Abstract
Background: Compared with open hepatectomy (OH), laparoscopic hepatectomy (LH) had better short-term outcomes in normal hepatocellular carcinoma (HCC) patients. Since liver cirrhosis is the major risk of HCC, serve postoperative complications can be observed after LH in HCC patients with cirrhosis. We conducted this systematic review to analysis the safety and the efficiency of LH in HCC patients with liver cirrhosis. Methods: MEDLINE, EMBASE, the Cochrane Library, the Chinese National Knowledge Infrastructure database, and clinical trial registries were searched through March 2015. Risk ratios (RRs), weigh mean difference (WMD) and 95% confidence intervals (CIs) were calculated. Results: The analysis included 7 retrospective trials, altogether involving 828 patients. Patients in LH group had wider tumor margin (WMD = 0.12, 95% CI 0.04 to 0.21, P = 0.003), less blood loss (WMD = -157.25, 95% CI -295.05 to -19.45, P = 0.03), less blood transfusion (RR = 0.41, 95% CI 0.22 to 0.74, P = 0.004), less postoperative mobility (RR = 0.48, 95% CI 0.35 to 0.66, P<0.001) and less hospital stay (WMD = -4.11, 95% CI -6.23 to -1.98, P<0.001). Overall survival (OS) and disease free survival (DFS) were similar between 2 groups, except LH had a better 5-year survival rate (RR = 1.28, 95% CI 1.01 to 1.62, P = 0.04). Conclusion: In HCC patients with liver cirrhosis, LH have short-term outcomes advantages of tumor margin, blood loss, blood transfusion, postoperative mobility, and hospital stay. OS and DFS were similar between LH and OH. LH is safe in HCC patients with liver cirrhosis.
Keywords: Hepatocellular carcinoma, meta-analysis, laparoscopic, open hepatectomy, surgery
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer worldwide and the third leading cause of cancer-related death [1]. Increasing incidence of HCC associated with the development of cirrhosis [2]. Nearly 80% of HCC develop the tumor from such chronic liver diseases [3]. Among varies therapies for HCC patients, hepatectomy is the most curative therapy [3,4]. Traditional surgical therapy is open hepatectomy (OH). Since the first laparoscopic use was reported in 1987 as laparoscopic cholecystectomy, laparoscopic surgery has been increasingly popular in all fields of general surgery [5].
For cirrhotic HCC patients, more postoperative adverse events (ADEs) would develop including infections, pleural effusion, or liver failure [6,7]. Compared with OH, laparoscopic hepatectomy (LH) seems to get decreased postoperative pain, less blood loss and shorter hospital stay [8-12]. No significant difference in survival outcomes is presented between LH and OH in normal HCC patients [13-19]. However, with the difficulties in techniques and bleeding control, LH should be carefully chosen for patients with liver cirrhosis. For liver cirrhosis patients, hepatectomy may lead to several serious ADEs related to poor hepatic function [20,21].
At present, several trials studied the safety and efficiency of LH comparing OH in patients with liver cirrhosis [7,8,22-24]. Meanwhile, a recent meta-analysis figured out that LH is safe and would improve outcomes [25]. Nevertheless, long-term outcomes were not clearly described and sensitivity analysis was not conducted.
In order to clearly described the short-/long-term outcomes in HCC patients with liver cirrhosis in LH and OH. We conducted this systematic review to evaluate the safety and efficacy of LH comparing OH.
Methods
This meta-analysis was conducted according to PRISMA guidelines (Checklist S1).
Literature search strategy
Systematic searches of the following electronic databases were conducted through March 2015 without language restrictions: MEDLINE, EMBASE, the Cochrane Library, and the Chinese National Knowledge Infrastructure (CNKI). We also searched five primary clinical trial registries recognized by the WHO International Clinical Trial Registry Platform: Australia and New Zealand Clinical Trial Registry (www.anzctr.org.au/), Chinese Clinical Trial Register (www.chictr.org), ISRCTN (www.controlled-trials.com/isrctn/), U.S. National Institutes of Health Clinical Trials Database (www.clinicaltrials.gov/), and Clinical Trials Registry-India (www.ctri.in:8080/Clinicaltrials/index.jsp) [26,27]. Eligible studies were identified using any of the following index words: hepatocellular carcinoma or HCC or hepatic tumor or liver tumor or hepatic cancer or liver cancer; open surgery or open hepatectomy or open liver resection or traditional surgery or traditional hepatectomy or traditional liver resection; laparoscopic surgery or laparoscopic hepatectomy or laparoscopic liver resection.
Relevant reviews and meta-analyses comparing OH and LH for HCC were manually examined in order to identify additional eligible studies.
Inclusion criteria
In order to be concluded, studies had to satisfy the following criteria: (1) the trial should conducted two kinds of hepatectomy for HCC patients which is LH and OH; (2) HCC patients in the trials should have liver cirrhosis (New European classification system was used to diagnose liver cirrhosis [28]); (3) the trial reported data on short-/long-term outcomes; (4) the trial reported sufficient data to allow calculation of risk ratios (RRs) or weigh mean difference (WMD) with 95% confidence intervals (CIs); (5) retrospective studies.
Types of outcome measures
Intraoperative outcomes were tumor margin, operative time, blood loss, and blood transfusion. Short-term outcomes were postoperative morbility and mortality, curative resection, and length of hospital stay. Long-term outcomes concluded overall survival and disease free survival.
Data extraction
Two reviewers (J.C. and T.B.) independently read potentially eligible studies and extracted the following data respectively: authors, publication year, study design, patient characteristics, and outcomes. Any disagreements were arbitrated by a third reviewer (L.Q.L.) [27].
