Abstract
Objective: The traditional Chinese medicinal puerarin, has long been used to treat cardiovascular diseases, however, the mechanism underlying its effects remain unclear. Here, this study would to investigate the role of puerarin on cardiac angiogenesis and myocardial function induced by myocardial infarction. Methods: Puerarin was treated in rats after left anterior descending coronary artery (LAD) ligation and maintained for 4 weeks (diets containing about 50 mg/kg/day or 100 mg/kg/day). After treatment, cardiac function was evaluated by echocardiography and markers of heart failure. Paraffin sections of the heart tissues were used for isolect in GS-IB4 staining. The Mrna and protein expression levels of VEGFA, Ang-1 and Ang-2 were detected by real-time polymerase chain reaction and western blot. Results: Significantly damaged angiogenesis and slightly increase of VEGFA, Ang-1 and Ang-2 were showed after LAD ligation. Impaired angiogenesis and cardiac function were remarkably improved in puerarin treatment rats with great increase of VEGFA, Ang-1 and Ang-2. Conclusion: The above results demonstrated that puerarin could accelerate cardiac angiogenesis and improve cardiac function of myocardial infarction rats by upregulating VEGFA, Ang-1 and Ang-2.
Keywords: Puerarin, cardiac angiogenesis, cardiac function, VEGFA, angiopoietin
Introduction
Coronary heart disease (CHD) is one of the contemporary senile diseases, and also is the important cause of death in the elderly [1]. As the development of coronary artery recanalization such as coronary artery stent, ischemic myocardium of the patients with severe myocardial infarction (MI) obtains a lot of benefits [2]. However, the rate of postoperative restenosis rate is greatly high, which limits wide development of coronary recanalization surgery. In recent years, drug-coated stents and novel anticoagulant drugs are widely used, which has significantly reducedthe rate of restenosis after coronary stent, but some foreign large-scale clinical trials show that restenosis rate of drug-coated stent is still around 5% [3,4]. In addition, with the results of BRAVE-2 and TOSCA-2, it is found that drug-coated stents play slight role in late mortality in patients with MI; showed by COURAGE, there is no difference in advantage for stable angina pectoris patients between drug therapy and drug-coated stents [5-8]. Therefore, it is particularly important to promote the formation of collateral circulation of myocardial ischemia region and angiogenesis in the current strategy of CHD. At this point, therapeutic angiogenesis has become a new target for the treatment of CHD. Chinese traditional drug, puerarin as vasodilation drugs, isisolated from the root of kudzu leguminous plant, are commonly used in the clinic for the treatment of myocardial ischemia, cerebral ischemia, dynamic and retinal vein occlusion [9-12]. However, the function of puerarin on left anterior descending coronary artery (LAD) ligation-induced cardiac damage is still unknown. The vascular endothelial growth factor A (VEGFA), angiopoietin-1 (Ang-1) and angiopoietin-2 (Ang-2) are the most powerful and well-studied molecular factors in cardiac angiogenesis [13-16]. And, whether puerarin achieve the aim of treatment of ischemic diseases by promoting new angiogenesis? However, development of a successful therapeutic strategy requires a detailed understanding of the molecular mechanisms involved in coronary angiogenesis, which must rely on extensive bench studies. Therefore, we establish MI model, from the angle of promoting angiogenesis, to identify function of puerarin on LAD ligation-induced cardiac damage and whether it could improve the levels of proangiogenic factors such as VEGFA, Ang-1 and Ang-2, which would be the critical point in this study.
Materials and methods
Materials
Primary antibody against VEGFA (ab51745) was obtained from Abcam (Cambridge, MA, USA). Primary antibodies against Ang-1 (ab118380) and Ang-2 (ab155106) were purchased from Abcam. GAPDH (#MB001) was got from Bioworld Technology (St. Louis Park, MN, USA). TRIZol (#15596018) was obtained from Life Technologies (Invitrogen, NY, USA). The bicinchoninic acid protein assay kit was bought from Pierce Biotechnology, Inc. (Rockford, IL, USA). Isolectin GS-IB4 staining (I21411) was got from Life Technologies (Invitrogen, NY, USA).
