction tubes were immediately moved to an ice bath and centrifuged again under the same conditions. About 1 mL of the supernatant was mixed with 3 mL of Drabkin’s solution. 10 min later, the absorbance of the solution was measured using a micro plate ELISA reader (PT-3502, Potenov Technology Corporation, Beijing, China) at 540 nm.
Determination of serum cytokine levels by the ELISA assay
The levels of IL-2, IL-6 and TNF-α in the serum of the mice from each group were determined using the mouse ELISA kits (Beyotime, Nantong, China) according to the manufacturer’s protocols.
Statistical analysis
Results were presented as mean ± S.D. All statistical comparisons were carried out using one-way ANOVA test followed by Tukey’s test. P-values less than 0.05 were considered to be a statistically significant difference.
Results
Effect of LRGP3 on spleen and thymus indices in Cy-treated mice
The immune function was diminished when the mice were treated with Cy alone. As shown in Table 1, the spleen indices were decreased significantly compared with normal group. When the mice were given both intraperitoneal injection of LRGP3 at 25, 50 or 100 mg/kg body weight once daily for ten days, the spleen indices of the mice increased obviously compared with Cy group. Moreover, consistent with the spleen index, LRGP3 also increase the thymus index in Cy-treated mice in a dose-dependent manner. These results suggested that LRGP3 protects the immune organs against the impairment caused by Cy.
Table 1.
Effects of LRGP3 on spleen and thymus indices in Cy-treated mice
| Group | Spleen index (mg/g) | Thymus index (mg/g) |
|---|---|---|
| Control | 4.43 ± 0.67 | 3.43 ± 0.34 |
| Cy | 3.27 ± 0.28* | 1.81 ± 0.17* |
| LRGP3 (25 mg/kg) + Cy | 4.38 ± 0.74# | 2.44 ± 0.19# |
| LRGP3 (50 mg/kg) + Cy | 4.94 ± 0.96# | 2.73 ± 0.21# |
| LRGP3 (100 mg/kg) + Cy | 4.65 ± 0.79# | 2.97 ± 0.27# |
Data are means ± SD of ten animals.
P < 0.05 compared to control group;
P < 0.05 compared to Cy group.
Effect of LRGP3 on mitogen-induced splenic lymphocyte proliferation in Cy-treated mice
It is well known that lectin stimulates T cells and LPS stimulates B cell proliferation. In this study, we investigated the effects of LRGP3 on lymphocyte proliferation with mitogen stimulation. As shown in Figure 1, the proliferative responses of splenic lymphocytes to lectin and LPS were reduced significantly in Cy-treated mice, as compared with the control group. In the presence of lectin, LRGP3 at 25, 50 and 100 mg/kg enhanced the proliferation of lymphocytes by 55%, 64% and 76%, respectively, compared with the vehicle group. In the presence of LPS, low-, intermediate- and high-dose LRGP3 elicited an increase in lymphocytes proliferation by 65%, 74% and 87%, respectively, as compared with the vehicle group. These results suggested that LRGP3 enhanced both T cell and B cell proliferation in a dose-dependent manner.
Figure 1.
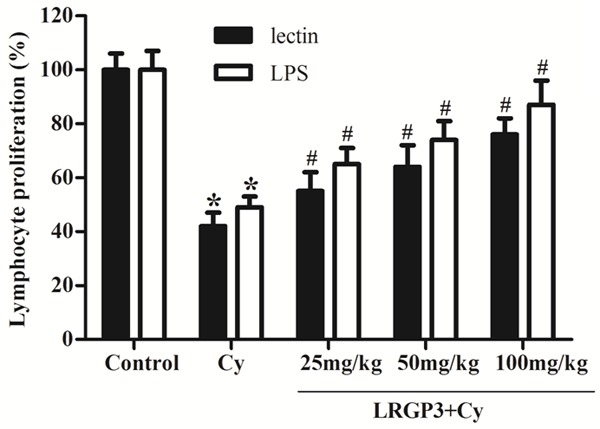
Effect of LRGP3 on mitogen-induced splenic lymphocyte proliferation in Cy-treated mice. LRGP3 enhanced both T cell and B cell proliferation in a dose-dependent manner. Data are means ± SD of ten animals. *P < 0.05 compared to control group; #P < 0.05 compared to Cy group.