Quality assessment
Two reviewers (J.C. and T.B.) independently assess the risk for every included trials using modified criteria suggested by the Newcastle-Ottawa quality assessment tool (NOS) [29]. Sensitivity analysis is conducted by omitting the biggest weigh trials.
Statistical analysis
All statistical calculations were performed using Review Manager 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.). Mantel-Haenszel RRs with corresponding 95% CIs were calculated for dichotomous, while WMD with 95% CIs were calculated for continuous variable. Medians were converted to means using the technique described by Hozo et al. [30]. P value of < 0.05 was considered statically significant.
Meta-analysis was carried out on an ‘intention-to-treat’ basis which means all patients were evaluated according to their initial group allocation. Patients with unknown endpoints were considered to have died or lost to follow up. Heterogeneity was assessed by calculating I2. When I2 was less than 50%, we used a fixed-effects model for meta-analysis; when I2 was more than 50%, a random-effects model was used. Homogeneity between trials was assessed using the χ2 test with the significance threshold set at P > 0.1. Moreover, I2 < 25% was defined to represent low heterogeneity, moderate heterogeneity was defined as a value between 25 and 50%, and I2 > 50% was of a high heterogeneity [31]. Subgroup was conducted depending on retrospective and retrospective matched trials. To evaluate the robustness of meta-analysis results, we repeated all meta-analyses using the other type of model (fixed- or random-effects); if both models gave the same meta-analysis results, we judged the result to be reliable.
Results
Characteristics of the included studies
After searching the database and trial registries, 928 published trials and 310 registered studies were initially presented (Figure 1). We removed 250 duplicates, and left with 988 trials (887 published and 101 registered trials), which were potentially eligible. With the screening of the titles and abstracts, 856 published trials and 101 registered studies were excluded because the design or outcomes data were not satisfied with the inclusion criteria (not related with our topic). The remaining 37 published trials were fully read, and 30 published trials were excluded. It is because the trials were systematic reviews, or meta-analyses. Finally, 7 trials involving 828 patients were included (Belli et al. [22], Cheung et al. [23], Kanazawa et al. [7], Memeo et al. [24], Siniscalchi et al. [32], Truant et al. [8] and Yamashita et al. [33]). In 828 patients, 281 patients were with LH, another 547 patients were under OH. The number of HCC patients ranged from 56 to 179. A total of 605 patients were men. All HCC patients had liver cirrhosis. Conversion rate of LH to OH ranged 7.0% to 19.4%. The characteristics of the included studies are shown in Table 1.
Figure 1.
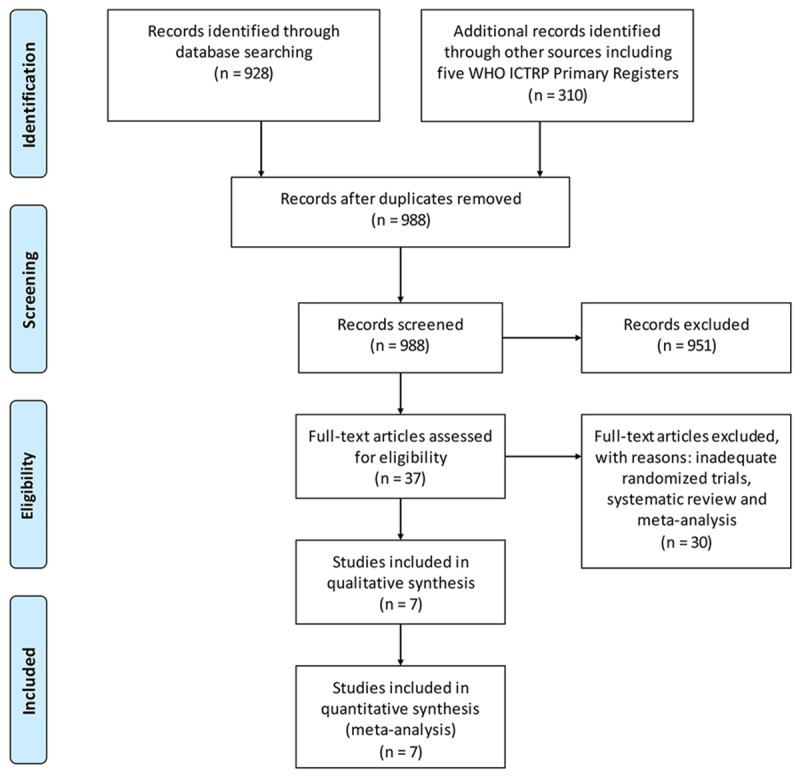
Selection process for trials included in this meta-analysis.
Table 1.