Experimental groups
This study was approved by the ethics committee of the Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology (Hubei, China). In the present study, the male Sprague-dawley (SD) rats were divided into five groups: the Sham group without any treatment (Sham); the MI group with LAD ligation (MI); the MI+S group treated with Saline after LAD ligation (MI+S); the MI+L group treated with puerarin at low dose (diets containing about 50 mg/kg/day) after LAD ligation (MI+L); the MI+H group treated with Puerarin at high dose (diets containing about 100 mg/kg/day) after LAD ligation (MI+H).
Left anterior descending coronary artery (LAD) ligation and puerarin treatment
The male SDrats (180-200 mg) were randomized to undergo LAD ligation or Sham surgery. All rats were anaesthetized with 3% pentobarbital sodium (40 mg/kg) by intraperitoneal injection and 1 ml of 1% lidocaine by local injection. Briefly, thoracotomy was performed via the third left intercostal and then the pericardium was torn open. The LAD was ligated by 6-0 polypropylene. It indicated successful ligation when the anterior wall of the left ventricle became visible blanching and swelling of the left atrium became hypokinesis. In Sham group, the suture was passed through the myocardium beneath but the LAD without ligation. All of the LAD ligation-induced cardiac damage lasted for 4 weeks. For the treatment groups, rats were treated with two doses of puerarin (diets containing about 50 mg/kg/day or 100 mg/kg/day) for 4 weeks after LAD ligation. MI+S group rats were received LAD ligation and normal saline.
Assessments of myocardial function
After 4 weeks treatment, myocardial function was evaluated by an echocardiograph equipped with a 15-MHz probe (Acuson SequoiaTM 512 ultrasound system, Siemens Medical Solution USA, Mountain View, CA, USA). Left ventricularend-systolic interior dimension (LVIDs), left ventricular enddiastolic interior dimension (LVIDd), left ventricula rejection fraction (LVEF) and left ventricular fractional shortening (LVFS) were measured in the present study. In addition, we detected the markers of heart failure including atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) in different groups.
Isolectin GS-IB4 staining
The hearts were harvested and arrested in diastole phase. After the processes of fixing, dehydration and embedding, to measure the levels of cardiac angiogenesis in different groups, paraffin sections of the whole heart were used for isolectin GS-IB4 staining according to the manufacturer’s instructions (Invitrogen, NY, USA). Pictures of isolectin GS-IB4 staining were taken under the microscope objective.
Real-time polymerase chain reaction (RT-PCR)
To evaluate the levels of VEGFA, Ang-1 and Ang-2, real-time polymerase chain reaction (RT-PCR) analysis was used to detect the mRNA levels of the above factors. Briefly, total mRNA was extracted from myocardium tissue using TRIZol, chloroform, isopropanol and 75% DEPC-ethanol. Synthesized cDNA was performed by using oligo (dT) primers with the Transcriptor First Strand cDNA Synthesis Kit. Selected gene differences were measured by quantitative PCR using SYBR green and normalized against gene expression of GAPDH. The sequences of primers in the present study were showed as follows: ANP: 5’-TTCAGACCTCCCGAAGCTAC-3’ and 5’-GCTCCAAGGTGCTGATATCT-3’; BNP: 5’-AGTTGTTACATCGTGTTGTGGA-3’ and 5’-CATCATTCTCGCTGGGAAGC-3’; VEGFA: 5’-TTGTTCAGAGCGGAGAAAGC-3’ and 5’-TTTAACTCAAGCTGCCTCGC-3’; Ang-1: 5’-TGATGGACTGGGAAGGGAAC-3’ and 5’-AGCGTCCTTTGTGCTGAAAT-3’; Ang-2: 5’-ACGGCTGTGATGATCGAGAT-3’ and 5’-GGTCTGGTCCAAAATCTGCTG-3’; GAPDH: 5’-ATGGTGAAGGTCGGTGTGAA-3’ and 5’-TGACTGTGCCGTTGAACTTG-3’.
