Abstract
Objective: The aim of this study is to examine and compare the efficacy of everolimus-eluting stents (EES) and zotarolimus-eluting stents (ZES) in coronary heart disease in diabetic or non-diabetic patients. Methods: A total of 666 patients needed for percutaneous coronary intervention were randomly selected from June 2008 to June 2013 in our hospital and were divided into two groups: (i) coronary heart disease with diabetes group and (ii) non-diabetes group. Patients in each group were further assigned to receive treatment of either EES or ZES. Then we observed the major adverse cardiac events, including mortality, nonfatal myocardial infarction and non-fatal cerebrovascular events over the period of 15 months after initial stent implantation. Results: Compared to the non-diabetic group, more patients in diabetic group had received anti-hypotensive treatment (72% vs. 49%, P < 0.0001) and hypolipemic treatment (80% vs. 67%, P < 0.0001) before the percutaneous coronary intervention. In both diabetic group and non-diabetic group, patients received ZES treatment had a much greater incidence rate of major adverse cardiac events compared to the patients received EES treatment (P < 0.05). Meanwhile, target lesion revascularization rate in the ZES group was also significantly higher than that in the EES group. The data showed big differences between ZES and EES groups with important statistical significance (P < 0.05). Conclusion: Patients with coronary heart disease and diabetes have a higher risk of major adverse cardiac events after stent implantation. EES treatment is safer with higher efficacy in our study, being a more effective stent for the patients merged with diabetes.
Keywords: Everolimus-eluting stent, zotarolimus-eluting stents, coronary heart disease, diabetes
Introduction
Interventional stent therapy has become the main treatment for coronary heart disease due to the small trauma and high repetitiveness. Now, the most commonly used stent is drug-eluting stents (DES). Compared to bare-metal stents (BMS), drug-eluting stents (DES) is more effective for diabetic patients with a higher safety [1,2]. Drug-eluting stents with a metal platform and the controlled release of a therapeutic agent from polymer matrix has reduced the risk of restenosis [3,4]. However, the first generation DESs still have biocompatible problems, they have been causing some reactions, such as allergic reactions and inflammation [5,6]. Incomplete strut endothelialization also led to early and late stent thrombosis [7,8]. Zotarolimus-eluting stent (ZES) and everolimus-eluting stents (EES) are used as a second-generation DES with lower rates of clinical restenosis, lower targeting lesion failure for 12 months after surgery [9,10]. The new-generation ZES was designed for a better mimicking the endothelial lining in order to reduce thrombosis rate. Recently, Michael et al. found that ZES has lower efficacy and safety than EES in the treatment of coronary heart disease [11]. The second-generation DES has significantly improved the outcomes with percutaneous coronary intervention [12,13]. Randomized trials and meta-analyses have shown that the use of EES greatly reduced mortality, nonfatal myocardial infarction, nonfatal cerebrovascular events, restenosis, and stent thrombosis, suggesting that EES is safer and more effective than first generation drug-eluting stents [14,15]. However, few reports were found on comparing the efficacy between ZES and EES in the treatment of coronary heart disease in diabetic patients. Here we compared the efficacy of ZES and EES in coronary artery disease in diabetic and non-diabetic patients, so as to provide future guidance for clinical treatment.
Methods
Patients
From June 2008 to June 2013, we randomly recruited 666 adult patients with chronic, stable coronary artery disease or acute coronary syndromes, including myocardial infarction with or without ST-segment elevation. Patients were eligible if they had at least one coronary lesion with stenosis of more than 50% in a vessel with a reference diameter of 2.25 to 4.0 mm. No restriction was placed on the total number of treated lesions, treated vessels, lesion length, or number of stents implanted. Analysts and authors were excluded in the study. The patients were divided into two different groups in this research. The first group is “non-diabetic group”. In this group, patients were randomly selected in Cardiology Department in our hospital, with the selection criteria showing below: (i) eligible patients were 18 years age or older; (ii) patients had chronic coronary artery disease or acute coronary syndrome; and (iii) patients were eligible if they had at least one vascular lesion requiring interventional stent therapy.
