Abstract
Background and objectives: Stroke volume variation (SVV) and the pulse pressure variation (PPV) have been found to be effective in prediction fluid responsiveness especially in high risk operations. The objective of this study is to validate the ability of SVV obtained by FloTrac/Vigileo system and PPV obtained by IntelliVue MP System to predict fluid responsiveness in patients with obstructive jaundice during mechanical ventilation. Methods: Twentyfive patients with obstructive jaundice (mean serum total bilirubin 175.0 ± 120.8 μmol/L), who accepted volume expansion and were hemodynamically stable after induction of anesthesia, were included in the study. SVV and PPV were recorded simultaneously before and after an intravascular volume expansion. Patients with a stroke volume index (SVI) increase of more than 10% after volume expansion were considered as responders. Results: The agreement (mean bias ± SD) between SVV and PPV was -0.2% ± 1.56%. Before volume expansion, SVV and PPV were significantly higher in responders compared to non-responders (P<0.001, P<0.001). Significant correlation was observed between the baseline value of SVV and PPV and the percent change in SVI after fluid expansion (r=0.654, P<0.001; r=0.592, P=0.002). Area under the receiver operating characteristic curves of SVV (0.955) and PPV (0.875) were comparable (P=0.09). The optimal threshold values in predicting fluid responsiveness were 10% for SVV and 8% for PPV. Conclusion: In conclusion, SVV obtained by FloTrac/Vigileo system and PPV obtained by IntelliVue MP System was able to predict fluid responsiveness in patients with obstructive jaundice.
Keywords: Obstructive jaundice, stroke volume variation, pulse pressure variation, fluid responsiveness
Introduction
The high morbidity and mortality following surgical intervention in patients with obstructive jaundice continues to be challenge despite recent advances in diagnosis and treatment [1,2]. Major post-surgical complications include hypotension, acute renal failure, multiple organ failure and endotoxemia. Potential reasons for this increased susceptibility include total body water and extracellular water depletion, defective vascular reactivity, subclinical myocardial dysfunction, and exaggerated release of pro-inflammatory cytokines [1-5]. Essential preventative strategies include reversal of coagulopathy by parenteral vitamin K and the replacement of clotting factors, antibiotic usage and fluid administration to control the postoperative complications and mortality in patients with obstructive jaundice [6].
The fundamental purpose of the fluid administration in perioperative period is to increase the left ventricular stroke volume in order to avoid cardiopulmonary complications, and interstitial edema [7,8]. Numerous studies demonstrated static variables of preload, such as central venous pressure (CVP) and pulmonary capillary wedge pressure (PCWP), or left ventricular end diastolic area (LVEDA) to be poor predictors of fluid responsiveness [9-11]. Therefore, an accurate and reliable criterion to predict fluid responsiveness is required. Dynamic variables such as stroke volume variation (SVV) and pulse pressure variation (PPV) have been shown to be reliable methods to indicate fluid responsiveness in different patient populations in several recent studies [12-18].
Lately, monitoring devices that can automatically calculate and continuously monitor the indices of SVV and PPV have been developed. FloTrac/Vigileo monitoring technology is a new type of system which is used to measure arterial pressure and cardiac output (CO), and can accurately and continuously measure CO, stroke volume (SV) and SVV by analyzing and calculating peripheral arterial pressure waveform [12]. IntelliVue MP Invigilator can be used to an automatically and continuously monitor PPV [13,18].
Previous studies have shown that the SVV obtained by FloTrac/Vigileo system and the PPV obtained by an IntelliVue MP monitor could be successfully used for predicting fluid responsiveness in surgical patients [12-15,18-21], but there is limited evidence showing that either PPV or SVV guided fluid management can improve mortality.
Most of the previous studies have focused on patients with coronary bypass operation, septic shock and other high risk operative patients [12-15,18,20,21]. The validation of SVV and PPV in patients with obstructive jaundice has not been studied so far. The main objective of this study is to analyze and compare the ability of SVV obtained by FloTrac/Vigileo system and PPV obtained by IntelliVue MP System to predict fluid responsiveness in patients with obstructive jaundice during mechanical ventilation so as to apply these dynamic indexes to optimize the volume management of perioperative jaundice patients.