Western blot
Total protein was extracted from the heart tissue and protein concentration was qualified by bicinchoninic acid protein assay kit. Protein was lysed in radio immunoprecipitation assay lysis buffer and then used for SDS-PAGE electrophoresis. The proteins were subsequently transferred to polyvinylidene fluoride membranes and blocked with 5% nonfat dry milk in Tris-buffered saline with Tween 20 (TBST) at room temperature. Membranes were respectively probed with primary antibodies including VEGFA, Ang-1 Ang-2 and GAPDH overnight. Next day, the membranes were washed with 1XTBST, and respectively incubated with corresponding 2nd antibodies (Cell Signaling Technology, Inc.). Following several washes, each image was captured on film, which was placed in LumiGLO® solution (Cell Signaling Technology, Inc.) for one minute. Following development, the images were placed into an automatic image analyzer (Bio-Rad Laboratories, Hercules, CA, USA) to determine the quantity of the proteins as well as the reference grayscale values. A monoclonal GAPDH antibody was used as a loading control and normalized standard.
Statistical analysis
Values are expressed as the mean ± standard error of the mean (SEM). One-way analysis of variance was used for comparisons following by LSD (equal variances assumed) or Tamhane’s T2 (equal variances not assumed) using SPSS version 13.0 software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered as a statistically significant difference.
Results
Puerarin improves myocardial function of ratsin response to LAD ligation
LAD ligation challenge resulted in dilated left ventricular chambers and significant decrease in myocardial function as measured by echocardio graphic analyses. Compared with Sham group, the LVIDs increased from 5.11 mm to 8.85 mm, the LVIDd increased from 7.29 mm to 10.51 mm, LVEF decreased from 81.62% to 33.28%, LVFS decreased from 40.52% to 21.82%, indicating that MIrats developed heart failure 4 weeks after LAD ligation challenge (Table 1). Exhilaratingly, the MI+L and MI+H groups displayed smaller ventricular chambers and greater cardiac function than MI group, which were evaluated by increases of LVIDs, LVIDd, LVEF and LVFS compared to MI rats (Table 1). Consistent with the results of echocardio graphic analyses, it was found that the markers of heart failure including ANP and BNP were decreased in puerarin treatment groups (Figure 1). The above results indicated that puerarin could improve myocardial function of rats in response to myocardial infarction challenge.
Table 1.
Echocardiography results for each group
| Sham | MI | MI+S | MI+L | MI+H | |
|---|---|---|---|---|---|
| LVIDs (mm) | 5.11±0.45 | 8.85±0.38 | 8.67±0.29 | 5.21±0.14 | 5.38±0.35 |
| LVIDd (mm) | 7.29±0.50 | 10.51±0.68 | 10.37±0.74 | 8.53±0.46 | 8.70±0.38 |
| EF (%) | 81.62±2.59 | 33.28±3.65 | 33.38±3.98 | 49.59±4.92 | 49.32±2.67 |
| FS (%) | 40.52±4.00 | 21.82±4.84 | 22.57±3.48 | 32.64±4.22 | 32.12±4.20 |
Figure 1.
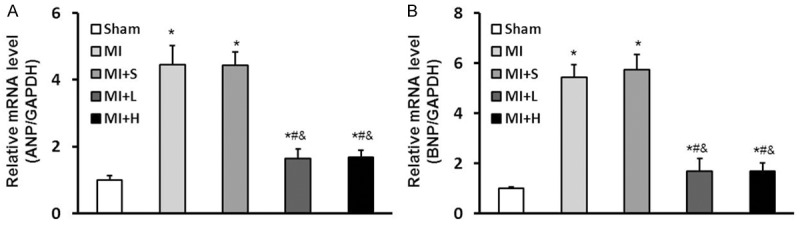
Puerarin inhibitsexpressions of markers of heart failure including ANP and BNP. A: The mRNA level of ANP increased in MI and MI+S groups, but the MI+L and MI+H groups displayed less expression than MI/MI+S group. B: The mRNA level of BNP significantly increased in MI and MI+S groups 4 weeks after LAD ligation surgery. However, the expression of BNP was inhibited in MI+L and MI+H groups compared to MI and MI+S groupsrats. Data are presented as the mean ± standard deviation. *P<0.05 vs. Sham group, #P<0.05 vs. MI group, &P<0.05 vs. MI+S group.