The second group is named “diabetic group”. In this group, patients not only met all three criteria showed above, but also, they had diabetes history before, and were being treated actively with insulin, oral hypoglycemic agents or diet therapy. The patient’s fasting blood glucose of less than 6.19 mmol/L during treatment is also required to be counted in this group. However, patients with life expectancy less than one year, patients allergic to everolimus or zotarolimus, and patients with rare complex lesions were excluded in this study. All patients provided written informed consent before treatment. All patients were either censored at the time of death or followed up for 15 months. The study was approved by the Hospital Clinical Trials Assessment and Management Committee.
Study procedures
Eligible patients were randomly assigned in a ~1:1 ratio and divided into two groups, one group received everolimus-eluting stent (EES, Medtronic), and the other group received zotarolimus-eluting stent (ZES, Abbott). Before stent implantation, patients were pretreated with clopidogrel at a dose of 300-600 mg per day and heparin at a dose of 70-100 IU/Kg per day. After stent implantation, all patients received clopidogrel at a dose of 75 mg per day for at least 12 months according to the instruction [16]. We evaluated the efficacy of stent therapy by observing patient death, cardiac death, myocardial infarction, stent thrombosis, vascular restenosis and target lesion revascularization within 15-month after stent therapy.
Statistical analysis
Data from the different groups were compared with the use of a two-sample t-test. Unless otherwise specified, a two-sided p value of less than 0.05 was considered to indicate statistical significance. SPSS software, version 17.0, was used for all the statistical analyses.
Results
Patients
A total of 666 patients were randomly assigned in a ~1:1 ratio to receive either ZES (333 patients) or EES (333 patients) as shown in Table 1. A total of 85 patients (25.5%) in the ZES group and 84 (25.2%) in the EES group were diabetic patients, leaving 248 patients in the ZES group and 249 patients in the EES group non-diabetic (Table 1).
Table 1.
Patient and procedure characteristics in diabetic and non-diabetic patients treated with zotarolimus-eluting stent (ZES) or everolimus-eluting stents (EES)
| Diabetic group | Non-diabetic group | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Variable | ZES | EES | P value | ZES | EES | P value |
| Patient (no.) | 85 | 84 | 248 | 249 | ||
| Lesion number | 0.59 | 0.27 | ||||
| 1 | 63 (74.12%) | 58 (69.05%) | 171 (68.95%) | 177 (71.08%) | ||
| 2 | 15 (17.65%) | 19 (22.62%) | 50 (20.16%) | 55 (22.09%) | ||
| 3 | 4 (4.71%) | 7 (8.33%) | 27 (10.89%) | 20 (8.03%) | ||
| Stent number | 0.56 | 0.31 | ||||
| 1 | 69 (81.18%) | 66 (78.57%) | 196 (79.03%) | 196 (78.71%) | ||
| 2 | 12 (14.12%) | 16 (19.05%) | 42 (16.94%) | 45 (18.07%) | ||
| 3 | 4 (4.71%) | 2 (2.38%) | 10 (4.33%) | 8 (3.21%) | ||
| Age (yr) | 64 (54-73) | 66 (56-74) | 0.71 | 62 (55-71) | 65 (57-72) | 0.87 |
| Male sex | 61 (71.76%) | 60 (72.61%) | 0.89 | 183 (73.79%) | 185 (74.30%) | 0.079 |
| Previous mcoyardial infarction | 36 (42.35%) | 30 (35.71%) | 0.28 | 93 (37.5%) | 96 (38.55%) | 0.38 |
| Previous stent therapy | 24 (28.24%) | 21 (25%) | 0.49 | 46 (18.54%) | 39 (15.66%) | 0.041 |
| ST segment elevation | 4 (4.71%) | 6 (7.05%) | 18 (7.26%) | 21 (8.43%) | ||
| Non-ST segment elevation | 33 (39.29%) | 35 (41.67%) | 103 (41.53%) | 109 (43.78%) | ||
| Stable angina | 47 (55.29%) | 41 (48.24%) | 123 (49.6%) | 118 (47.39%) | ||
| Others | 1(1.18%) | 2(2.38%) | 4 (1.61%) | 1 (0.4%) | ||
| Anti-hypotensive treatment | 67 (78.82%) | 64 (76.19%) | 0.38 | 161 (64.92%) | 157 (63.05%) | 0.41 |