Effect of LRGP3 on peritoneal macrophage phagocytosis in Cy-treated mice
As shown in Figure 2, peritoneal macrophage phagocytosis was significantly lower in Cy-treated mice than in the control group. This inhibitory effect of Cy was obviously reversed by pretreatment with LRGP3. In the low-, intermediate- and high-dose LRGP3 groups, the phagocytic indexes were significantly higher than in the Cy-treated, exhibiting a dose-dependent manner.
Figure 2.
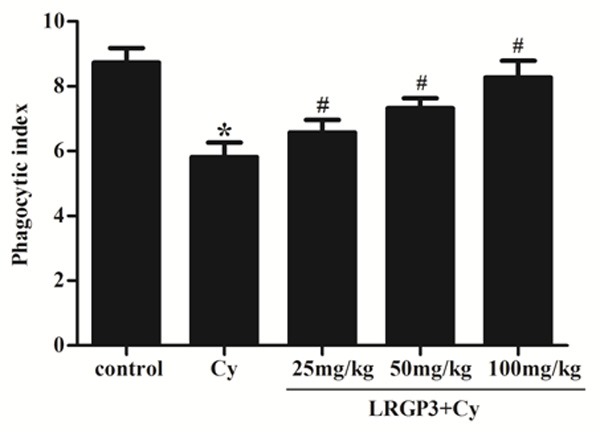
Effect of LRGP3 on peritoneal macrophage phagocytosis in Cy-treated mice. LRGP3 promotes peritoneal macrophage phagocytosis in a dose-dependent manner. Data are means ± SD of ten animals. *P < 0.05 compared to control group; #P < 0.05 compared to Cy group.
Effect of LRGP3 on serum hemolysin formation
In order to investigate the effect of on the regulating humoral immunity, the serum hemolysin levels were determined among all the groups. As shown in Figure 3, the serum hymolysin level was significantly decreased in the Cy group compared with that of the control group, which shows that Cy could suppress humoral immunity. In contrast, the serum hemolysin formation was significantly increased in the LRGP3-treated groups, as compared with the Cy group.
Figure 3.
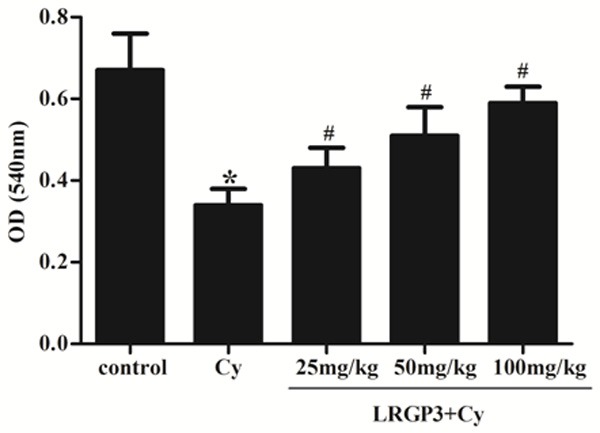
Effect of LRGP3 on serum hemolysin formation in Cy-treated mice. LRGP3 promotes serum hemolysin formation in a dose-dependent manner. Data are means ± SD of ten animals. *P < 0.05 compared to control group; #P < 0.05 compared to Cy group.
Effect of LRGP3 on the levels of cytokines in Cy-treated mice
The effects of LRGP3 on IL-2, IL-6 and TNF-α level in the immunosuppressed mice were examined. As shown in Figure 4, the levels of IL-2, IL-6 and TNF-α were inhibited significantly in Cy-treated mice, as compared with the control group. Treatment with low-, intermediate- and high-dose LRGP3 increases the levels of IL-2, IL-6 and TNF-α in immunosuppressed mice serum, which indicates that LRGP3 could promote the secretion of IL-2, IL-6 and TNF-α.
Figure 4.
