Abstract
In this study the antitumor effects of polyphyllin I (PPI) were investigated in hepatocellular carcinoma HepG2 cells. Our data showed that PPI treatment exerted dose-dependent cytotoxicity on HepG2 cells as previously reported. Furthermore, PPI could sensitize HepG2 cells to cisplastin treatment in concentration-dependent manner. The molecular mechanisms of PPI actions involved nuclear factor-κB (NF-κB) and its downstream gene products. PPI treatment dose-dependently could decrease the constitutive phosphorylation of NF-κB subunit p65 protein and its downstream target genes expression, such as Bcl-2, c-Myc and VEGF. PPI could also inhibit cisplatin-evoked increase of p65 protein phosphorylation and its downstream genes expression, which could be further decreased by combination with NF-κB specific inhibitor, PDTC. The cytotoxicity and chemosensitization effects of PPI on HepG2 cells were greatly potentiated by concomitant treatment with PDTC. Taken together, our data confirmed the cytotoxicity of PPI on hepatocellular carcinoma HepG2 cells and provided new findings about PPI sensitizing HepG2 cells to chemotherapy. Moreover, our data also indicated the involvement of NF-κB signaling pathway in PPIactions for the first time.
Keywords: Hepatocellular carcinoma, polyphyllin I (PPI), cisplatin, nuclear factor--κB (NF-κB), cytotoxicity, chemotherapy, chemosensitizing effects
Introduction
Hepatocellular carcinoma (HCC) is the main form of liver cancer, which represents the third and fifth leading cause of death from cancer worldwide in men and women, respectively. The main attributable factors of HCC include persistent infection of hepatitis virus and contamination of foodstuff with aflatoxins [1,2]. For many HCC patients the cancers are considered as non-resectable when diagnosed. And for these patients chemotherapy is the only choice of treatment. However, drug resistance developed in cancer cells after treatment is always a major obstacle to the successful management of liver cancer [3,4]. So patients with HCC usually show poor prognosis and low 5-year survival rate (approximately 5-6%) because of the low effectiveness of available treatments [1]. And therefore, development of new adjuvant therapeutic agents that can chemosensitize cancer cells is still in great demand.
Traditional Chinese medical herb, Rhizoma of Paris polyphyllin, also known as Chong-Lou in China, has been widely prescribed by herbal practitioners to treat a number of tumors including pancreas, urinary bladder and liver tumor [5]. As one of the main active components of Rhizoma of Paris polyphyllin [6], polyphyllin I (PPI), a steroidal saponin, has been studied to show antitumor effects in many types of cancers, such asgastric cancer [7,8], breast cancer [9], glioblastoma [10], ovarian cancer [11], lung cancer [6] and hepatocellular cancer [12,13] through inhibiting tumor cell growth, metastasis [6,9-12,14], and inducing cell cycle arrest [8] and mitochondrial pathway-mediated apoptosis [13,15]. However, whether PPI cansensitize tumor cells to chemotherapy has not been investigated so far. In this study we determined the chemosensitizing effects of PPI on hepatocellular carcinoma HepG2 cells and further explored the molecular mechanisms.
Materials and methods
Materials
HepG2 hepatocellular carcinoma cells were purchased from American Type Culture Collection (ATCC, Manassas, Virginia, USA). Polyphyllin I (PPI) was bought from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Ammonium pyrrolidinedithiocarbamate (PDTC), Aprotinin, leupeptin and cisplatinwere purchased from Sigma Chemical Co. (St. Louis, MO, USA). Primary antibodies against actin, phosphor-p65, Bcl-2, c-Myc, VEGF were purchase from Cell Signaling Technology (Denver, Colorado, USA). Secondary antibodies were obtained from Jackson ImmunoResearch (Baltimore, Maryland, USA).
Cell culture
HepG2 cells were cultured in RPMI 1640 medium (Gibco-BRL, Gasthersburg, MD, USA) supplemented with 10% (v/v) heat-inactivated fetal calf serum (FCS) (Gibco-BRL, Gasthersburg, MD, USA), 2% penicillin/streptomycin (10,000 U/ml penicillin, 10 mg/ml streptomycin), at 37°C, 5% CO2.