| Hypolipematic treatment | 59 (69.41%) | 58 (69.05%) | 0.91 | 122 (49.19%) | 114 (45.78%) | 0.09 |
| Smoker | 51 (60%) | 38 (41.67%) | 0.16 | 148 (69.68%) | 152 (61.04%) | 0.92 |
| Body mass index | 29.3 | 29.4 | 0.91 | 27.2 | 26.8 | 0.87 |
| Coronary artery disease location | 0.57 | 0.48 | ||||
| Left main | 2 (2.35%) | 2 (2.38%) | 5 (2.02%) | 6 (2.41%) | ||
| Left anterior descending | 33 (39.19%) | 32 (41.32%) | 106 (43.08%) | 99 (40.04%) | ||
| Left circumflex | 21 (24.35%) | 21 (28.46%) | 63 (25.46%) | 67 (27.75%) | ||
| Right coronary | 29 (34.03%) | 29 (38.29%) | 74 (30.34%) | 75 (30.37%) | ||
| Mean lesion length (mm) | 17 (12-23) | 17 (13-25) | 0.68 | 17 (12-23) | 17 (13-25) | 0.26 |
| Lesion diameter (mm) | 3.2 ± 0.3 | 3.2 ± 0.5 | 0.37 | 3.2 ± 0.5 | 3.2 ± 0.4 | 0.41 |
Baseline patient, procedure characteristics and lesion characteristics were listed in Table 1, showing differences between the groups treated with ZES versus EES. In order to more effectively compare zotarolimus-eluting stent (ZES) and everolimus-eluting stents (EES) in diabetic and non-diabetic treatment group, in general, we balanced the conditions between the two groups, including patient’s age, sex, coronary lesion location, number and average lesion length and diameter, etc., there is no significant difference (P > 0.05) between the two groups as shown in Table 1. In diabetic group, more patients received anti-hypotensive treatment (72% vs. 49%, P < 0.0001) and hypolipemic treatment (80% vs. 67%, P < 0.0001) before percutaneous coronary intervention compared to non-diabetic group. In addition, in diabetic group, patients received more times of percutaneous coronary intervention (PCI) treatment (27% vs. 18%, P < 0.0001) and had higher body mass index (BMI, 29.6 vs. 27.5 kg/m, P < 0.0001) compared to non-diabetic group.
Clinical outcomes
Data on cardiac and non-cardiac deaths, myocardial infarction, and stent thrombosis are listed in Table 2. Plots for adverse cardiac events over 15 months of follow-up are shown in Figure 1. The 15-month cumulative incidence of adverse cardiac events showed significant difference between ZES group and EES group. Numbers and relative risk for adverse cardiac events are listed in Table 2. In diabetic group, rate of adverse cardiac events for patients received ZES (18.8%, n = 16) is much higher than that in patients treated with EES (4.8%, n = 4), relative risk 4.95, 95% CI 1.86 to 8.82, P = 0.0003. In non-diabetic group, the adverse cardiac events rate for patients received ZES (8.5%, n = 21) is also much higher than patients treated with EES (3.2%, n = 8), relative risk 1.96, 95% CI 1.37 to 2.69, P = 0.008 (Table 2 and Figure 1).
Table 2.
Comparing the clinical outcomes at 15 months of relative risk estimates for death, myocardial infarction, and stent thrombosis in diabetic and non-diabetic patients treated with ZES (n = 333) or EES (n = 333)
| Diabetic patients | Non-diabetic patients | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Outcome | ZES | EES | Relative risk (95% CI) | P Value | ZES | EES | Relative risk (95% CI) | P Value |
| Adverse cardiac events | 16 (18.8%) | 4 (4.8%) | 4.95 (1.86-8.82) | 0.0003 | 21 (8.5%) | 8 (3.2%) | 1.96 (1.37-2.69) | 0.008 |
| All-cause death | 7 (8.2%) | 4 (4.7%) | 1.57 (0.47-3.28) | 0.32 | 9 (3.6%) | 6 (2.4%) | 1.61 (0.87-2.37) | 0.069 |
| Cardiac death | 3 (3.5%) | 1 (1.2%) | 3.12 (0.28-14.9) | 0.21 | 3 (1.2%) | 2 (0.8%) | 1.32 (0.51-2.96) | 0.71 |
| Myocardial infarction | 4 (4.7%) | 1 (1.2%) | 8.09 (1.32-64.7) | 0.03 | 4 (1.6%) | 2 (0.8%) | 1.73 (0.39-3.95) | 0.31 |
| Stent thrombosis | 1 (1.2%) | 0 (0%) | - | - | 2 (0.8%) | 1 (0.4%) | 1.79 (0.69-5.65) | 0.39 |
| Target lesion revascularization | 12 (14.1%) | 1 (1.2%) | 11.7 (2.59-47.1) | 0.002 | 13 (5.2%) | 4 (1.6%) | 2.85 (1.67-4.89) | 0.001 |
Figure 1.