Materials and methods
Study population
This prospective study was approved by institutional Ethics Committee of People’s Liberation Army General Hospital, Beijing, China. Informed, written consent was taken from the study participants. Twenty-five consecutive patients in the age group 18-65 years, with obstructive jaundice (serum total bilirubin >20 μmol/L) caused by tumor or stone in the bile duct or in the head of the pancreas were included in the study between January and September 2012. All the participating patients were scheduled for elective surgery for the underlying diseases and were characterized as physical status I or II by the American Society of Anesthesiologists (ASA). Patients with body mass index >30 kg/m2 or <18 kg/m2, acid–base disturbance, blood electrolyte abnormality, diabetes or sepsis, respiratory, peripheral vascular, renal diseases or hepatic encephalopathy, arrhythmias and intra-cardiac shunts, heart rate (HR)/respiratory rate (RR) >3.6 and above the age of 65 years and below 18 years were excluded from the study.
It was not possible to blind this study to the attending anesthetist but we employed an independent observer to measure and record the perioperative data.
Procedure for anesthesia
Patient were fasted overnight and given an intramuscular injection of 0.1 g phenobarbital 30 min before surgery. Ringer’s lactate solution (RLS) 500 mL was administered to the patients on the ward the day of surgery. On arrival to the operating theatre, a baseline fluid administration of RLS 2 mL/kg/h was started.
Midazolam (0.04 to 0.05 mg/kg), fentanyl (2 to 3 μg/kg), and propofol (1 to 1.5 mg/kg) were used to induce anesthesia. Orotracheal intubation was facilitated with rocuronium (0.6 to 0.9 mg/kg). After the induction of anesthesia, a catheter (REFRA-04220, Arrow international Inc, USA) was inserted in the left radial artery as part of the standard monitoring and connected additionally to the FloTrac/Vigileo system (Edwards Life sciences, USA, version 1.14). Vigileo monitor system computed SV from the patient’s arterial pressure signal and displayed SV, stroke volume index (SVI), CO, cardiac index (CI) and SVV continuously. A two lumen French central venous catheter (Arrow International Inc, USA) was inserted in the right internal jugular vein. Pressure transducers were leveled at the mid axillary line and affixed to the operating room table and zeroed to atmospheric pressure before each step of the protocol.
Anesthesia was maintained with sevoflurane (0.7 to 0.9 minimum alveolar concentrations, MAC) and a continuous infusion of remifentanil (0.1 to 0.15 μg/kg/min). The Bispectral index (BIS, Aspect 1000, Aspect Medical Systems Inc., Natick, MA, USA) was kept between 40 and 60. Fentanyl (1 to 1.5 μg/kg) was administered as bolus injection when required. Patients’ lungs were ventilated in a volume controlled mode with a tidal volume of 8 mL/kg of body weight, an inspiratory/expiratory ratio of 0.5, and ventilatory frequency between 10 and 12 bpm to achieve an end-tidal carbon dioxide partial pressure between 4.66 and 5.3 kPa. Positive end-expiratory pressure was set at 0 cm H2O.
Analytical measurements
The FloTrac/Vigileo system enables the continuous monitoring of SV, SVI, CO, CI and SVV without external calibration [22-24]. The CO was calculated from SV×HR and SVV using the equation: SVV (%)=(SVmax - SVmin)/SVmean. The mean, minimum and maximum SV were determined by this system over a window of 20 s. The parameters were set to display continuously in 1-min intervals on the Vigileo monitor.
The automated PPV was displayed in real-time as a percentage by a Philips IntelliVue MP70 monitor (Philips Medical Systems, Boeblingen, Germany) using the algorithm described by Aboy [25]. PPV was calculated by: PPV (%)=(pulse pressure max-pulse pressure min)/pulse pressure mean. PPV was calculated and averaged over four cycles of 8 s.
At each step of the study protocol, mean arterial blood pressure (MAP), HR, end-expiratory CVP, systemic vascular resistance (SVR) and systemic vascular resistance index (SVRI) were recorded simultaneously.
Study protocol
All patients were studied immediately after induction of anesthesia and after a 5-min period of hemodynamic stability with no changes in anesthetic protocol and no intravascular volume expansion. Baseline hemodynamic measurements were obtained and then followed by an IV intravascular volume expansion with 250 mL hydroxyethyl starch 130/0.4 6% (Voluven, Fresenius Kabi GmbH, Graz, Austria) for 5 to 10 min [26]. Hemodynamic measurements were performed within 3 min after intravascular volume expansion [13]. Arrhythmia was not observed during the experiment and no vasoactive drugs were used during the study.