Puerarin improves damaged angiogenesis after LAD ligation challenge
In order to observe whether puerarin play a role on cardiac angiogenesis, we performed isolect in GS-IB4 staining in the present study. Compared to Sham group, significantly fewer capillary number sand smaller capillary areas were showed in the hearts of MI rats. Compensatory increases in the capillary area and capillary number were found in puerarin treatment rats in response to myocardial infarction stress (Figure 2). These results strongly indicated that puerarin could improves everely damaged coronary angiogenes is in response to LAD ligation.
Figure 2.
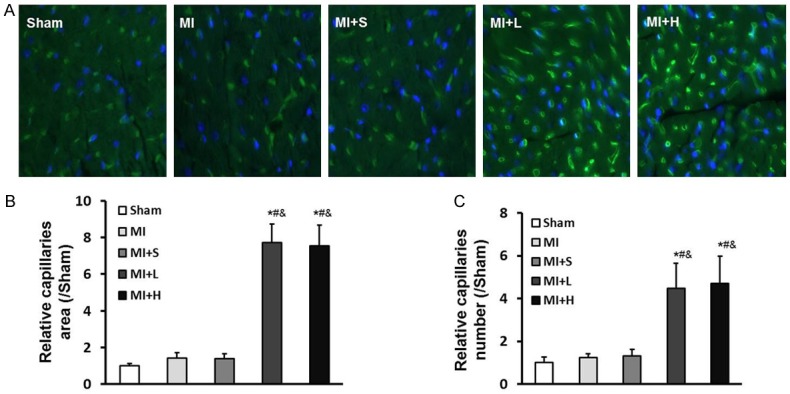
Puerarinimproves damaged cardiac angiogenesis in response to LAD ligation challenge. A: Fewer capillaries were observed in the post-MI heart byisolectin staining shown in green. Nuclear DAPI staining was shown in blue. However, the MI+L and MI+H groups displayed great improvement in capillary number compared to MI or MI+S group rats. B, C: Cardiac capillaries were quantified. Smaller capillary areas and fewer capillary numbers were observed in the MI and MI+S groups compared to the puerarin treatment groups after LAD ligation. Data are presented as the mean ± standard deviation. *P<0.05 vs. Sham group, #P<0.05 vs. MI group, &P<0.05 vs. MI+S group.
Puerarinupregulates key proangiogenic factorsVEGFA, Ang-1 and Ang-2 in response to LAD ligation stress
VEGFA and its downstream targets Ang-1 and Ang-2 are critical for cardiac angiogenesis in response to ischemic stress. Therefore, we measured the expression levels of these factors in every group to better understand the role of puerarin oncardiac angiogenesis. Consistent with impaired cardiac angiogenesis after stress, the mRNA and protein levels of VEGFA, Ang-1 and Ang-2 were lightly increased in MIand MI+S groups compared to the Shamgroup. However, remarkably increase of VEGFA, Ang-1 and Ang-2 were showed in MI+L and MI+H groups after LAD ligation challenge (Figure 3). These data suggested that puerarin could upregulate key proangiogenic factors VEGFA, Ang-1 and Ang-2 in response to LAD ligation stress.
Figure 3.
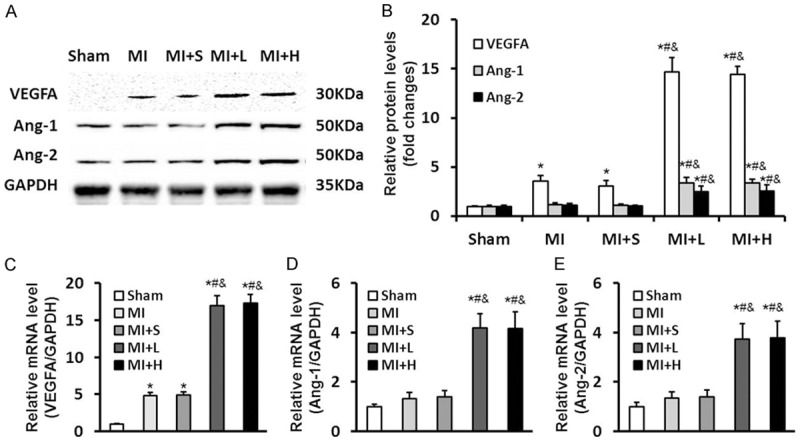
Puerarin upregulates key proangiogenic factors VEGFA, Ang-1 and Ang-2 in response to LAD ligation stress. A: Slightly increase of VEGFA, Ang-1 and Ang-2 protein in the MI and MI+S groups in response to MI was detected by western blot. However, protein expression of VEGFA, Ang-1 and Ang-2 were greatly increased in the MI+L and MI+H groups. B: Quantitative results for western blot ofVEGFA, Ang-1 and Ang-2. C-E: The mRNA levels of VEGFA, Ang-1 and Ang-2 in the rats following Sham or LAD ligation surgery with or without puerarin treatment. The puerarin treatment groups displayed great increase in mRNA levels of VEGFA, Ang-1 and Ang-2 compared to MI or MI+S groups. Data are presented as the mean ± standard deviation. *P<0.05 vs. Sham group, #P<0.05 vs. MI group, &P<0.05 vs. MI+S group.