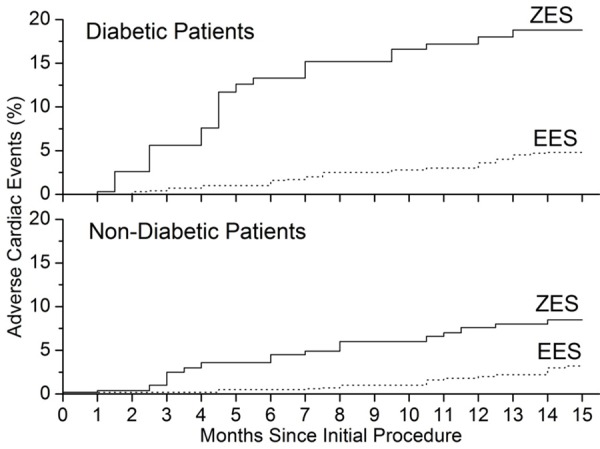
Kaplan-Meier plots for adverse cardiac events over 15 Months of Follow-up, showing a big difference in the incidence of the adverse cardiac events between the ZES (solid line) and EES (dot line) group.
Overall mortality after 15 months of follow-up was lower in patients received EES than that in patients received ZESs in both diabetic and non-diabetic groups (Table 2 and Figure 2). This differences were not significant after adjustment for covariates (adjusted relative risk 1.57, 95% CI 0.47 to 3.28, P = 0.32) in diabetic group and (adjusted relative risk 1.61, 95% CI 0.87 to 2.37, P = 0.069) in non-diabetic group. Analysis of all-cause deaths and cardiac death before and after 15 months showed similar trends in both diabetic and non-diabetic groups (Figure 2), however, the p values are greater than 0.05, suggesting that there is no significant difference between the EES and ZES groups in the risk of all cause death and cardiac death. For the target lesion revascularization, we also found significant difference between ZES group and EES group, data were shown in Table 2 and Figure 3. The cumulative incidence of target lesion revascularization (Figure 3) differs significantly between the ZES group and EES group after adjustment for covariates (Table 2). In diabetic group, target lesion revascularization within 15 months occurred much less frequently in patients received EES than those with a ZES treatment (1.2% vs. 14.1%; relative risk 11.7, 95% CI 2.59 to 47.1, P = 0.002). In non-diabetic group, incidence of target lesion revascularization was 1.6% for patients received EES, which is much lower than 5.2% in ZES group, relative risk 2.85, 95% CI 1.67 to 4.89, P = 0.001) (Table 2; Figure 3). In other end points of the events, the incidence rate of myocardial infarction in diabetic group received ZES is higher than that in diabetic group received EES (P < 0.05), but not for non-diabetic group. For other events, there was no significant difference between ZES and EES groups (P > 0.05) (Table 2).
Figure 2.
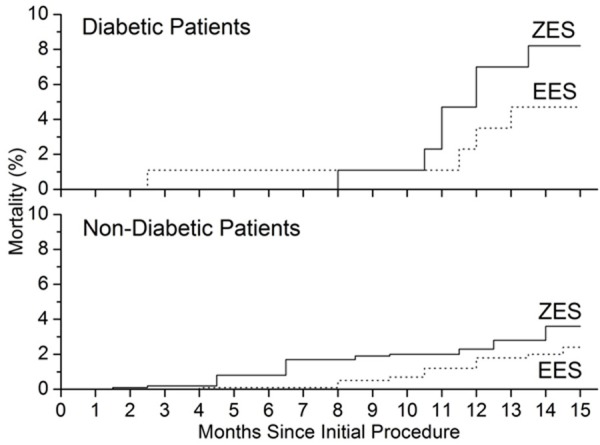
Kaplan-Meier plots show the unadjusted risk of all-cause mortality, showing a difference of mortality incidence between ZES (solid line) and EES (dot line) group.