Statistical analysis
Patients were divided into two groups, based on their response to fluids. Fluid responders were defined as patients demonstrating an increase in SVI of at least 10% after volume expansion [26] and non-responders as patients whose SVI changed less than 10%. Quantitative variables are presented as mean ± SD. Continuous variables were assessed for normal distribution (Kolmogorov-Smirnov test for normality) and variance determined by paired Student’s t-test and two-sample Student’s t-test when appropriate. Linear regression analysis was performed between the baseline values of SVV, PPV, CVP, MAP and CI and the percentage value of changes in SVI. Bland-Altman analysis was performed to assess agreement between SVV and PPV [27]. Receiver operating characteristic (ROC) curves were generated for SVV, PPV, MAP, CVP, and CI, varying the discriminating threshold of each parameter. Area under the ROC curves were calculated and compared using MedCalc 12.3.0.0 (MedCalc Software, Mariakerke, Belgium) [28]. Power analysis showed that twenty-five patients were necessary to detect a difference of 0.15 between SVV and PPV areas under the ROC curves (5% type I error rate, 80% power, two-tailed test) [17]. P value <0.05 was considered as statistically significant. All statistic analyses were performed using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA).
Results
The baseline characteristics of twenty -five patients are shown in Table 1. Agreement (mean bias ± SD) between SVV and PPV (Bland-Altman analysis) was -0.2% ± 1.56% (Figure 1) over the fifty pairs of data.
Table 1.
Baseline patient characteristics
| Project | Values |
|---|---|
| Sex, M/F | 15/10 |
| Age (yr) | 54.8 ± 7.3 |
| BMI (kg/m2) | 23.0 ± 2.8 |
| ASA physical status, I/II | 9/16 |
| Total bilirubin (μmol/L) | 175.0 ± 120.8 |
| SVRI (dyn·s·cm-5/m2) | 1836.3 ± 367.1 |
| Type of surgery (n) | |
| Whipple’s procedure | 10 |
| Hepatic resection | 6 |
| Radical resection of hilar chlangiocarcinoma | 9 |
M, male; F, female; BMI, body mass index; SVRI, systemic vascular resistance index; All values, except n, sex and ASA physical status, are expressed as mean ± SD.
Figure 1.
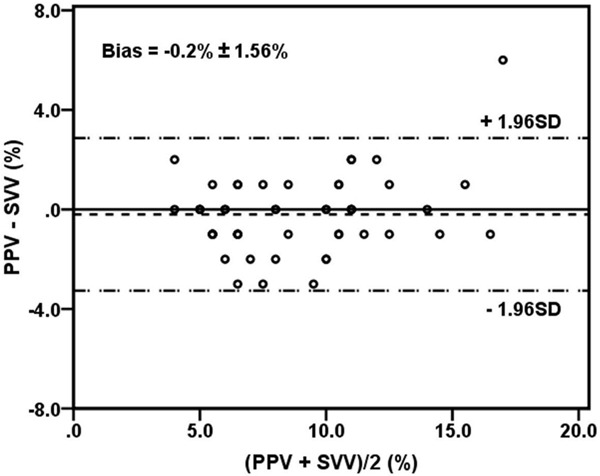
Relationship between PPV and SVV, and Bland Altman analysis for the agreement between PPV and SVV. PPV, pulse pressure variation; SVV, stroke volume variation.
Changes in hemodynamic variables after volume expansion
Twelve (48%) patients were found to be responders and thirteen patients were non- responders to volume expansion. Their hemodynamic data is shown in Table 2. Before volume expansion, SVV and PPV was significantly higher in responders than in non-responders (P<0.001, P<0.001 respectively). No significant differences in HR, MAP, CVP, CI, SVI, and SVRI were observed between the two groups. After VE, there was significant changes in HR, CVP, CI, SVI, SVV, PPV and SVRI (P=0.004, P=0.017, P=0.007, P<0.001, P<0.001, P<0.001, P=0.002 respectively) in responders, while in non-responders, showed significant changes of HR, CVP, SVV, and PPV (P<0.001, P=0.006, P=0.001, P=0.004 respectively).
Table 2.