Treatment and cell viability assay
The day before treatment HepG2 cells were plated in 96-wells culture plate at the density of 2×104 cells per well. Then cells were treated with drugs for 24 hours or 48 hours. After that, cell viability was determined by CCK-8 assay kits purchased from Dojindo (Kumamoto, Japan).The percentage of cell viability was calculated as ODdrug/ODcontrol ×100%.
Western blot
HepG2 cells were lysed in lysis buffer (1% (w/v) SDS, 1 mM Na3VO4, 10 mM Tris. HCl pH 7.4, 5 mM MgCl2) supplemented with 2 ug/ml aprotinin, 5 ug/ml leupeptin and 1 mM PMSF. Subsequently, 25 ug of protein were separated by 12% sodium dodecyl sulfate-polyacrylamide gelelectrophoresis (SDS-PAGE) and transferred to PVDF (polyvinylidene fluoride) membrane. Membranes were incubated with appropriate dilutions of specific primary antibodies overnight at 4°C. After incubation of horseradish peroxidase (HRP)-conjugated secondary antibody (dilution, 1:20000), the blots were developed with enhanced chemiluminescence detection kit (ECL, Millipore, Darmstadt, Germany).
Statistical analysis
IBM SPSS statistics 21.0 (SPSS Inc., Chicago, Illinois, USA) was utilized to analyze the data obtained in this study. Numeric variables were compared between groups through one-way analysis of variance (one-way ANOVA). P<0.05 was considered as statistically significant.
Results
Cytotoxic effects of polyphyllin I (PPI) on HepG2 cell line
Previous reports have indicated that polyphillin I harbored antitumor activities in many types of cancers, including gastric cancer [7,8], breast cancer [9], glioblastoma [10], ovarian cancer [11], lung cancer [6] and hepatocellular cancer [12,13] through inhibiting tumor cell growth, metastasis [6,9-12,14], and inducing cell cycle arrest [8] and mitochondrial pathway-mediated apoptosis [13,15]. In this research we confirmed the cytotoxic effects of polyphyllin I in hepatocellular carcinoma cell line HepG2 cells. As illustrated in Figure 1, PPI could induce HepG2 cells death in dose-dependent manner.
Figure 1.
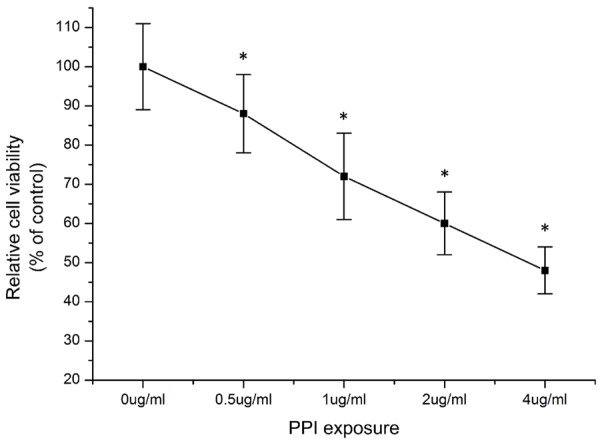
Polyphillin I (PPI) induced cytotoxicity in human hepatoma HepG2 cells. HepG2 cells were treated with different dose of PPI (0.5 ug/ml-4 ug/ml) for 24 hours. Cell viability was measured using commercially available CCK-8 kit as described in Materials and Methods and presented as the percentage of control. Data were presented as mean ± standard deviation (SD). *P<0.05 vs. control (without PPI treatment).
Chemosensitizing effects of polyphyllin I (PPI) on HepG2 cell line
Although a lot of works have been done to investigate the antitumor activities of PPI, so far no research works were performed to explore the sensitizing effects of PPI on tumor cells. In this study our data showed that cell death induced by cisplatin treatment was further enhanced by combination with PPI treatment in concentration-dependent manner (Figure 2), which proved the chemosensitizing effects of polyphyllin I on HepG2 cells.