Figure 3.
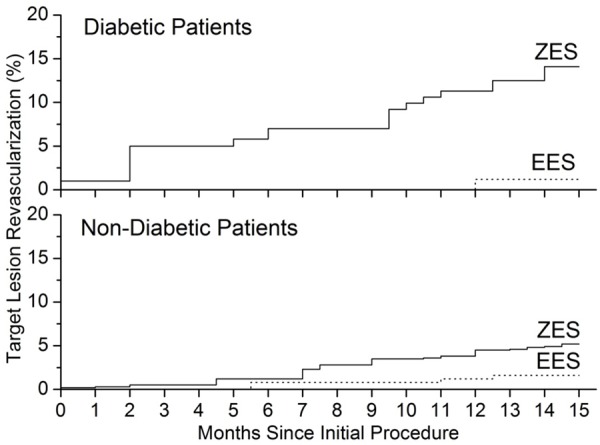
Kaplan-Meier plots show the unadjusted risk of target lesion revascularization. Significant difference between ZES (solid line) and EES (dot line) groups were shown in incidence of target lesion revascularization over 15 months of follow-up.
Discussion
Diabetes is a high risk factor for coronary heart disease, in addition, diabetes is associated with more than 70% of coronary heart disease, which lead to a great number of patients combined coronary heart disease with diabetes [17]. Stent implantation is the primary therapy method in the treatment of coronary heart disease, but now very few literatures were published on the effect of diabetes on coronary stent treatment. The Cardiology Department in our hospital allowed us to include all patients treated in our city to ensure complete follow-up of patients and accurate linkage between coronary heart disease and diabetes. Moreover, to our knowledge, our study is the first report on clinical outcomes in diabetic/non-diabetic patients after receiving EES and ZES treatment after a 15 months follow-up. We found significant between-group difference by comparing diabetic group and non-diabetic group. Although we did not see significant between-group difference in overall rates of stent thrombosis, there were significant differences in rates of adverse cardiac events, mortality, and target lesion revascularization. The patients with diabetes after stent treatment having a higher risk of major adverse cardiac events may be due to more severe vascular lesions in diabetic patients with atherosclerosis, resulting in a smaller diameter of blood vessels. Meanwhile, severe impairment in stem cell in vivo and endothelial cell function in diabetic patients, make it more difficult in the treatment and recovery of vascular injury after stent implantation [18]. Therefore, patients with both coronary heart disease and diabetes should be given special attentions, as a result, we should actively assess the condition and treat the patients with antihypertensive and hypolipemic agents before surgery, we also should closely observe any changes in patient’s condition after surgery and provide timely treatment at the time of the occurrence of adverse cardiac events.
Everolimus-eluting stents (EES) and zotarolimus-eluting stents (ZES) are the two new generation stents that commonly used in clinical trial. Park et al. [19] found that EES showed higher cumulative incidence rate of target lesion failure than ZES in diabetic patients, however, Mehilli et al. [20] reported that ZES had a slightly higher cumulative incidence rate than EES at 1-year follow-up. A large-scale clinical study [21] showed no significant differences between EES and ZES in treating patients with coronary artery disease. Meta-analysis of the results, however, suggests EES and ZES effectively reduce the rebuilding target vessel, and EES has a higher efficacy with a lower rate of stent thrombosis [22]. Our study is consistent with the Meta-anaylsis, we found that both in diabetic and non-diabetic group, the patients received EES had a much lower rate in major adverse cardiac events and target lesion revascularization compared to the patients received ZES. In details, in comparison to ZES treatment, EES treatment in both diabetic and non-diabetic patients was associated with reduced adverse cardiac events, mortality, myocardial infarction and target lesion revascularization, but not stent thrombosis after 15 months of follow-up. In both ZES and EES groups, the patients with diabetes have a much higher risk of adverse cardiac events, much higher mortality, myocardial infarction, and target lesion revascularization than the non-diabetic patients, indicating that in both diabetic and non-diabetic patient populations, EES treatment reduced target lesion revascularization without affecting the risk of stent thrombosis. In a pooled analysis of 666 patients who had been randomly assigned to ZES versus EES, an improvement in mortality rates was observed in favor of the EES group over the ZES group in the diabetic subpopulation (n = 333). In our non-diabetic population, which is as many as diabetic patients (n = 333), we did find that EES implantation was associated with decreased mortality, which is similar to our results for diabetic population (Table 2). However, we can’t conclude that diabetes is an independent risk factor for stent thrombosis in both ZES group and EES group from Table 2. This finding likely reflected the impact of diabetes on the higher risk of death and adverse cardiac events in diabetic patients. Therefore, we suggest that for coronary heart disease and diabetes, EES treatment has better safety and higher efficacy than ZES treatment.