Hemodynamic date at baseline and after intravascular volume expansion
| Responders (n=12) | Nonresponders (n=13) | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Baseline | Volume expansion | P value1 | Baseline | Volume expansion | P value2 | P value3 | |
| HR (beats/min) | 74.9 ± 14.3 | 67.5 ± 9.6 | 0.004 | 71.2 ± 11.5 | 68.0 ± 10.1 | <0.001 | 0.474 |
| MAP (mmHg) | 72.2 ± 9.6 | 72.4 ± 9.0 | 0.704 | 74.0 ± 9.1 | 73.7 ± 8.1 | 0.746 | 0.629 |
| CVP (mmHg) | 7.0 ± 1.7 | 7.4 ± 1.7 | 0.017 | 6.9 ± 1.9 | 7.6 ± 2.2 | 0.006 | 0.916 |
| CI (L/min/m2) | 2.9 ± 0.6 | 3.1 ± 0.7 | 0.007 | 3.1 ± 0.7 | 3.0 ± 0.7 | 0.147 | 0.438 |
| SVI (mL/beat/m2) | 38.3 ± 6.0 | 45.4 ± 6.7 | <0.001 | 44.9 ± 13.7 | 45.2 ± 13.4 | 0.584 | 0.138 |
| SVV (%) | 12.8 ± 2.1 | 8.4 ± 2.1 | <0.001 | 8.1 ± 2.4 | 6.8 ± 2.4 | 0.001 | <0.001 |
| PPV (%) | 12.9 ± 3.4 | 8.1 ± 2.4 | <0.001 | 7.9 ± 2.8 | 6.5 ± 2.0 | 0.004 | <0.001 |
| SVRI (dyn·s·cm-5/m2) | 1870.8 ± 360.6 | 1735.1 ± 358.9 | 0.002 | 1806.9 ± 382.2 | 1820 ± 376.5 | 0.726 | 0.665 |
HR, heart rate; MAP, mean arterial pressure; CVP, central venous pressure; CI, cardiac index; SVI, stroke volume index; SVV, stroke volume variation; PPV, pulse pressure variation; SVRI, systemic vascular resistance index; All values are expressed as mean ± SD.
P value, before and after volume expansion in responders.
P value, before and after volume expansion in nonresponders.
P value, before volume expansion in responders and nonresponders.
Dynamic indices and static indices to quantify response to intravascular volume expansion (ΔSVI)
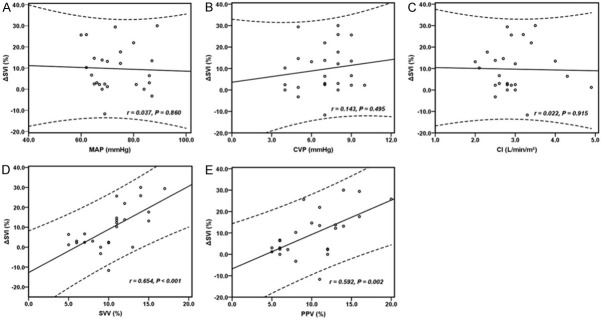
There was no significant correlation between baseline values of MAP, CVP and CI and the percent change in SVI (ΔSVI) after fluid expansion (r=0.037, P=0.860; r=0.143, P=0.459; r=0.022, P=0.915 respectively). However, the baseline value of SVV and PPV demonstrated a significant correlation to ΔSVI (r=0.654, P<0.001; r=0.592, P=0.002 respectively) (Figure 2).
Figure 2.
Relationships between MAP (A), CVP (B), CI (C), SVV (D) and PPV (E) at baseline and ΔSVI. MAP, mean arterial pressure; CVP, central venous pressure; CI, cardiac index; SVV, stroke volume variation; PPV, pulse pressure variation; SVI, stroke volume index; ΔSVI, percent increase in SVI after volume expansion.
Dynamic indices and static indices to predict fluid responsiveness
The areas under the ROC curve and thresholds for each variable with the highest sum of sensitivity and specificity, showing the ability of the hemodynamic parameters to discriminate between responders and non-responders, are shown in Table 3 and Figure 3. The areas for SVV, PPV were significantly higher than the areas for MAP, CVP and CI (P<0.05). There were no significant differences between the areas for SVV and PPV (P=0.09). The optimal threshold value for discrimination between responders and non-responders was 10% for SVV (sensitivity 100.0% and specificity 92.3%) and 8% for PPV (sensitivity 91.7% and specificity 69.2%).
Table 3.
Results of receiver operating characteristics (ROC) curve analysis
| Area (95% CI) | P value | Threshold (Sensitivity/Specificity, %) | |
|---|---|---|---|
| SVV | 0.955 (0.789 to 0.998) | <0.001 | 10 (100.0/92.3) |
| PPV | 0.875 (0.682 to 0.972) | <0.001 | 8 (91.7/69.2) |
| MAP | 0.545 (0.335 to 0.743) | 0.712 | 62 (25.0/100.0) |
| CVP | 0.519 (0.312 to 0.721) | 0.871 | 7 (50.0/61.5) |
| CI | 0.551(0.341 to 0.749) | 0.671 | 2.3 (25.0/100.0) |
Area (95% CI), area under the ROC curve with 95% asymptotic confidence interval; threshold, value with highest sum of sensitivity and specificity to predict a positive response to volume loading; SVV, stroke volume variation; PPV, pulse pressure variation; MAP, mean arterial pressure; CVP, central venous pressure; CI, cardiac index. P value, area under the curve compare with 0.5.