Characteristics of included studies comparing LH and OH to treat HCC patients with liver cirrhosis
| Study | Study design | No. of patients | Mean age, y | Child-Pugh (A/B) | Convention to open, (n, %) | |||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| LH (M, %) | OH (M, %) | LH | OH | LH | OH | |||
| Belli et al. | R | 54 (31, 57.4%) | 125 (78, 62.4%) | 63.3±6.1* | 61.5±7.8 | 49/5 | 117/8 | 4, 7.0% |
| Cheung et al. | RM | 32 (22, 68.8%) | 64 (50, 78.1%) | 59.5 (39-79)** | 61 (29-82) | 32/0 | 62/4 | 3, 18.8% |
| Kanazawa et al. | R | 28 (16, 57.1%) | 28 (17, 60.7%) | 69 (40-85) | 68 (29-82) | 20/8 | 21/7 | 3, 10.7% |
| Memeo et al. | RM | 45 (37, 82.2%) | 45 (35, 77.8%) | 60 (43-80) | 62 (34-75) | 43/2 | 44/1 | NA |
| Siniscalchi et al. | R | 23 (15, 65.2%) | 133 (104. 78.2%) | 57.9 (30-73) | 63.3 (41-77) | NA | NA | NA |
| Truant et al. | RM | 36 (31, 86.1%) | 53 (47, 88.7%) | 60.6±10.2 | 63.3±7.6 | 36/0 | 53/0 | 7, 19.4% |
| Yamashita et al. | R | 63 (48, 76.2%) | 99 (74, 74.7%) | 67.5±9.5 | 65.2±10.1 | 59/4 | 96/3 | NA |
Abbreviations: LH = laparoscopic hepatectomy; OH = open hepatectomy; R = retrospective study; RM = retrospective matching study; NA = not available.
Mean ± SD.
Median (range).
Quality assessment results were presented as Supplementary Table 1. NOS was used to assess the risk of bias for quality assessment of non-randomized studies. Overall quality of the included studies was of good quality that the NOS scores varied between 7 and 8 out of 9.
Therapy outcomes
Intraoperative outcomes
During surgery, tumor margin was significantly wider in LH than OH (WMD = 0.12, 95% CI 0.04 to 0.21, P = 0.002, I2 = 0%). Operating time seems to be similar between LH and OH (WMD = -10.36, 95% CI -26.21 to 5.49, P = 0.20, I2 = 36%). Patients in LH get less blood loss (WMD = -157.25, 95% CI -295.05 to -19.45, P = 0.03, I2 = 84%) and blood transfusion (RR = 0.41, 95% CI 0.22 to 0.74, P = 0.004, I2 = 40%) than patients in OH. (Tables 2 and 3; Supplementary Figure 1).
Table 2.
Intraoperative data and surgical results comparing LH and OH to treat HCC patients with liver cirrhosis
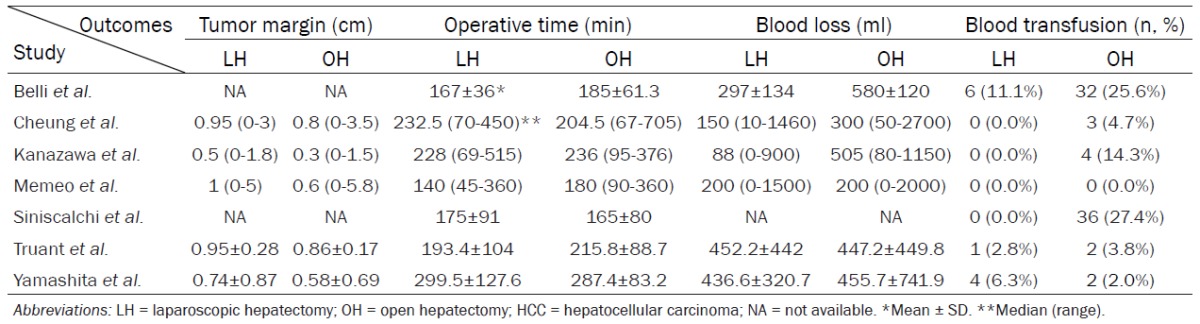
|
Table 3.
Subgroup analysis of outcomes depending on retrospective studies and retrospective match studies
| Comparison | Pooled estimates | 95% CI | P | I2 |
|---|---|---|---|---|
| Tumor margin | WMD 0.12 | 0.04-0.21 | 0.03 | 28% |
| Studies with RM | WMD 0.10 | 0.01-0.20 | 0.06 | 7% |
| Studies without RM | WMD 0.18 | 0.02-0.35 | 0.03 | 0% |
| Omit Truant et al. | WMD 0.19 | 0.05-0.33 | 0.01 | 0% |
| Operating time | WMD -10.36 | -26.21-5.49 | 0.20 | 36% |
| Studies with RM | WMD -15.95 | -52.86-20.96 | 0.40 | 60% |
| Studies without RM | WMD -8.06 | -23.80-7.69 | 0.32 | 17% |
| Omit Belli et al. | WMD -6.12 | -27.66-15.43 | 0.58 | 42% |
| Blood loss | WMD -157.25 | -295.05-19.45 | 0.03 | 84% |
| Studies with RM | WMD -41.11 | -151.95-69.74 | 0.47 | 0% |
| Studies without RM | WMD -250.12 | -420.36-79.89 | 0.004 | 85% |
| Omit Belli et al. | WMD -121.63 | -308.77-65.50 | 0.20 | 83% |
| Blood transfusion | RR 0.41 | 0.22-0.74 | 0.004 | 40% |
| Studies with RM | RR 0.47 | 0.08-2.86 | 0.41 | 0% |
| Studies without RM | RR 0.45 | 0.10-1.98 | 0.29 | 63% |
| Omit Belli et al. | RR 0.38 | 0.16-0.93 | 0.20 | 83% |
| Postoperative morbility | RR 0.48 | 0.35-0.66 | < 0.001 | 40% |
| Studies with RM | RR 0.52 | 0.33-0.81 | 0.004 | 0% |
| Studies without RM | RR 0.45 | 0.17-1.16 | 0.10 | 71% |
| Omit Belli et al. | RR 0.48 | 0.26-0.86 | 0.01 | 52% |
| Death | RR 0.72 | 0.28-1.81 | 0.48 | 19% |
| Studies with RM | RR 1.10 | 0.35-3.49 | 0.87 | 47% |
| Studies without RM | RR 0.36 | 0.07-1.98 | 0.24 | 0% |
| Omit Truant et al. | RR 0.96 | 0.34-2.70 | 0.68 | 36% |
| Curative resection | RR 1.15 | 0.90-1.47 | 0.26 | 90% |
| Studies with RM | RR 1.13 | 0.98-1.30 | 0.08 | NA |
| Studies without RM | RR 1.17 | 0.72-1.91 | 0.52 | 95% |
| Omit Siniscalchi et al. | RR 1.25 | 0.99-1.58 | 0.06 | 75% |
| Length of hospital stay | WMD -4.11 | -6.23-1.98 | < 0.001 | 82% |
| Studies with RM | WMD -3.11 | -4.42-1.80 | < 0.001 | 0% |
| Studies without RM | WMD -5.23 | -9.70-0.76 | 0.02 | 88% |
| Omit Belli et al. | WMD -4.78 | -6.68-2.88 | < 0.001 | 50% |
Abbreviations: LH = laparoscopic hepatectomy; OH = open hepatectomy; RM = retrospective matching study; RR = risk ratio; WMD = weigh mean difference CI = confidence interval.