Based on the 15 months follow-up in large patient populations, we showed definitive conclusions with sufficient statistical power to detect differences between ZES and EES. Our relative risk estimates were based on population-based databases, largely ruling out referral and diagnostic biases. However, it is important to note that the follow-up period of 15 months may be insufficient to completely quantify possible long term risks associated with the use of EES and ZES in a diabetic population, so, a longer term follow-up will be required to confirm these 15 months follow-up results.
In summary, our study shows that the patients with both coronary heart disease and diabetes have higher risk to develop major adverse cardiac events after stent implantation. We conclude that the new-generation zotarolimus- eluting stent was found to be less safe and less effective as compared to the everolimus-eluting stent in the patients with or without diabetes. However, everolimus is a derivative of rapamycin, which may cause adverse effects in stent treatments, in future studies, we will further investigate its clinical application in coronary heart disease.
Disclosure of conflict of interest
None.
References
- 1.Maeng M, Jensen LO, Kaltoft A, Hansen HH, Bøttcher M, Lassen JF, Thayssen P, Krusell LR, Rasmussen K, Pedersen L, Sørensen HT, Johnsen SP, Thuesen L. Comparison of stent thrombosis, myocardial infarction, and mortality following drug-eluting versus bare-metal stent coronary intervention in patients with diabetes mellitus. Am J Cardiol. 2008;102:165–172. doi: 10.1016/j.amjcard.2008.03.034. [DOI] [PubMed] [Google Scholar]
- 2.Pendyala LK, Loh JP, Kitabata H, Minha S, Torguson R, Chen F, Satler LF, Suddath WO, Pichard AD, Waksman R. The impact of diabetes mellitus on long-term clinical outcomes after percutaneous coronary saphenous vein graft interventions in the drug-eluting stent era. J Interv Cardiol. 2014;27:391–398. doi: 10.1111/joic.12136. [DOI] [PubMed] [Google Scholar]
- 3.Raja SG, Benedetto U, Ilsley CD, Amrani M. Multiple arterial grafting confers survival advantage compared to percutaneous intervention with drug-eluting stents in multivessel coronary artery disease: A propensity score adjusted analysis. Int J Cardiol. 2015;189:153–158. doi: 10.1016/j.ijcard.2015.04.059. [DOI] [PubMed] [Google Scholar]
- 4.Tian W, Lhermusier T, Minha S, Waksman R. Rational use of rotational atherectomy in calcified lesions in the drug-eluting stent era: Review of the evidence and current practice. Cardiovasc Revasc Med. 2015;16:78–83. doi: 10.1016/j.carrev.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Kereiakes DJ, Meredith IT, Windecker S, Lee Jobe R, Mehta SR, Sarembock IJ, Feldman RL, Stein B, Dubois C, Grady T, Saito S, Kimura T, Christen T, Allocco DJ, Dawkins KD. Efficacy and Safety of a Novel Bioabsorbable Polymer-Coated, Everolimus-Eluting Coronary Stent: The EVOLVE II Randomized Trial. Circ Cardiovasc Interv. 2015;8:e002372. doi: 10.1161/CIRCINTERVENTIONS.114.002372. [DOI] [PubMed] [Google Scholar]