Figure 3.
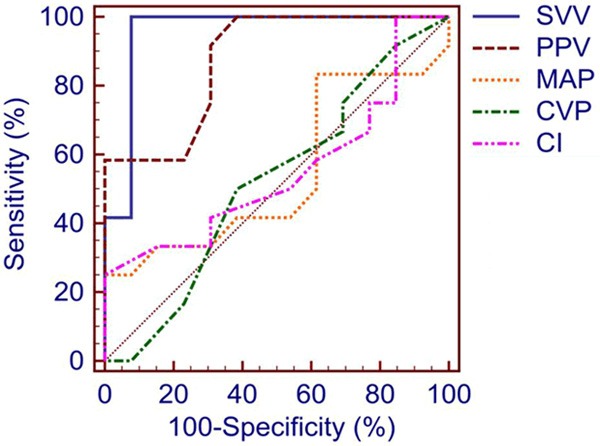
ROC curves comparing the ability of hemodynamic indices at baseline to predict fluid responsiveness. ROC, receiver operating characteristics; SVV, stroke volume variation; PPV, pulse pressure variation; MAP, mean arterial pressure; CVP, central venous pressure; CI, cardiac index.
Discussion
Our study showed that SVV obtained by FloTrac/Vigileo system and PPV obtained by IntelliVue MP system have the ability to predict fluid responsiveness in patients with obstructive jaundice during mechanical ventilation, which may perhaps guide volume management in the patients with jaundice. To the best of our knowledge, this study is the first time to demonstrate the ability of dynamic parameters to predict fluid responsiveness in patients with obstructive jaundice.
In the past twenty years, the effectiveness of SVV and of PPV to predict fluid responsiveness and reduce the complications and days of hospitalization has been demonstrated by several clinical studies [12-17,19]. However, these parameters have not been found to be very useful in some recent studies [29-31]. While several confounders like decreased in SVR and cardiac function are known to interfere with these dynamic variables to predict fluid responsiveness, monitoring CO at low SVR by FloTrac/Vigileo system have also been under criticism [32,33].
Decrease in cardiac function has been reported in animal models and patients with obstructive jaundice [2,5]. Additionally, patients with obstructive jaundice usually have low SVR due to an increased atrial natriuretic peptide in blood and an attenuated response to catecholamine [2,5]. All these pathophysiological characteristics may have effects on the accuracy of dynamic indices in prediction of fluid responsiveness. Therefore in order to guide the volume management of jaundice patients the effectiveness of SVV and PPV in prediction of fluid responsiveness needs to be studied in this group.
Our results show that SVV obtained by second generation FloTrac/Vigileo system have ideal ability of prediction fluid responsiveness in jaundice patients with mechanical ventilation, the area under the AUC curve was 0.955 (95% CI, 0.789-0.998), the optimal threshold to determine fluid responsiveness was 10% (sensitivity 100%, specificity 92.3%). This result are in concordance with previous results of predicting fluid responsiveness in patients with coronary artery bypass grafting, septic shock and other high risk surgeries [12,14-16,22]. Our results further confirm the accuracy and reliability of SVV in predicting fluid responsiveness in different clinical environment. However, Lanner et al observed a decrease in SVR in patients undergoing abdominal operation and found that SVV could not predict fluid responsiveness in these patients [29]. In this study all the patients had normal range of SVR, although it has been reported that SVR could reduce in patients with obstructive jaundice. The effect of SVR changes on SVV to predict fluid responsiveness would be interesting to explore.
The ability of PPV to predict fluid responsiveness have been confirmed by many researchers and gradually become the gold standard to predict fluid responsiveness. Several methods are used for automatic continuous monitoring of PPV. The PiCCO system and LiDCO system require special device [34], while IntelliVue MP monitoring system does not need special device and can automatically calculate and monitor PPV through the arterial waveform signal [25]. Cannesson and Derichard et al proved that this automatic monitoring of PPV and artificial calculation results of PPV have good consistency [13,18]. PPV obtained by IntelliVue MP System has been reported to have good clinical application in patients with heart operation and critically ill patients [35,36]. Our study shows that PPV has the ability to predict fluid responsiveness in jaundice patients with mechanical ventilation with a sensitivity of 91.7%, and specificity of 69.2%, with a threshold of 8% slightly lower that that reported earlier [13,14,18,20,21]. Gouvea et al found that PPV failed to predict fluid responsiveness in liver transplantation operation which may be caused due to decrease in SVR, heart function, CO monitoring method and operation stimulation [30].