Postoperative outcomes
Postoperative mobility was significantly decreased in LH (RR = 0.48, 95% CI 0.35 to 0.66, P<0.0001, I2 = 40%. Postoperative mortality was similar between LH and OH (RR = 0.72, 95% CI 0.28 to 1.81, P = 0.48, I2 = 19%). Curative resection in LH was no significantly better than patients in OH (RR = 1.15, 95% CI 0.90 to 1.47, P = 0.26, I2 = 90%). Patients in OH had significantly longer hospital stay than LH (WMD = -4.11, 95% CI -6.23 to -1.98, P = 0.0002, I2 = 82%) (Tables 3 and 4; Supplementary Figure 2).
Table 4.
Short-term outcomes comparing LH and OH to treat HCC patients with liver cirrhosis
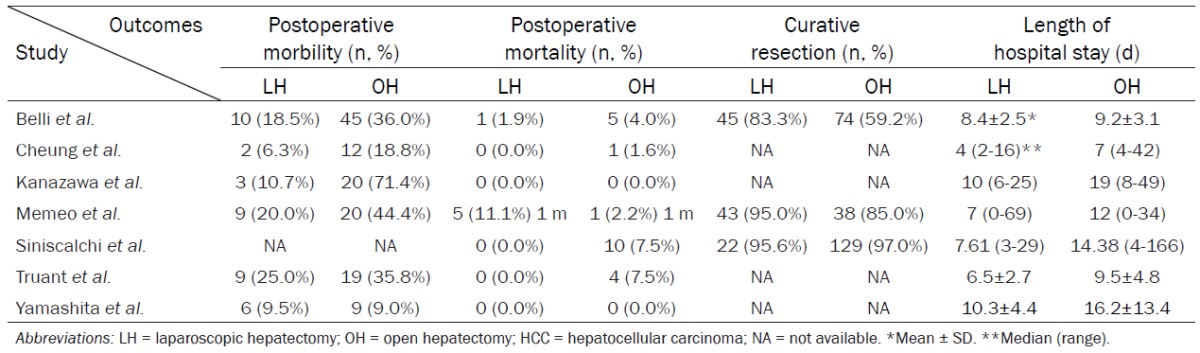
|
Belli et al. [22] reported 8 patients suffered postoperative ascites, 2 patients developed postoperative haemorrhage, 1 patient had infectious and 1 patient had cardiovascular complications, and one patient had an abdominal wall complication. In Cheung et al. [23], 2 patients suffered chest infections, 11 patients had pleural effusion, and 1 patient suffered subphrenic abscess. In Yamashita et al. [33], 3 patients suffered bile leakage, 7 patients had ascites, and 11 patients had infections.
Overall survival
Patients in LH got similar 1-year survival (RR = 1.13, 95% CI 0.96 to 1.34, P = 0.15, I2 = 83%) and 3-year survival (RR = 1.06, 95% CI 0.73 to 1.55, P = 0.75, I2 = 85%) as patients in OH. However, 5-year survival in LH seemed to be significantly higher than OH (RR = 1.28, 95% CI 1.01 to 1.62, P = 0.04, I2 = 62%) (Figure 2).
Figure 2.
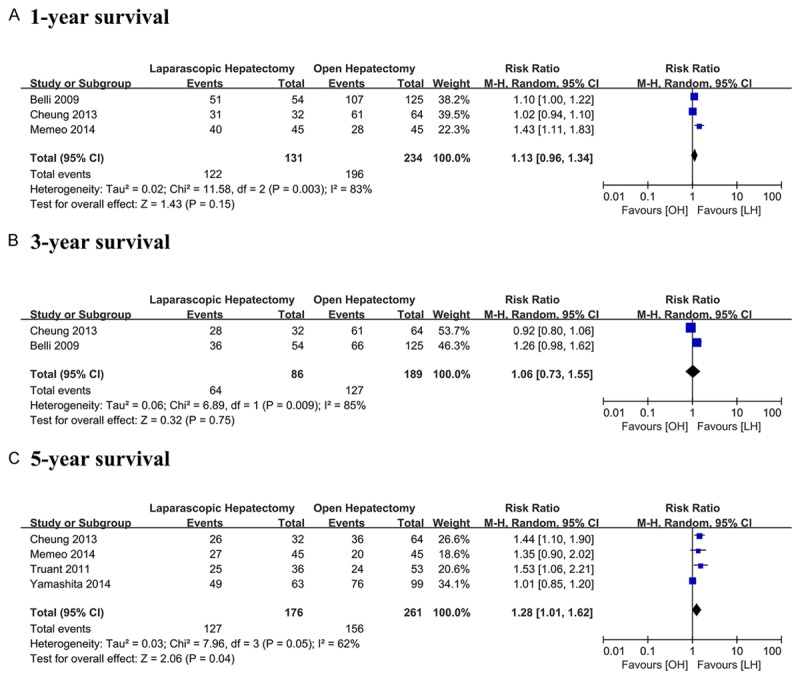
Meta-analysis of data on overall survival in LH and OH.