- 6.Maciejewski D, Tekieli Ł, Kabłak-Ziembicka A, Paluszek P, Trystuła M, Wójcik-Pędziwiatr M, Machnik R, Pieniążek P. Transradial approach for vertebral artery stenting. Postepy Kardiol Interwencyjnej. 2015;11:32–36. doi: 10.5114/pwki.2015.49182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foin N, Gutiérrez-Chico JL, Nakatani S, Torii R, Bourantas CV, Sen S, Nijjer S, Petraco R, Kousera C, Ghione M, Onuma Y, Garcia-Garcia HM, Francis DP, Wong P, Di Mario C, Davies JE, Serruys PW. Incomplete stent apposition causes high shear flow disturbances and delay in neointimal coverage as a function of strut to wall detachment distance: implications for the management of incomplete stent apposition. Circ Cardiovasc Interv. 2014;7:180–189. doi: 10.1161/CIRCINTERVENTIONS.113.000931. [DOI] [PubMed] [Google Scholar]
- 8.Karjalainen P, Kiviniemi TO, Lehtinen T, Nammas W, Ylitalo A, Saraste A, Mikkelsson J, Pietila M, Biancari F, Airaksinen JK. Neointimal coverage and vasodilator response to titanium-nitride-oxide-coated bioactive stents and everolimus-eluting stents in patients with acute coronary syndrome: insights from the BASE-ACS trial. Int J Cardiovasc Imaging. 2013;29:1693–1703. doi: 10.1007/s10554-013-0285-8. [DOI] [PubMed] [Google Scholar]
- 9.Valgimigli M, Patialiakas A, Thury A, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, Menozzi A, de Cesare N, Garbo R, Meliga E, Testa L, Gabriel HM, Airoldi F, Ferlini M, Liistro F, Dellavalle A, Vranckx P, Briguori C. Randomized comparison of Zotarolimus-Eluting Endeavor Sprint versus bare-metal stent implantation in uncertain drug-eluting stent candidates: rationale, design, and characterization of the patient population for the Zotarolimus-eluting Endeavor Sprint stent in uncertain DES candidates study. Am Heart J. 2013;166:831–838. doi: 10.1016/j.ahj.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Kandzari DE, Eisenstein EL, Anstrom KJ, Mauri L, Cutlip DE, Nikolsky E, O’Shaughnessy C, Overlie PA, Kirtane AJ, McLaurin BT, Solomon SL, Douglas JS Jr, Popma JJ. Late safety, efficacy, and cost-effectiveness of a zotarolimus-eluting stent compared with a paclitaxel-eluting stent in patients with de novo coronary lesions: 2-year follow-up from the ENDEAVOR IV trial (Randomized, Controlled Trial of the Medtronic Endeavor Drug [ABT-578] Eluting Coronary Stent System Versus the Taxus Paclitaxel-Eluting Coronary Stent System in De Novo Native Coronary Artery Lesions) JACC Cardiovasc Interv. 2009;2:1208–1218. doi: 10.1016/j.jcin.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Maeng M, Jensen LO, Tilsted HH, Kaltoft A, Kelbaek H, Abildgaard U, Villadsen A, Aarøe J, Thayssen P, Krusell LR, Christiansen EH, Bøtker HE, Kristensen SD, Ravkilde J, Madsen M, Sørensen HT, Rasmussen K, Thuesen L, Lassen JF. Outcome of sirolimus-eluting versus zotarolimus-eluting coronary stent implantation in patients with and without diabetes mellitus (a SORT OUT III Substudy) Am J Cardiol. 2011;108:1232–1237. doi: 10.1016/j.amjcard.2011.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Shah N, Cheng VE, Cox N, Soon K. Percutaneous Coronary Intervention of an Anomalous Left Main Coronary Artery Arising from the Right Sinus of Valsalva. Heart Lung Circ. 2015;24:e123–6. doi: 10.1016/j.hlc.