Discussion
Coronary heart disease is a kind of cardiovascular disease, which is due to stenosis or obstruction of coronary artery (fixitylesions including atherosclerosis, or dynamic lesions such as vasospasm), could induce coronary circulation disorder, and then cause imbalances of myocardial oxygen supply and demand and lead to a lack of myocardial ischemia or necrosis, also known as ischemic heart disease [17,18]. In spite of remarkably development in the preventive health care, cardiovascular medication and cardiac surgery, the incidence and mortality of coronary heart disease remain very high. Therefore, abnormal myocardial circulation and perfusion in ischemic heart disease play a vital role in the development and/or transition from coronary heart disease to heart failure. It was proved that there was a close relationship between depressed cardiac perfusion and damaged cardiac angiogenesis in the myocardium of ischemic heart disease patients [19-21]. Thus it can be seen improvement of coronary microvasculature and acceleration of cardiac angiogenesis might be an important therapeutic target for the treatment of heart failure induced by ischemic heart disease.
The traditional Chinese medicine, puerarin is a kind of flavonoids, which is extracted from the root of a leguminous plant known as puerarin lobata or the root of dried kudzu vine (puerarinthomsonii) [22]. Since 1992, more and more researchers committed to study the functions of puerarin. With the progress of medicines and health products, puerarin has been developed as injection, capsules, tablets, and eye drops or other agents, which is widely used in clinical application to treat coronary heart disease, arrhythmia, hypertension, retinal vein occlusion, sudden deafness, high cholesterol and high blood glucose and shows remarkable curative effect [23,24]. However, the specific mechanism of puerarin in treating coronary heart disease is still not very clear. In the present study, it is showed that puerarin could improve myocardial function of rats with myocardial infarction, and the application of puerarin playsthe protective roleon cardiac angiogenes is in response to LAD ligation challenge. More importantly, we demonstrated that puerarin functions as a new mediator in the process of compensatory angiogenesis by upregulating the expressions of key proangiogenic factors VEGFA, Ang-1 and Ang-2 during cardiac stress induced by myocardial infarction.
Establishment of coronary collateral circulation includes the formation of original collateral and the formation of new branch. In recent years, it was confirmed the mRNA levels of VEGF and its receptor was increased significantly in myocardial ischemia and hypoxia, suggesting that VEGF is closely related to the formation coronary collateral circulation. VEGF can specifically act on vascular endothelial cells and promote its mitotic division, which can promote angiogenesis in vitro; and also it can effectively promote the establishment of collateral circulation in animals. Although the existence of spontaneous formation of collateral circulation after myocardial ischemia or infarction, the compensatory enhancement of angiogenesis in response to these pathological stimuli appears to be insufficient, and this insufficiency is one of the critical factors responsible for the transition of the heart to heart failure [25]. Exhilaratingly, our research has given the evidence that puerarin can promote the opening and formation of coronary collateral circulation, form abundant collateral circulation to improve the damaged cardiacangiogenesis, a process of sprouting new capillaries, is an adaptive response to myocardial infarction, and this role may be related to upregulation of VEGFA expression and further promotion of Ang-1 and Ang-2 expression.