Disease-free survival
Patients in LH got similar results as OH no matter 1-year disease free survival (RR = 1.21, 95% CI 0.99 to 1.48, P = 0.07, I2 = 61%), 3-year disease free survival (RR = 1.19, 95% CI 0.85 to 1.68, P = 0.31, I2 = 62%) or 5-year disease free survival (RR = 0.97, 95% CI 0.75 to 1.25, P = 0.81, I2 = 0%) (Figure 3).
Figure 3.
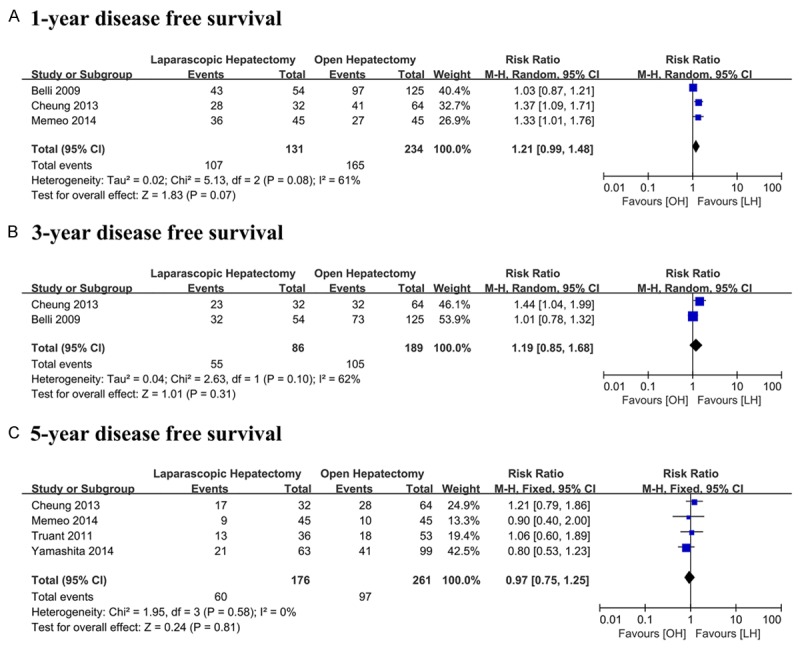
Meta-analysis of data on disease free survival in LH and OH.
Subgroup analysis
According to the trials were retrospective or retrospective matched studies, subgroup analysis were conducted. Different results were found in blood loss, blood transfusion, and postoperative morbility (Table 3).
Sensitivity analysis
Omitting the trial which has the biggest weigh (Table 3). Different results were found in blood loss.
Discussion
LH has been proved to have better short-term outcomes and have similar long-term outcomes as OH in normal HCC patients [14,17,18,34-38]. Initially, ascribe to the difficulties of technique, LH should be carefully for liver cirrhosis patients [15,39]. With the huge development of laparoscopic technique and equipment, LH seemed to provide reduced surgical trauma comparing with OH in HCC patients with cirrhotic liver [8,23,24,32]. Our systematic reviews suggest LH could perform better short-term outcomes, and may prolong survival benefit.
For the intraoperative outcomes in HCC patients with liver cirrhosis, Patients in LH group have significantly wide tumor margin, less blood loss and blood transfusion. Meanwhile, operating time was similar between LH and OH. This result may associate with the study design. Since the study design is not randomized, selection bias may be presented. Surgeons would like to perform preoperative evaluation; patients with huge HCC, higher degree of cirrhosis, improper tumor location that had high risk of blood loss and life threaten were inclined to perform OH. For LH, patients with solitary lesion, 5 cm or less, located in liver segment 2-6 would be suitable [40]. Patients in LH group suffer less postoperative morbility, and shorter hospital stay than OH. Curative resection and postoperative mortality was similar between OH and LH. LH has the advantage of reduction of surgery-induced injuries [36,41], thus patients are more likely to have less postoperative complications and to recover soon. Compared with OH, LH has other advantages in several studies. LH make subsequent surgical procedures easier which could reduce the adhesion [42,43]. Also, LH used as repeat operation could also perform better short-term outcomes [12,44].
Survival benefit was also familiar between LH and OH, except 5-year survival (RR = 1.26, 95% CI 1.01 to 1.62, P = 0.04, I2 = 62%). We conducted sensitivity analysis, it showed LH had better 5-year survival (RR = 1.44, 95% CI 1.18 to 1.76, P < 0.001, I2 = 0%). It may associated with laparoscopic equipment which make us more easily detect micro-vascular invasion. Integrity resection of micro invasion may prolong the survival. Moreover, in HCC patients under LH, the locations of tumors were more easier than patients under OH. Thus sometimes it would be more curative. But a high heterogeneity was observed, and limited sample size, the results still need to be confirmed. If the original data could be collected and analyzed hazard ratio could be calculated, the result would be more convincible. Thus the results need further certifications.