In this study SVR of patients with obstructive jaundice was in the normal range, in addition, unfortunately, we did not collect data about cardiac function of patients. However, based on our present results we believe that PPV can guide the volume management of patients with jaundice and improve the prognosis. We also observed that the prediction of fluid responsiveness by static hemodynamic indexes (MAP, CVP, CI) were inferior to SVV and PPV, as reported earlier [10-12,20].
Earlier reports by Monge Garcia et al have shown that vascular Eadyn (dynamic arterial elastance) value before fluid expansion which is the ratio of PPV and SVV, could predict changes of MAP after infusion with a sensitivity of 93.75% and a specificity of 100% [37]. Unfortunately that baseline Eadyn value had no correlation with changes of MAP after infusion in our study, probably because of automatic monitoring of PPV in our study versus artificial method of PPV calculation by Monge Garcia.
Our study has some limitations. We applied Vigileo monitoring system to detect SVI and CI instead of the standard thermo dilution measuring method. Vigileo system to detect CI was reported to be inaccurate when there is a decline in SVR or decrease of cardiac function [32,33]. But the second generation Vigileo system was reported to be comparable with the standard method by Marik et al [11]. In addition, SVV calculation does not rely on the absolute value of SV but on SV changes in respiration cycle. So, even if SVI and CI monitored by Vigileo system are inconsistence it could still monitor SVV to predict fluid responsiveness accurately [12]. The study participants were patients who were scheduled for operation with ASA I to II, and we have no data on floating catheter and its related complications [38]. Secondly, our study only detected SVV and PPV to predict fluid responsiveness in steady state after induction but did not detect efficiency of these indicators during intraoperative period. Many studies obtained positive results that assessment of dynamic indexes before operation [12-14,20,21], but the results under condition of intraoperative period are not consistent [29,30]. Further we did not study the effect of SVR changes and cardiac function on SVV and PPV to predict fluid responsiveness. Our future studied will be directed at addressing these limitations.
Conclusion
In conclusion, after induction of anesthesia and before operation, we found the SVV measured by FloTrac/Vigileo system and PPV obtained by IntelliVue MP System could predict fluid responsiveness in patients with obstructive jaundice. In future research we should further explore the ability of SVV and PPV to predict the fluid responsiveness in patients with jaundice during intraoperative period, and further explore whether apply dynamic indices of such as SVV and PPV to guide perioperative volume management can improve the prognosis of patients with obstructive jaundice.
Acknowledgements
The authors thank the many research staff, nursing staff, surgical and anesthesiology colleagues who have helped with the conduct of the study in Chinese People’s Liberation Army General Hospital. This work was supported by a Hainan Society Development Fund (to Dr Qiang Fu).
Disclosure of conflict of interest
None.
References
- 1.Dixon JM, Armstrong CP, Duffy SW, Davies GC. Factors affecting morbidity and mortality after surgery for obstructive jaundice: a review of 373 patients. Gut. 1983;24:845–852. doi: 10.1136/gut.24.9.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green J, Better OS. Systemic hypotension and renal failure in obstructive jaundice-mechanistic and therapeutic aspects. J Am Soc Nephrol. 1995;5:1853–1871. doi: 10.1681/ASN.V5111853. [DOI] [PubMed] [Google Scholar]
- 3.Padillo FJ, Rodriguez M, Gallardo JM, Andicoberry B, Naranjo A, Martin-Malo A, Mino G, Sitges-Serra A, Pera-Madrazo C. Preoperative assessment of body fluid disturbances in patients with obstructive jaundice. World J Surg. 1999;23:681–687. doi: 10.1007/pl00012368. discussion 687. [DOI] [PubMed] [Google Scholar]
- 4.Sewnath ME, van der Poll T, van Noorden CJ, ten Kate FJ, Gouma DJ. Cholestatic interleukin-6-deficient mice succumb to endotoxininduced liver injury and pulmonary inflammation. Am J Respir Crit Care Med. 2004;169:413–420. doi: 10.1164/rccm.200303-311OC. [DOI] [PubMed] [Google Scholar]