Figure 2.
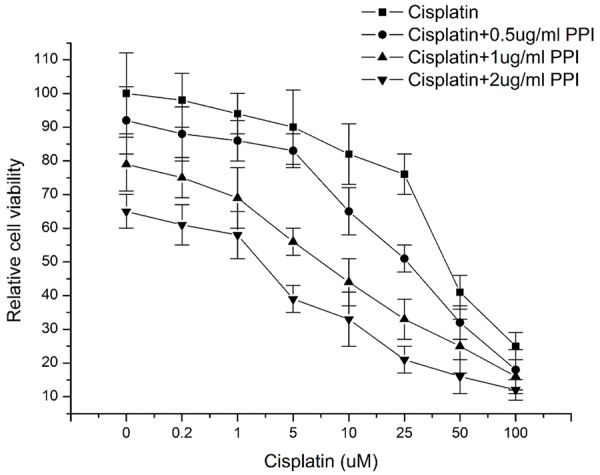
Polyphyllin I (PPI) sensitized HepG2 cells to cisplatin-induced cytotoxicity. HepG2 cells were treated with different concentration of cisplatin (0.2 uM-100 uM) for 24 hours in the presence or absence of different dose of PPI (0.5 ug/ml-2 ug/ml). Cell viability was measured using commercially available CCK-8 kit as described in Materials and Methods and presented as the percentage of control. Data were presented as mean ± standard deviation (SD).
Involvement of nuclear factor-κB (NF-κB) signaling pathway in the anticancer activities of polyphyllin I (PPI)
In order to further elucidate the molecular mechanisms of PPI actions, we determined the influence of PPI on the NF-κB signaling pathway. As indicated in Figure 3A, PPI treatment could greatly inhibit the activation of NF-κB in concentration dependent manner, which was indicated by the phosphorylation level of p65 protein. The NF-κB regulated gene products such as Bcl-2, c-Myc, VEGF, were also dose-dependently decreased by PPI treatment (Figure 3B). PPI treatment not only inhibited the constitutive NF-κB activation in HepG2, but also inhibited cisplatin-evoked NF-κB activation (Figure 3C) and up-regulation of NF-κB regulated gene products including Bcl-2, c-Myc, VEGF (Figure 3D), all of which could alsobe further decreased by combination with 50 uM PDTC, an NF-κB specific inhibitor (Figure 3D).
Figure 3.
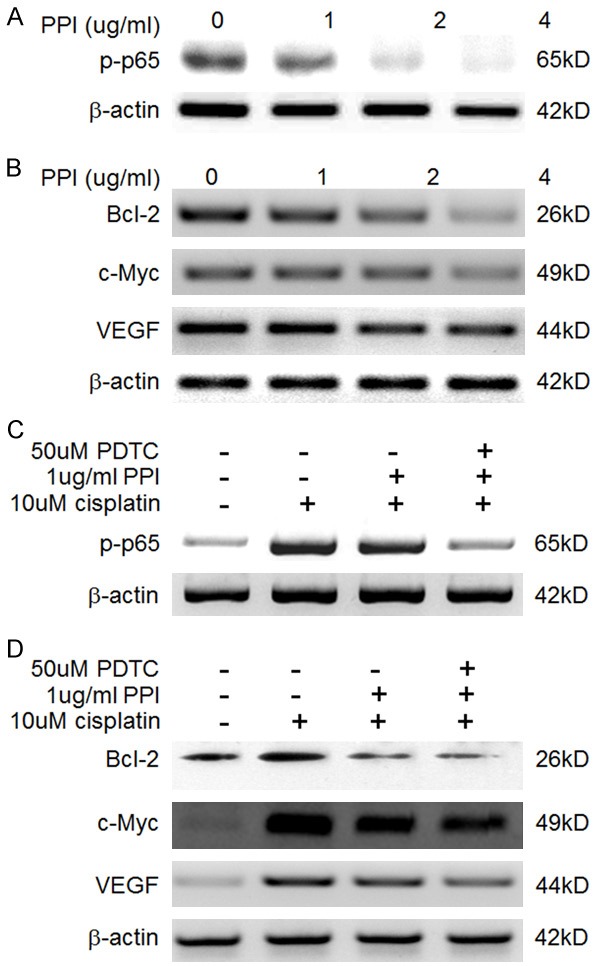
Inhibitory effects of polyphyllin I (PPI) on nuclear factor-κB (NF-κB) activation (A) and its downstream target genes expression (B) HepG2 cells were incubated with PPI (0.5 ug/ml-4 ug/ml) for 24 hours and then were harvested for further western blotting analysis. Inhibitory effects of PPI (1 ug/ml) and its combination with PDTC (50 uM) on cisplatin (10 uM)-induced NF-κB activation (C) and its downstream target genes expression (D) HepG2 cells were incubated with cisplatin (10 uM) for 24 hours in the presence or absence of PPI (1 ug/ml) or PPI (1 ug/ml) + PDTC (50 uM). Then cells were harvested for further western blotting analysis.