Compared with normal HCC patients, patients with liver cirrhosis have more serious postoperative ADEs. In our included trials, several serious ADEs were reported above. In Cheung et al. [23], Truant et al. [8], and Kanazawa et al. [33], Clavien-Dindo score system [45] was performed to evaluate postoperative complications between 2 groups. However, no significant difference was found. We often concern gas embolization and blood controlling in LH. The risk of gas embolism due to lesions of the hepatic veins has been suggested during parenchymal transection. However, the incidence of this ADE is relatively low [46,47]. The main technical challenge of LH remains intraoperative bleeding when parenchymal transection happens. These were mainly related to hepatic veins injuries [48-50]. In our systematic review, the rate of convention to OH is from 7% to 19.4%. Main reasons are technically related issues (difficult exposure, or fragile tumor with risk of rupture) and difficulties of bleeding control [50].
A recent meta-analysis [25] is performed in HCC patients with liver cirrhosis between LH and OH. Several results were different from us because some points in their review may be improper. In Twaij et al. [25], standard mean difference (WMD) was performed to calculate continues variable. However, SMD is recommended when different easurement scales in the studies are used to reflect the outcomes [31]. Here WMD is more suitable which studies use the same scale to report the outcomes. In our study, we use WMD to calculate the variables and add several new trials. In addition, we performed sensitivity analysis to test the robustness of our results which showed our results was reliable. In their review, no details were about survival benefits. As for the original data was not available, we only calculate the given data, and showed LH had similar survival benefit as OH. Another systematic review comparing LH and OH in normal HCC patients also showed similar results as ours like the outcome of postoperative morbility and blood loss [51]. This global analysis not only testify the safety and efficiency of LH in normal HCC patients but also convinced our results in liver cirrhosis HCC patietns.
Sensitivity analysis result (omitting Belli et al. [22]) was different on the outcome of blood transfusion and 1-year disease free survival. This may cause by patient compose in Belli et al. [22]. In Belli et al. [22], patients were without serve portal hypertension, which may result in better liver function inefficiency endurance. It could explain why only 7% convention rate was observed in their study. And Belli et al. [22]’s study had the largest sample size, its results would affect the final results a lot.
The biggest limitation in our systematic review is the included trials were retrospective, non-randomized studies which would increase the selection bias. Moreover, the sample size is small which decrease the reliability of the final results. We select trials carefully with strict include and exclude criteria. Newcastle-Ottawa quality assessment tool [29] was performed to evaluate the quality which our final quality is high. In addition, subgroup analysis was performed to list the detail data of our review. Sensitivity analysis was conducted to conformed the reliability of the pooled estimates in the meta-analysis. And the basic characteristics between 2 groups was almost no significantly different. Thus, the selection bias would play little role in our final results. With the limitations shown in our systematic review, further large sample size, well designed randomized or controlled trials should perform.
In conclusion, LH may provide better intraoperative and short-term outcomes than OH in HCC patients with liver cirrhosis. However, no significant survival benefit was shown between them. But a tendency to have better survival benefit still could be found of LH in HCC patients with liver cirrhosis.
Acknowledgements
This work was supported by Guangxi Natural Science Foundation (no. 2011GXNSFD018032), Key Laboratory for High-Incidence Tumor Prevention and Treatment, Ministry of Education, and Self-Funded Research Project of Guangxi Zhuang Autonomous Region National Health and Family Planning Commission (no. Z2015570).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Cherqui D, Laurent A, Tayar C, Chang S, Van Nhieu JT, Loriau J, Karoui M, Duvoux C, Dhumeaux D, Fagniez PL. Laparoscopic liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: midterm results and perspectives. Ann Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–27. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 5.Mouret P. How I developed laparoscopic cholecystectomy. Ann Acad Med Singapore. 1996;25:744–7. [PubMed] [Google Scholar]
- 6.Farges O, Malassagne B, Flejou JF, Balzan S, Sauvanet A, Belghiti J. Risk of major liver resection in patients with underlying chronic liver disease: a reappraisal. Ann Surg. 1999;229:210–5. doi: 10.1097/00000658-199902000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanazawa A, Tsukamoto T, Shimizu S, Kodai S, Yamazoe S, Yamamoto S, Kubo S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surg Endosc. 2013;27:2592–7. doi: 10.1007/s00464-013-2795-9. [DOI] [PubMed] [Google Scholar]