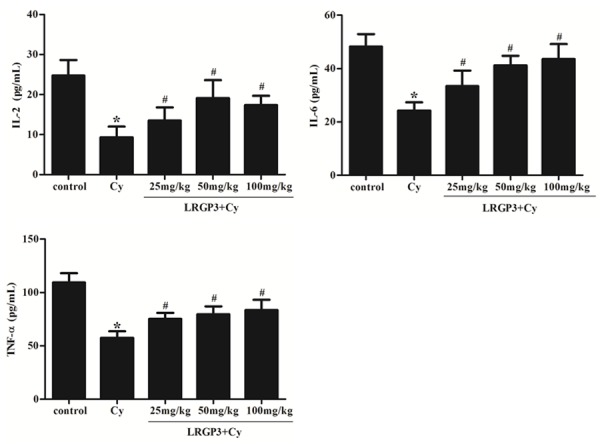
Effect of LRGP3 on the levels of cytokines in Cy-treated mice. LRGP3 increases the levels of IL-2, IL-6 and TNF-α in immunosuppressed mice serum. Data are means ± SD of ten animals. *P < 0.05 compared to control group; #P < 0.05 compared to Cy group.
Discussion
The modulation of immune cell activities by molecules from medicinal plant origin is an area of active interest for inflammation, autoimmunity and cancer therapy. The application natural immunostimulants is a promising approach because they are biodegradable, biocompatible and safe for human health. Polysaccharides isolated from various traditional medical plants had been demonstrated to profoundly affect the immune system both in vivo and in vitro through their ability to modulate immune function. In this study, we assessed the immunomodulatory activity of orally administrated LRGP3 on various indices in immunosuppressed mice, and our results demonstrated that treatment with LRGP3 accelerated recovery of immunosuppression in Cy-treated mice.
Cy, a well-known alkylating agent, acts as an important chemotherapeutic drug in tumor treatment [15], but administration of Cy leads to myelosuppression and immunosuppression [16]. It can damage the structure of DNA, kill the immune cells, interfere with the proliferation and differentiation of B and T cells, and restrain the humoral and cellular immune response. Therefore, we used Cy to create immunosuppressive animal model in this study. Immune function and immune prognosis are reflected by the spleen and thymus indices, because these organs play important roles in nonspecific immunity. Spleen contains T-cells and B-cells, while thymus is the organ in which T lymphocytes develop, differentiate and mature [17]. In this study, we found that LRGP3 increases the spleen and thymus indices in the Cy-treated mice, indicating that LRGP3 was able to counteract the effect of immunosuppression on immune organs development. It was reported that some Chinese herbal medicines or their ingredients also could significantly improve the immune organs index of mice and inhibit the atrophy of immune organs.
Lymphocytes are the key effector cells of mammalian adaptive immune system. Lymphocyte proliferation is the direct indicator reflecting the state of cellular immunity in animal. Cell-mediated immunity can exert the actions of anti-infection, anti-tumor, helping lymphocyte produce antibody by sensitized lymphocyte against corresponding antigen [18]. Lectin acts directly on lymphocyte T cells, which are involved in cell mediated immunity, while LPS acts on lymphocyte B cells that are responsible for humoral immune response [19]. Our results demonstrated that the co-mitogenic activities of LRGP3, both with lectin and LPS, were dose-dependent in Cy-treated mice. Therefore, LRGP3 could enhance both cellular and humoral immunity in Cy-treated mice.
Macrophages play critical roles in immune response and in host defense. It can function as antigen-presenting cells and can interact with T lymphocytes to regulate the adaptive immune response. Activated macrophages not only participate in both specific and non-specific immune reactions, but also is the “bridge cell” of these two immune reactions [20]. Furthermore, macrophages contribute to the innate immune response to invading microorganisms by engulfing them through the process of phagocytosis [21]. In this study, our results showed that LRGP3 effectively stimulated the phagocytic activity of macrophages in Cy-treated mice, indicating that LRGP3 may initiate the activation of macrophage function.
The formation of serum hemolysin with SRBC immunization reflects humoral immunologic function [22]. In this study, our results showed that LRGP3 obviously increased serum hemolysin formation in Cy-immunosuppressed mice, indicating that LRGP3 can enhance humoral immunity activities.