How did NF-κB signaling pathway influence the cytotoxic and chemosensitizing effects of PPI on HepG2 cells? In order to answer these questions, HepG2 cells were treated with different combination of cisplatin, PPI and PDTC for 24 hours and 48 hours; then the cells viability was determined by CCK-8 assay kits. As shown in Figure 4, inhibition of NF-κB pathway by 50 uM PDTC could enhance the cytotoxic effects of PPI on HepG2 cells (Figure 4). Moreover, the chemosensitizing effects of PPI were also potentiated by 50 uM PDTC treatment (Figure 4).
Figure 4.
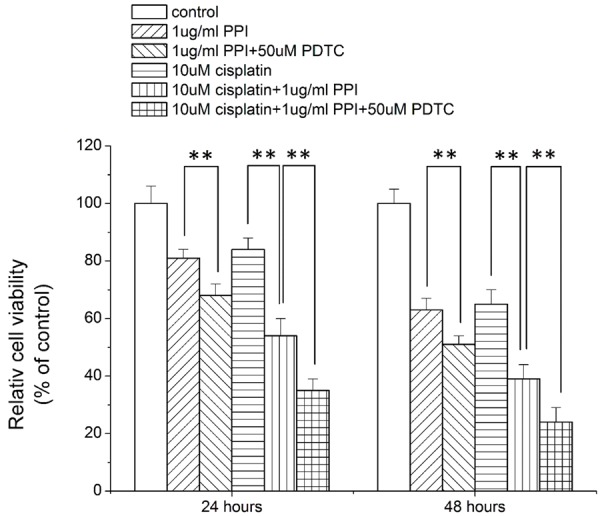
Nuclear factor-κB (NF-κB) inhibitor potentiated polyphyllin I (PPI) induced cytotoxicity and chemosensitization in HepG2 cells. In these assays NF-κB specific inhibitor PDTC was used. The cytotoxic enhancement of NF-κB inhibitor was investigated by incubation of HepG2 cells with 1 ug/ml PPI in the presence or absence of 50 uM PDTC. The chemosensitization enhancement of NF-κB inhibitor was studied by treating HepG2 cells with 10 uM cisplatin and 1 ug/ml PPI in the presence or absence of 50 uM PDTC. 24 hours or 48 hours later cell viability was measured and expressed as % of control. **P<0.05.
Discussion
Because of multi-drug resistance and severe toxic side effects of current chemotherapy for cancers, developing new therapeutic agents against tumor is always needed. Nowadays, traditional Chinese medical herbs, as an important complementary and alternative therapy, attract more and more eyeballs, and more and more natural chemical compounds from Chinese medical herbs have been isolated and identified with antitumor activities [16-19]. Polyphyllin I (PPI), one of the main active steroidal saponins from Chinese medical herbs, Rhizoma of Paris polyphyllin, was previously proved to harbor antitumor effects in many cancers [6,7,13,14,20,21]. However, the antitumor activities of PPI and its molecular mechanisms have not been thoroughly investigated so far. In present study, our results not only confirmed the previously reported cytotoxic effects of PPI on HepG2 cells [13,22] (Figure 1), but also provided new fidnings that PPI could sensitize HepG2 cells to cisplatin treatment (Figure 2).