- 8.Truant S, Bouras AF, Hebbar M, Boleslawski E, Fromont G, Dharancy S, Leteurtre E, Zerbib P, Pruvot FR. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: a case-matched study. Surg Endosc. 2011;25:3668–77. doi: 10.1007/s00464-011-1775-1. [DOI] [PubMed] [Google Scholar]
- 9.Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385–92. doi: 10.1097/SLA.0b013e318146996c. discussion 92-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buell JF, Thomas MT, Rudich S, Marvin M, Nagubandi R, Ravindra KV, Brock G, McMasters KM. Experience with more than 500 minimally invasive hepatic procedures. Ann Surg. 2008;248:475–86. doi: 10.1097/SLA.0b013e318185e647. [DOI] [PubMed] [Google Scholar]
- 11.Topal B, Fieuws S, Aerts R, Vandeweyer H, Penninckx F. Laparoscopic versus open liver resection of hepatic neoplasms: comparative analysis of short-term results. Surg Endosc. 2008;22:2208–13. doi: 10.1007/s00464-008-0023-9. [DOI] [PubMed] [Google Scholar]
- 12.Kazaryan AM, Pavlik Marangos I, Rosseland AR, Røsok BI, Mala T, Villanger O, Mathisen O, Giercksky KE, Edwin B. Laparoscopic liver resection for malignant and benign lesions: tenyear Norwegian single-center experience. Arch Surg. 2010;145:34–40. doi: 10.1001/archsurg.2009.229. [DOI] [PubMed] [Google Scholar]
- 13.Ahn KS, Kang KJ, Kim YH, Kim TS, Lim TJ. A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. J Laparoendosc Adv Surg Tech A. 2014;24:872–7. doi: 10.1089/lap.2014.0273. [DOI] [PubMed] [Google Scholar]
- 14.Aldrighetti L, Guzzetti E, Pulitano C, Cipriani F, Catena M, Paganelli M, Ferla G. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: short and middle term results. J Surg Oncol. 2010;102:82–6. doi: 10.1002/jso.21541. [DOI] [PubMed] [Google Scholar]
- 15.Hu BS, Chen K, Tan HM, Ding XM, Tan JW. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World J Gastroenterol. 2011;17:4725–8. doi: 10.3748/wjg.v17.i42.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Suh KS, Lee KW, Yi NJ, Hong G, Suh SW, Yoo T, Park MS, Choi Y, Lee HW. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: a case-controlled study with propensity score matching. Surg Endosc. 2014;28:950–60. doi: 10.1007/s00464-013-3254-3. [DOI] [PubMed] [Google Scholar]
- 17.Kim HH, Park EK, Seoung JS, Hur YH, Koh YS, Kim JC, Cho CK, Kim HJ. Liver resection for hepatocellular carcinoma: case-matched analysis of laparoscopic versus open resection. J Korean Surg Soc. 2011;80:412–9. doi: 10.4174/jkss.2011.80.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–56. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 19.Yoon S, Kim K, Jung D, Yu A, Lee SG. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surg Endosc. 2015;29:2628–34. doi: 10.1007/s00464-014-3980-1. [DOI] [PubMed] [Google Scholar]
- 20.Violi F, Leo R, Basili S, Ferro D, Cordova C, Balsano F CALC Group. Association between prolonged bleeding time and gastrointestinal hemorrhage in 102 patients with liver cirrhosis: results of a retrospective study. Haematologica. 1994;79:61–5. [PubMed] [Google Scholar]
- 21.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey WD Practice Guidelines Committee of American Association for Study of Liver Diseases; Practice Parameters Committee of American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Am J Gastroenterol. 2007;102:2086–102. doi: 10.1111/j.1572-0241.2007.01481.x. [DOI] [PubMed] [Google Scholar]
- 22.Belli G, Limongelli P, Fantini C, D’Agostino A, Cioffi L, Belli A, Russo G. Laparoscopic and open treatment of hepatocellular carcinoma in patients with cirrhosis. Br J Surg. 2009;96:1041–8. doi: 10.1002/bjs.6680. [DOI] [PubMed] [Google Scholar]
- 23.Cheung TT, Poon RT, Yuen WK, Chok KS, Jenkins CR, Chan SC, Fan ST, Lo CM. Long-term survival analysis of pure laparoscopic versus open hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a single-center experience. Ann Surg. 2013;257:506–11. doi: 10.1097/SLA.0b013e31827b947a. [DOI] [PubMed] [Google Scholar]
- 24.Memeo R, de’Angelis N, Compagnon P, Salloum C, Cherqui D, Laurent A, Azoulay D. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: a case-control study. World J Surg. 2014;38:2919–26. doi: 10.1007/s00268-014-2659-z. [DOI] [PubMed] [Google Scholar]
- 25.Twaij A, Pucher PH, Sodergren MH, Gall T, Darzi A, Jiao LR. Laparoscopic vs open approach to resection of hepatocellular carcinoma in patients with known cirrhosis: systematic review and meta-analysis. World J Gastroenterol. 2014;20:8274–81. doi: 10.3748/wjg.v20.i25.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong JH, Li LQ. Postoperative adjuvant transarterial chemoembolization for participants with hepatocellular carcinoma: A meta-analysis. Hepatol Res. 2010;40:943–53. doi: 10.1111/j.1872-034X.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- 27.Xie ZB, Wang XB, Peng YC, Zhu SL, Ma L, Xiang BD, Gong WF, Chen J, You XM, Jiang JH, Li LQ, Zhong JH. Systematic review comparing the safety and efficacy of conventional and drugeluting bead transarterial chemoembolization for inoperable hepatocellular carcinoma. Hepatol Res. 2015;45:190–200. doi: 10.1111/hepr.12450. [DOI] [PubMed] [Google Scholar]
- 28.Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513–20. [PubMed] [Google Scholar]
- 29.GA W, Shea BO, D C, et al., editors. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 29 2012.