- 5.Padillo J, Puente J, Gomez M, Dios F, Naranjo A, Vallejo JA, Mino G, Pera C, Sitges-Serra A. Improved cardiac function in patients with obstructive jaundice after internal biliary drainage: hemodynamic and hormonal assessment. Ann Surg. 2001;234:652–656. doi: 10.1097/00000658-200111000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke DL, Pillay Y, Anderson F, Thomson SR. The current standard of care in the periprocedural management of the patient with obstructive jaundice. Ann R Coll Surg Engl. 2006;88:610–616. doi: 10.1308/003588406X149327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, Dwane P, Glass PS. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002;97:820–826. doi: 10.1097/00000542-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–646. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 9.Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, Teboul JL. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007;35:64–68. doi: 10.1097/01.CCM.0000249851.94101.4F. [DOI] [PubMed] [Google Scholar]
- 10.Cole R. Does central venous pressure predict fluid responsiveness? Chest. 2008;134:1351–1352. doi: 10.1378/chest.08-1846. author reply 1352-1353. [DOI] [PubMed] [Google Scholar]
- 11.Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 12.Cannesson M, Musard H, Desebbe O, Boucau C, Simon R, Henaine R, Lehot JJ. The ability of stroke volume variations obtained with Vigileo/FloTrac system to monitor fluid responsiveness in mechanically ventilated patients. Anesth Analg. 2009;108:513–517. doi: 10.1213/ane.0b013e318192a36b. [DOI] [PubMed] [Google Scholar]
- 13.Cannesson M, Slieker J, Desebbe O, Bauer C, Chiari P, Henaine R, Lehot JJ. The ability of a novel algorithm for automatic estimation of the respiratory variations in arterial pulse pressure to monitor fluid responsiveness in the operating room. Anesth Analg. 2008;106:1195–1200. doi: 10.1213/01.ane.0000297291.01615.5c. table of contents. [DOI] [PubMed] [Google Scholar]
- 14.Khwannimit B, Bhurayanontachai R. Prediction of fluid responsiveness in septic shock patients: comparing stroke volume variation by FloTrac/Vigileo and automated pulse pressure variation. Eur J Anaesthesiol. 2012;29:64–69. doi: 10.1097/EJA.0b013e32834b7d82. [DOI] [PubMed] [Google Scholar]
- 15.Fu Q, Mi WD, Zhang H. Stroke volume variation and pleth variability index to predict fluid responsiveness during resection of primary retroperitoneal tumors in Hans Chinese. Biosci Trends. 2012;6:38–43. doi: 10.5582/bst.2012.v6.1.38. [DOI] [PubMed] [Google Scholar]
- 16.Cecconi M, Monti G, Hamilton MA, Puntis M, Dawson D, Tuccillo ML, Della Rocca G, Grounds RM, Rhodes A. Efficacy of functional hemodynamic parameters in predicting fluid responsiveness with pulse power analysis in surgical patients. Minerva Anestesiol. 2012;78:527–533. [PubMed] [Google Scholar]
- 17.Michard F, Boussat S, Chemla D, Anguel N, Mercat A, Lecarpentier Y, Richard C, Pinsky MR, Teboul JL. Relation between respiratory changes in arterial pulse pressure and fluid responsiveness in septic patients with acute circulatory failure. Am J Respir Crit Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 18.Derichard A, Robin E, Tavernier B, Costecalde M, Fleyfel M, Onimus J, Lebuffe G, Chambon JP, Vallet B. Automated pulse pressure and stroke volume variations from radial artery: evaluation during major abdominal surgery. Br J Anaesth. 2009;103:678–684. doi: 10.1093/bja/aep267. [DOI] [PubMed] [Google Scholar]
- 19.Benes J, Chytra I, Altmann P, Hluchy M, Kasal E, Svitak R, Pradl R, Stepan M. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care. 2010;14:R118. doi: 10.1186/cc9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keller G, Sinavsky K, Desebbe O, Lehot JJ. Combination of continuous pulse pressure variation monitoring and cardiac filling pressure to predict fluid responsiveness. J Clin Monit Comput. 2012;26:401–405. doi: 10.1007/s10877-012-9365-x. [DOI] [PubMed] [Google Scholar]