Previous investigations have demonstrated that PPI exertedits antitumor activities through inhibiting tumor cell growth, proliferation and metastasis [6,9-12,14], inducing cell cycle arrest [8] and mitochondrial pathway-mediated apoptosis [13,15]. In present study NF-κB signaling pathway was found to be involved in PPI actions. Our results indicated that PPI treatment could inhibit not only the constitutive activation of NF-κB signaling pathway (Figure 3A) in HepG2 cells, but also the adaptive activation of NF-κB signaling pathway evoked by cisplatin treatment (Figure 3C), both of which were represented by phosphorylation of p65. Furthermore, PPI treatment decreased NF-κB regulated genes expression, such as Bcl-2, c-Myc, VEGF (Figure 3B and 3D). Because NF-κB and its regulated gene products were involved in chemo-resistance and radio-resistance in many tumors [23,24], we suggested that it was through inhibition of NF-κB activation PPI exerted its antitumor and chemosensitizing effects in HepG2 cells. Exactlyas we supposed, combination treatment of PPI with NF-κB specific inhibitor, PDTC, the cytotoxic effects and chmosensitizing activities were both greatly enhanced (Figure 4).
In conclusion, our results confirmed that PPI harbored cytotoxic effects on hepatocellular carcinoma HepG2 cells as previously reported [13,22]. And furthermore, our study indicated that PPI could sensitize hepatocellular carcinoma HepG2 cells to chemotherapy. The cytotoxic and chemosensitizing activities of PPI might be accomplished through down-regulation of NF-κB signaling pathway.
Disclosure of conflict of interest
None.
References
- 1.Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol. 2013;59:897–903. doi: 10.1016/j.jhep.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 2.Chuang SC, La Vecchia C, Boffetta P. Liver cancer: descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 3.Foo J, Michor F. Evolution of acquired resistance to anti-cancer therapy. J Theor Biol. 2014;355:10–20. doi: 10.1016/j.jtbi.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottesman MM. Mechanisms of Cancer Drug Resistance. Annu Rev Med. 2002;53:615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 5.Pharmacopoeia Commission of the Ministry of Public Health. The Pharmacopoeia of Peoplef the Ministry of Public Healt [Google Scholar]
- 6.Kong M, Fan J, Dong A, Cheng H, Xu R. Effects of polyphyllin I on growth inhibition of human non-small lung cancer cells and in xenograft. Acta Biochim Biophys Sin (Shanghai) 2010;42:827–833. doi: 10.1093/abbs/gmq091. [DOI] [PubMed] [Google Scholar]
- 7.Yue G, Wei J, Qian X, Yu L, Zou Z, Guan W, Wang H, Shen J, Liu B. Synergistic anticancer effects of polyphyllin I and evodiamine on freshly-removed human gastric tumors. PLoS One. 2013;8:e65164. doi: 10.1371/journal.pone.0065164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao M, Dai X, He X, Zhou R, Zhang B, Hu G, Huang Z, Fan X. Paris saponin I induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma SGC7901 cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31:768–772. doi: 10.1007/s11596-011-0674-y. [DOI] [PubMed] [Google Scholar]
- 9.Lee MS, Yuet-Wa JC, Kong SK, Yu B, Eng-Choon VO, Nai-Ching HW, Chung-Wai TM, Fung KP. Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther. 2005;4:1248–1254. doi: 10.4161/cbt.4.11.2136. [DOI] [PubMed] [Google Scholar]