- 30.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.JP H, S G. Cochrane handbook for systematic reviews of interventions version 5.1.0. March 2011. Cochrane Collaboration 2011 Available at: http://wwwcochrane-handbookorg Accessed March 29 2012. [Google Scholar]
- 32.Siniscalchi A, Ercolani G, Tarozzi G, Gamberini L, Cipolat L, Pinna AD, Faenza S. Laparoscopic versus Open Liver Resection: Differences in Intraoperative and Early Postoperative Outcome among Cirrhotic Patients with Hepatocellular Carcinoma-A Retrospective Observational Study. HPB Surg. 2014;2014:871251. doi: 10.1155/2014/871251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamashita Y, Ikeda T, Kurihara T, Yoshida Y, Takeishi K, Itoh S, Harimoto N, Kawanaka H, Shirabe K, Maehara Y. Long-term favorable surgical results of laparoscopic hepatic resection for hepatocellular carcinoma in patients with cirrhosis: a single-center experience over a 10-year period. J Am Coll Surg. 2014;219:1117–23. doi: 10.1016/j.jamcollsurg.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 34.Chan AC, Poon RT, Cheung TT, Chok KS, Dai WC, Chan SC, Lo CM. Laparoscopic versus open liver resection for elderly patients with malignant liver tumors: a single-center experience. J Gastroenterol Hepatol. 2014;29:1279–83. doi: 10.1111/jgh.12539. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko H, Takagi S, Otsuka Y, Tsuchiya M, Tamura A, Katagiri T, Maeda T, Shiba T. Laparoscopic liver resection of hepatocellular carcinoma. Am J Surg. 2005;189:190–4. doi: 10.1016/j.amjsurg.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 36.Lai EC, Tang CN, Ha JP, Li MK. Laparoscopic liver resection for hepatocellular carcinoma: ten-year experience in a single center. Arch Surg. 2009;144:143–7. doi: 10.1001/archsurg.2008.536. discussion 8. [DOI] [PubMed] [Google Scholar]
- 37.Martin RC 2nd, Mbah NA, Hill RS, Kooby D, Weber S, Scoggins CR, Maithel SK. Laparoscopic Versus Open Hepatic Resection for Hepatocellular Carcinoma: Improvement in Outcomes and Similar Cost. World J Surg. 2015;39:1519–26. doi: 10.1007/s00268-015-2974-z. [DOI] [PubMed] [Google Scholar]
- 38.Tranchart H, Di Giuro G, Lainas P, Roudie J, Agostini H, Franco D, Dagher I. Laparoscopic resection for hepatocellular carcinoma: a matched-pair comparative study. Surg Endosc. 2010;24:1170–6. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- 39.Gigot JF, Glineur D, Santiago Azagra J, Goergen M, Ceuterick M, Morino M, Etienne J, Marescaux J, Mutter D, van Krunckelsven L, Descottes B, Valleix D, Lachachi F, Bertrand C, Mansvelt B, Hubens G, Saey JP, Schockmel R Hepatobiliary and Pancreatic Section of the Royal Belgian Society of Surgery and the Belgian Group for Endoscopic Surgery. Laparoscopic liver resection for malignant liver tumors: preliminary results of a multicenter European study. Ann Surg. 2002;236:90–7. doi: 10.1097/00000658-200207000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, Wakabayashi G, Belli G, Kaneko H, Ker CG, Scatton O, Laurent A, Abdalla EK, Chaudhury P, Dutson E, Gamblin C, D’Angelica M, Nagorney D, Testa G, Labow D, Manas D, Poon RT, Nelson H, Martin R, Clary B, Pinson WC, Martinie J, Vauthey JN, Goldstein R, Roayaie S, Barlet D, Espat J, Abecassis M, Rees M, Fong Y, McMasters KM, Broelsch C, Busuttil R, Belghiti J, Strasberg S, Chari RS World Consensus Conference on Laparoscopic Surgery. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–30. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 41.Endo Y, Ohta M, Sasaki A, Kai S, Eguchi H, Iwaki K, Shibata K, Kitano S. A comparative study of the long-term outcomes after laparoscopyassisted and open left lateral hepatectomy for hepatocellular carcinoma. Surg Laparosc Endosc Percutan Tech. 2009;19:e171–4. doi: 10.1097/SLE.0b013e3181bc4091. [DOI] [PubMed] [Google Scholar]
- 42.Soubrane O, Goumard C, Laurent A, Tranchart H, Truant S, Gayet B, Salloum C, Luc G, Dokmak S, Piardi T, Cherqui D, Dagher I, Boleslawski E, Vibert E, Sa Cunha A, Belghiti J, Pessaux P, Boelle PY, Scatton O. Laparoscopic resection of hepatocellular carcinoma: a French survey in 351 patients. HPB. 2014;16:357–65. doi: 10.1111/hpb.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laurent A, Tayar C, Andreoletti M, Lauzet JY, Merle JC, Cherqui D. Laparoscopic liver resection facilitates salvage liver transplantation for hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:310–4. doi: 10.1007/s00534-009-0063-0. [DOI] [PubMed] [Google Scholar]
- 44.Belli G, Fantini C, D’Agostino A, Cioffi L, Langella S, Russolillo N, Belli A. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: short- and middle-term results. Surg Endosc. 2007;21:2004–11. doi: 10.1007/s00464-007-9503-6. [DOI] [PubMed] [Google Scholar]
- 45.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang CN, Tsui KK, Ha JP, Yang GP, Li MK. A single-centre experience of 40 laparoscopic liver resections. Hong Kong Med J. 2006;12:419–25. [PubMed] [Google Scholar]
- 47.Bazin JE, Gillart T, Rasson P, Conio N, Aigouy L, Schoeffler P. Haemodynamic conditions enhancing gas embolism after venous injury during laparoscopy: a study in pigs. Br J Anaesth. 1997;78:570–5. doi: 10.1093/bja/78.5.570. [DOI] [PubMed] [Google Scholar]
- 48.Dagher I, Proske JM, Carloni A, Richa H, Tranchart H, Franco D. Laparoscopic liver resection: results for 70 patients. Surg Endosc. 2007;21:619–24. doi: 10.1007/s00464-006-9137-0. [DOI] [PubMed] [Google Scholar]
- 49.Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67–72. doi: 10.1002/bjs.5150. [DOI] [PubMed] [Google Scholar]
- 50.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–11. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 51.Yin Z, Fan X, Ye H, Yin D, Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1203–15. doi: 10.1245/s10434-012-2705-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