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Kozuch M, Kralisz P, Rog-Makal M, Bachorzewska-Gajewska H, Dobrzycki S. Significant narrowing of the circumflex artery leads to worse outcomes than right coronary artery narrowing in patients with anterior myocardial infarction treated invasively. Neth Heart J. 2015;23:258–262. doi: 10.1007/s12471-015-0678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai CL, Wu CF, Kuo RN, Yang YY, Chen MF, Chan KA, Lai MS. Clinical outcomes in low risk coronary artery disease patients treated with different limus-based drug-eluting stents-a nationwide retrospective cohort study using insurance claims database. PLoS One. 2015;10:e0122860. doi: 10.1371/journal.pone.0122860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sim DS, Jeong MH, Park DS, Kim JH, Lim KS, Kim HK, Kim SS, Cho JY, Jeong HC, Park KH, Hong YJ, Kim JH, Ahn Y, Cho JG, Park JC. Effect of pretreatment of ezetimibe/simvastatin on arterial healing and endothelialization after drug-eluting stent implantation in a porcine coronary restenosis model. Korean Circ J. 2015;45:110–116. doi: 10.4070/kcj.2015.45.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Costa M, Kereiakes D, Smith R, Wu K, Yu X, Cannon L, Wang J, Sudhir K. Two-Year Outcomes after Implantation of XIENCE PRIME and XIENCE PRIME Long Lesion Stents in Patients with Coronary Artery Disease: Results of the SPIRIT PRIME Multicenter Pivotal Clinical Trial. J Am Coll Cardiol. 2012;60:B176–B177. [Google Scholar]
- 17.Yeboah J, Erbel R, Delaney JC, Nance R, Guo M, Bertoni AG, Budoff M, Moebus S, Jöckel KH, Burke GL, Wong ND, Lehmann N, Herrington DM, Möhlenkamp S, Greenland P. Development of a new diabetes risk prediction tool for incident coronary heart disease events: The Multi-Ethnic Study of Atherosclerosis and the Heinz Nixdorf Recall Study. Atherosclerosis. 2014;236:411–417. doi: 10.1016/j.atherosclerosis.2014.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ledford H. Stem-cell success aids diabetes fight. Nature. 2014;514:281–281. doi: 10.1038/514281a. [DOI] [PubMed] [Google Scholar]
- 19.Park KW, Lee JM, Kang SH, Ahn HS, Kang HJ, Koo BK, Rhew JY, Hwang SH, Lee SY, Kang TS, Kwak CH, Hong BK, Yu CW, Seong IW, Ahn T, Lee HC, Lim SW, Kim HS. Everolimus-Eluting Xience V/Promus Versus Zotarolimus-Eluting Resolute Stents in Patients With Diabetes Mellitus. JACC Cardiovasc Interv. 2014;7:471–481. doi: 10.1016/j.jcin.2013.12.201. [DOI] [PubMed] [Google Scholar]
- 20.Mehilli J, Richardt G, Valgimigli M, Schulz S, Singh A, Abdel-Wahab M, Tiroch K, Pache J, Hausleiter J, Byrne RA, Ott I, Ibrahim T, Fusaro M, Seyfarth M, Laugwitz KL, Massberg S, Kastrati A. Zotarolimus- Versus Everolimus-Eluting Stents for Unprotected Left Main Coronary Artery Disease. J Am Coll Cardiol. 2013;62:2075–2082. doi: 10.1016/j.jacc.2013.07.044. [DOI] [PubMed] [Google Scholar]
- 21.Piccolo R, Stefanini GG, Franzone A, Spitzer E, Blöchlinger S, Heg D, Jüni P, Windecker S. Safety and efficacy of resolute zotarolimuseluting stents compared with everolimus-eluting stents: a meta-analysis. Circ Cardiovasc Interv. 2015;8:e002223. doi: 10.1161/CIRCINTERVENTIONS.114.002223. [DOI] [PubMed] [Google Scholar]
- 22.Baber U, Mehran R, Sharma SK, Brar S, Yu J, Suh JW, Kim HS, Park SJ, Kastrati A, de Waha A, Krishnan P, Moreno P, Sweeny J, Kim MC, Suleman J, Pyo R, Wiley J, Kovacic J, Kini AS, Dangas GD. Impact of the everolimus-eluting stent on stent thrombosis: a meta-analysis of 13 randomized trials. J Am Coll Cardiol. 2011;58:1569–1577. doi: 10.1016/j.jacc.2011.06.049. [DOI] [PubMed] [Google Scholar]