- 21.Yang SY, Shim JK, Song Y, Seo SJ, Kwak YL. Validation of pulse pressure variation and corrected flow time as predictors of fluid responsiveness in patients in the prone position. Br J Anaesth. 2013;110:713–720. doi: 10.1093/bja/aes475. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann M, Feibicke T, Keyl C, Prasser C, Moritz S, Graf BM, Wiesenack C. Accuracy of stroke volume variation compared with pleth variability index to predict fluid responsiveness in mechanically ventilated patients undergoing major surgery. Eur J Anaesthesiol. 2010;27:555–561. doi: 10.1097/EJA.0b013e328335fbd1. [DOI] [PubMed] [Google Scholar]
- 23.Senn A, Button D, Zollinger A, Hofer CK. Assessment of cardiac output changes using a modified FloTrac/Vigileo algorithm in cardiac surgery patients. Crit Care. 2009;13:R32. doi: 10.1186/cc7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayer J, Boldt J, Poland R, Peterson A, Manecke GR Jr. Continuous arterial pressure waveform-based cardiac output using the FloTrac/Vigileo: a review and meta-analysis. J Cardiothorac Vasc Anesth. 2009;23:401–406. doi: 10.1053/j.jvca.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Aboy M, McNames J, Thong T, Phillips CR, Ellenby MS, Goldstein B. A novel algorithm to estimate the pulse pressure variation index deltaPP. IEEE Trans Biomed Eng. 2004;51:2198–2203. doi: 10.1109/TBME.2004.834295. [DOI] [PubMed] [Google Scholar]
- 26.Cecconi M, Parsons AK, Rhodes A. What is a fluid challenge? Curr Opin Crit Care. 2011;17:290–295. doi: 10.1097/MCC.0b013e32834699cd. [DOI] [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 28.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 29.Lahner D, Kabon B, Marschalek C, Chiari A, Pestel G, Kaider A, Fleischmann E, Hetz H. Evaluation of stroke volume variation obtained by arterial pulse contour analysis to predict fluid responsiveness intraoperatively. Br J Anaesth. 2009;103:346–351. doi: 10.1093/bja/aep200. [DOI] [PubMed] [Google Scholar]
- 30.Gouvea G, Diaz R, Auler L, Toledo R, Martinho JM. Evaluation of the pulse pressure variation index as a predictor of fluid responsiveness during orthotopic liver transplantation. Br J Anaesth. 2009;103:238–243. doi: 10.1093/bja/aep123. [DOI] [PubMed] [Google Scholar]
- 31.Kronas N, Kubitz JC, Forkl S, Kemming GI, Goetz AE, Reuter DA. Functional hemodynamic parameters do not reflect volume responsiveness in the immediate phase after acute myocardial ischemia and reperfusion. J Cardiothorac Vasc Anesth. 2011;25:780–783. doi: 10.1053/j.jvca.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Junttila EK, Koskenkari JK, Ohtonen PP, Ala-Kokko TI. Uncalibrated arterial pressure waveform analysis for cardiac output monitoring is biased by low peripheral resistance in patients with intracranial haemorrhage. Br J Anaesth. 2011;107:581–586. doi: 10.1093/bja/aer170. [DOI] [PubMed] [Google Scholar]
- 33.Biais M, Nouette-Gaulain K, Cottenceau V, Vallet A, Cochard JF, Revel P, Sztark F. Cardiac output measurement in patients undergoing liver transplantation: pulmonary artery catheter versus uncalibrated arterial pressure waveform analysis. Anesth Analg. 2008;106:1480–1486. doi: 10.1213/ane.0b013e318168b309. table of contents. [DOI] [PubMed] [Google Scholar]
- 34.Cannesson M, de Backer D, Hofer CK. Using arterial pressure waveform analysis for the assessment of fluid responsiveness. Expert Rev Med Devices. 2011;8:635–646. doi: 10.1586/erd.11.30. [DOI] [PubMed] [Google Scholar]
- 35.Kim IB, Bellomo R, Fealy N, Baldwin I. A pilot study of the epidemiology and associations of pulse pressure variation in cardiac surgery patients. Crit Care Resusc. 2011;13:17–23. [PubMed] [Google Scholar]
- 36.Kim IB, Fealy N, Baldwin I, Bellomo R. A pilot study of the epidemiology and associations of pulse pressure variation among non-cardiac surgery critically ill patients. Crit Care Resusc. 2011;13:156–161. [PubMed] [Google Scholar]
- 37.Monge Garcia MI, Gil Cano A, Gracia Romero M. Dynamic arterial elastance to predict arterial pressure response to volume loading in preload-dependent patients. Crit Care. 2011;15:R15. doi: 10.1186/cc9420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marik PE. Obituary: pulmonary artery catheter 1970 to 2013. Ann Intensive Care. 2013;3:38. doi: 10.1186/2110-5820-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]