Cytokines play an important role in cell-cell communication in the immune system. Many natural products modulated cytokine production. Polysaccharides could stimulate production of both pro-inflammatory and anti-inflammatory cytokines [23]. IL-2 is secreted by Type 1 helper T cells mediating cellular immune response and can induce proliferation of B and natural killer (NK) cells [24]. IL-6 is a pleiotropic cytokine that modulates the proliferation and differentiation of B-cells and T-cells diverse cell functions, such as proliferation, differentiation [25]. TNF-α also play an important in host defense and can induce the expression of a deal of other immunoregulatory mediators [26]. In the present study, our results showed that LRGP3 raised the levels of IL-2, IL-6 and TNF-α, indicating that LRGP3 may have regulatory effects on inflammation and lymphocyte functions through these cytokines secretion.
In conclusion, LRGP3 possessed reliable immunoenhancement, it could resist immunosuppression by promoting immune organs development, lymphocyte proliferation and macrophage phagotytosis, up-regulating the levels of serum cytokines in immunosuppressive mice induced by Cy. Therefore, our findings suggest that LRGP3 is a potent immunomodulator for prevention and treatment of immunosuppressive diseases.
Acknowledgements
This study was supported by the grants from National Natural Science Foundation of China (No. 81171845) and Songjiang foundation (No. 2011PD13).
Disclosure of conflict of interest
None.
References
- 1.Chen JR, Yang ZQ, Hu TJ, Yan ZT, Niu TX, Wang L, Cui DA, Wang M. Immunomodulatory activity in vitro and in vivo of polysaccharide from Potentilla anserina. Fitoterapia. 2010;81:1117–1124. doi: 10.1016/j.fitote.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Shuai XH, Hu TJ, Liu HL, Su ZJ, Zeng Y, Li YH. Immunomodulatory effect of a Sophora subprosrate polysaccharide in mice. Int J Biol Macromol. 2010;46:79–84. doi: 10.1016/j.ijbiomac.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Tang J, Wang X, Sun F, Liang S. An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz. ) Baill Int J Biol Macromol. 2012;50:844–848. doi: 10.1016/j.ijbiomac.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Lv X, Wang C, Cheng Y, Huang L, Wang Z. Isolation and structural characterization of a polysaccharide LRP4-A from Lycium ruthenicum Murr. Carbohydr Res. 2013;365:20–25. doi: 10.1016/j.carres.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z, Dang J, Wang Q, Yu M, Jiang L, Mei L, Shao Y, Tao Y. Optimization of polysaccharides from Lycium ruthenicum fruit using RSM and its anti-oxidant activity. Int J Biol Macromol. 2013;61:127–134. doi: 10.1016/j.ijbiomac.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 6.Ni W, Gao T, Wang H, Du Y, Li J, Li C, Wei L, Bi H. Anti-fatigue activity of polysaccharides from the fruits of four Tibetan plateau indigenous medicinal plants. J Ethnopharmacol. 2013;150:529–535. doi: 10.1016/j.jep.2013.08.055. [DOI] [PubMed] [Google Scholar]
- 7.Wang JH, Chen XQ, Zhang WJ. Study on Hypoglycemic Function of Polysaccharides from Lycium ruthenicum Murr. Fruit and Its Mechanism. Food Sci. 2009;5:057. [Google Scholar]
- 8.Cheung JK, Li J, Cheung AW, Zhu Y, Zheng KY, Bi CW, Duan R, Choi RC, Lau DT, Dong TT. Cordysinocan, a polysaccharide isolated from cultured Cordyceps, activates immune responses in cultured T-lymphocytes and macrophages: Signaling cascade and induction of cytokines. J Ethnopharmacol. 2009;124:61–68. doi: 10.1016/j.jep.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Xu HS, Wu YW, Xu SF, Sun HX, Chen FY, Yao L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J Ethnopharmacol. 2009;125:310–317. doi: 10.1016/j.jep.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Peng Q, Liu H, Shi S, Li M. Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-κB signaling pathway. Int J Biol Macromol. 