In conclusion, we revealed a critical role of puerarin in the responsive cardiac angiogenesis with myocardial infarction, which restrain the transition of the heart to heart failure. These findings in the present study will provide the basic and clinical evidence and value in clinical treatment of coronary heart disease. Nevertheless, after ischemia and hypoxia, it can lead toa series of pathophysiological reactions including the release of inflammatory factors, apoptosis and necrosis of cardiomyocytes, collagen fibers accumulation of myocardial interstitial etc. Therefore, it could be studied whether and how puerarin could inhibit cardiac apoptosis, inflammation response and cardiac remodeling in future, which will further clarify the function and mechanism of puerarin on coronary heart disease and so as to lay the theoretical foundation for clinical treatment of myocardial infarction.
Acknowledgements
The authors would like to thank all members of the Department of Emergency (The Central Hospital of Wuhan, Wuhan, China) for their expert technical assistance and advice. The present study was supported by grants from the Natural Science Foundation of Hubei Province (No. 2012FFA107).
Disclosure of conflict of interest
None.
References
- 1.Vefali H, Manda Y, Shirani J. Myocardial viability in coronary artery chronic total occlusion. Curr Cardiol Rep. 2015;17:552. doi: 10.1007/s11886-014-0552-x. [DOI] [PubMed] [Google Scholar]
- 2.Visconti G, Focaccio A, Donahue M, Briguori C. Elective versus deferred stenting following subintimal recanalization of coronary chronic total occlusions. Catheter Cardiovasc Interv. 2015;85:382–390. doi: 10.1002/ccd.25509. [DOI] [PubMed] [Google Scholar]
- 3.Sarode K, Spelber DA, Bhatt DL, Mohammad A, Prasad A, Brilakis ES, Banerjee S. Drug delivering technology for endovascular management of infrainguinal peripheral artery disease. JACC Cardiovasc Interv. 2014;7:827–839. doi: 10.1016/j.jcin.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 4.Mamuti W, Jiamali A, Rao F, Zhang W, Pei X, Ablimit A, Kelimu W, Zhang F. Drug-coated balloon angioplasty for drug-eluting stent restenosis: insight from randomized controlled trials. Ann Med. 2014;46:679–683. doi: 10.3109/07853890.2014.952329. [DOI] [PubMed] [Google Scholar]
- 5.Parodi G, Ndrepepa G, Kastrati A, Conti A, Mehilli J, Sciagra R, Schwaiger M, Antoniucci D, Schomig A Beyond 12 hours Reperfusion AlternatiVe Evaluation Trial Investigators. Ability of mechanical reperfusion to salvage myocardium in patients with acute myocardial infarction presenting beyond 12 hours after onset of symptoms. Am Heart J. 2006;152:1133–1139. doi: 10.1016/j.ahj.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Schomig A, Mehilli J, Antoniucci D, Ndrepepa G, Markwardt C, Di Pede F, Nekolla SG, Schlotterbeck K, Schuhlen H, Pache J, Seyfarth M, Martinoff S, Benzer W, Schmitt C, Dirschinger J, Schwaiger M, Kastrati A Beyond 12 hours Reperfusion AlternatiVe Evaluation Trial Investigators. Mechanical reperfusion in patients with acute myocardial infarction presenting more than 12 hours from symptom onset: a randomized controlled trial. JAMA. 2005;293:2865–2872. doi: 10.1001/jama.293.23.2865. [DOI] [PubMed] [Google Scholar]
- 7.Dzavik V, Buller CE, Devlin G, Carere RG, Mancini GB, Cantor WJ, Buszman PE, Rankin JM, Vozzi C, Ross JR, Forman S, Barton BA, Lamas AG, Hochman JS. Angiographic and clinical outcomes of drug-eluting versus bare metal stent deployment in the Occluded Artery Trial. Catheter Cardiovasc Interv. 2009;73:771–779. doi: 10.1002/ccd.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzavik V, Buller CE, Lamas GA, Rankin JM, Mancini GB, Cantor WJ, Carere RJ, Ross JR, Atchison D, Forman S, Thomas B, Buszman P, Vozzi C, Glanz A, Cohen EA, Meciar P, Devlin G, Mascette A, Sopko G, Knatterud GL, Hochman JS TOSCA-2 Investigators. Randomized trial of percutaneous coronary intervention for subacute infarct-related coronary artery occlusion to achieve long-term patency and improve ventricular function: the Total Occlusion Study of Canada (TOSCA)-2 trial. Circulation. 2006;114:2449–2457. doi: 10.1161/CIRCULATIONAHA.106.669432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng M, Song D, Luo Z, Lu Z, Wu Y, Wang W. Effect of puerarin on expression of Fas/FasL mRNA in pulmonary injury induced by ischemia-reperfusion in rabbits. Nat Prod Commun. 2015;10:253–256. [PubMed] [Google Scholar]