- 10.Zheng B, Wang S, Qu H, Dong B, Mao Y. Inhibitory effects of Polyphyllin D and formosanin C on human glioblastoma U251 cells and its mechanism. Chin J Neuromed. 2013;12:1220–1223. [Google Scholar]
- 11.Linhui G, Jianguo F, Lijuan Q, Shenglin M. Research on Proliferation Inhibitory Effect of Paris Saponin I on High Metastatic Human Ovarian Cancer Cell Line HO -8910PM in vitro. Zhong Hua Zhong Yi Yao Xue Kan. 2012;10:2212–2215. [Google Scholar]
- 12.Xiao M, Dai X, He X, Zhou R, Zhang B, Hu G, Huang Z, Fan X. Growth and Apoptosis Effects of Paris Saponin I on Human Hepatocellular Carcinoma Cells. Life Sci Res. 2011;15:519–523. [Google Scholar]
- 13.Ong RC, Lei J, Lee RK, Cheung JY, Fung KP, Lin C, Ho HP, Yu B, Li M, Kong SK. Polyphyllin D induces mitochondrial fragmentation and acts directly on the mitochondria to induce apoptosis in drug-resistant HepG2 cells. Cancer Lett. 2007;261:158–164. doi: 10.1016/j.canlet.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Gu L, Feng J, Xu H, Luo M, Su D. Polyphyllin I inhibits proliferation and metastasis of ovarian cancer cell line HO-8910PM in vitro. J Tradit Chin Med. 2013;33:325–333. doi: 10.1016/s0254-6272(13)60174-0. [DOI] [PubMed] [Google Scholar]
- 15.Xiao X, Bai P, Bui Nguyen TM, Xiao J, Liu S, Yang G, Hu L, Chen X, Zhang X, Liu J, Wang H. The antitumoral effect of Paris Saponin I associated with the induction of apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2009;8:1179–1188. doi: 10.1158/1535-7163.MCT-08-0939. [DOI] [PubMed] [Google Scholar]
- 16.Parekh HS, Liu G, Wei MQ. A new dawn for the use of traditional Chinese medicine in cancer therapy. Mol Cancer. 2009;8:21. doi: 10.1186/1476-4598-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan W, Lu J, Huang M, Li Y, Chen M, Wu G, Gong J, Zhong Z, Xu Z, Dang Y, Guo J, Chen X, Wang Y. Anti-cancer natural products isolated from chinese medicinal herbs. Chin Med. 2011;6:27. doi: 10.1186/1749-8546-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Yang G, Li X, Zhang Y, Yang J, Chang J, Sun X, Zhou X, Guo Y, Xu Y. Traditional Chinese medicine in cancer care: A review of controlled clinical studies published in Chinese. PLoS One. 2013;8:e60338. doi: 10.1371/journal.pone.0060338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang CY, Bai XY, Wang CH. Traditional chinese medicine: a treasured natural resource of anticancer drug research and development. Am J Chin Med. 2014;42:543–559. doi: 10.1142/S0192415X14500359. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Liu BR, Hu WJ, Yu LX, Qian XP. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytother Res. 2007;21:1102–1104. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- 21.Negi JS, Bisht VK, Bhandari AK, Bhatt VP, Singh P, Singh N. Paris polyphylla: chemical and biological prospectives. Anticancer Agents Med Chem. 2014;14:833–839. doi: 10.2174/1871520614666140611101040. [DOI] [PubMed] [Google Scholar]
- 22.Cheung JY, Ong RC, Suen YK, Ooi V, Wong HN, Mak TC, Fung KP, Yu B, Kong SK. Polyphyllin D is a potent apoptosis inducer in drug-resistant HepG2 cells. Cancer Lett. 2005;217:203–211. doi: 10.1016/j.canlet.2004.06.042. [DOI] [PubMed] [Google Scholar]
- 23.Shishodia S, Aggarwal BB. Nuclear factorkappaB activation mediates cellular transformation, proliferation, invasion angiogenesis and metastasis of cancer. Cancer Treat Res. 2004;119:139–173. doi: 10.1007/1-4020-7847-1_8. [DOI] [PubMed] [Google Scholar]
- 24.Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]