2014;67:330–335. doi: 10.1016/j.ijbiomac.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Peng Q, Song J, Lv X, Wang Z, Huang L, Du Y. Structural Characterization of an Arabinogalactan-Protein from the Fruits of Lycium ruthenicum. J Agr Food Chem. 2012;60:9424–9429. doi: 10.1021/jf302619c. [DOI] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Meng XY, Yang R L, Qin T, Wang XY, Zhang KY, Fei CZ, Li Y, Xue FQ. Cordyceps militaris polysaccharides can enhance the immunity and antioxidation activity in immunosuppressed mice. Carbohydr Polym. 2012;89:461–466. doi: 10.1016/j.carbpol.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Tang J, Wang X, Sun F, Liang S. An immunostimulatory polysaccharide (SCP-IIa) from the fruit of Schisandra chinensis (Turcz. ) Baill Int J Biol Macromol. 2012;50:844–848. doi: 10.1016/j.ijbiomac.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer Res. 2011;71:661–665. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]
- 16.Chen W, Zhang W, Shen W, Wang K. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell Immunol. 2010;262:69–74. doi: 10.1016/j.cellimm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Yang X, Lv Y, Tian L, Zhao Y. Composition and systemic immune activity of the polysaccharides from an herbal tea (Lycopus lucidus Turcz) J Agr Food Chem. 2010;58:6075–6080. doi: 10.1021/jf101061y. [DOI] [PubMed] [Google Scholar]
- 18.Fan Y, Lu Y, Wang D, Liu J, Song X, Zhang W, Zhao X, Nguyen TL, Hu Y. Effect of epimedium polysaccharide-propolis flavone immunopotentiator on immunosuppression induced by cyclophosphamide in chickens. Cell Immunol. 2013;281:37–43. doi: 10.1016/j.cellimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Xu HS, Wu YW, Xu SF, Sun HX, Chen FY, Yao L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J Ethnopharmacol. 2009;125:310–317. doi: 10.1016/j.jep.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen W, Zhang W, Shen W, Wang K. Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell Immunol. 2010;262:69–74. doi: 10.1016/j.cellimm.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Maekawa A, Austen KF, Kanaoka Y. Targeted gene disruption reveals the role of cysteinyl leukotriene 1 receptor in the enhanced vascular permeability of mice undergoing acute inflammatory responses. J Biol Chem. 2002;277:20820–20824. doi: 10.1074/jbc.M203163200. [DOI] [PubMed] [Google Scholar]
- 22.Wang Z, Li H, Xu H, Yue XL, Cheng XQ, Hou WJ, Zhang YY, Chen DF. Beneficial effect of Bupleurum polysaccharides on autoimmune disease induced by Campylobacter jejuni in BALB/c mice. J Ethnopharmacol. 2009;124:481–487. doi: 10.1016/j.jep.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 23.Liu CJ, Lin JY. Anti-inflammatory and anti-apoptotic effects of strawberry and mulbery fruit polysaccharides on lipopolysaccharide-stimulated macrophages through modulating pro-/anti-inflammatory cytokines secretion and Bcl-2/Bak protein ratio. Food Chem Toxicol. 2012;50:3032–3039. doi: 10.1016/j.fct.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 24.Lu Z, Jin M, Huang M, Wang Y, Wang Y. Bioactivity of selenium-enriched exopolysaccharides produced by Enterobacter cloacae Z0206 in broilers. Carbohydr Polym. 2013;96:131–136. doi: 10.1016/j.carbpol.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 25.Samoilova EB, Horton JL, Hilliard B, Liu TST, Chen Y. IL-6-deficient mice are resistant to experimental autoimmune encephalomyelitis: roles of IL-6 in the activation and differentiation of autoreactive T cells. J Immunol. 1998;161:6480–6486. [PubMed] [Google Scholar]
- 26.Yu Q, Nie SP, Wang JQ, Yin PF, Huang DF, Li WJ, Xie MY. Toll-like receptor 4-mediated ROS signaling pathway involved in Ganoderma atrum polysaccharide-induced tumor necrosis factor-α secretion during macrophage activation. Food Chem Toxicol. 2014;66:14–22. doi: 10.1016/j.fct.2014.01.018. [DOI] [PubMed] [Google Scholar]