- 10.Liu J, Zhang H, Ji B, Cai S, Wang R, Zhou F, Yang J, Liu H. A diet formula of Puerariae radix, Lycium barbarum, Crataegus pinnatifida, and Polygonati rhizoma alleviates insulin resistance and hepatic steatosis in CD-1 mice and HepG2 cells. Food Funct. 2014;5:1038–1049. doi: 10.1039/c3fo60524h. [DOI] [PubMed] [Google Scholar]
- 11.Lu Q, Xiang DX, Yuan HY, Xiao Y, Yuan LQ, Li HB. Puerarin attenuates calcification of vascular smooth muscle cells. Am J Chin Med. 2014;42:337–347. doi: 10.1142/S0192415X14500220. [DOI] [PubMed] [Google Scholar]
- 12.Wu XD, Wang C, Zhang ZY, Fu Y, Liu FY, Liu XH. Puerarin attenuates cerebral damage by improving cerebral microcirculation in spontaneously hypertensive rats. Evid Based Complement Alternat Med. 2014;2014:408501. doi: 10.1155/2014/408501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chung HJ, Kim JT, Kim HJ, Kyung HW, Katila P, Lee JH, Yang TH, Yang YI, Lee SJ. Epicardial delivery of VEGF and cardiac stem cells guided by 3-dimensional PLLA mat enhancing cardiac regeneration and angiogenesis in acute myocardial infarction. J Control Release. 2015;205:218–230. doi: 10.1016/j.jconrel.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Zhao W, Zhao T, Chen Y, Sun Y. Angiotensin 1-7 promotes cardiac angiogenesis following infarction. Curr Vasc Pharmacol. 2015;13:37–42. doi: 10.2174/15701611113119990006. [DOI] [PubMed] [Google Scholar]
- 15.Daly KP, Seifert ME, Chandraker A, Zurakowski D, Nohria A, Givertz MM, Karumanchi SA, Briscoe DM. VEGF-C, VEGF-A and related angiogenesis factors as biomarkers of allograft vasculopathy in cardiac transplant recipients. J Heart Lung Transplant. 2013;32:120–128. doi: 10.1016/j.healun.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao T, Zhao W, Chen Y, Ahokas RA, Sun Y. Vascular endothelial growth factor (VEGF)-A: role on cardiac angiogenesis following myocardial infarction. Microvasc Res. 2010;80:188–194. doi: 10.1016/j.mvr.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagai M, Kario K. [Ischemic heart disease, heart failure, and their effects on cognitive function] . Nihon Rinsho. 2014;72:715–720. [PubMed] [Google Scholar]
- 18.Wong JM, Welles CC, Azarbal F, Whooley MA, Schiller NB, Turakhia MP. Relation of left atrial dysfunction to ischemic stroke in patients with coronary heart disease (from the heart and soul study) Am J Cardiol. 2014;113:1679–1684. doi: 10.1016/j.amjcard.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Sun L, Huan Y, Zhao H, Deng J. Application of bFGF and BDNF to improve angiogenesis and cardiac function. J Surg Res. 2006;136:85–91. doi: 10.1016/j.jss.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 20.Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, Peng H, Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677–1684. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 21.Westermann D, Schultheiss HP, Tschope C. New perspective on the tissue kallikreinkinin system in myocardial infarction: role of angiogenesis and cardiac regeneration. Int Immunopharmacol. 2008;8:148–154. doi: 10.1016/j.intimp.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Xu L, Zheng N, He Q, Li R, Zhang K, Liang T. Puerarin, isolated from Pueraria lobata (Willd. ), protects against hepatotoxicity via specific inhibition of the TGF-beta1/Smad signaling pathway, thereby leading to anti-fibrotic effect. Phytomedicine. 2013;20:1172–1179. doi: 10.1016/j.phymed.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Zhao Y, Gao E, Zhao X, Liu Z, Yu Z. Pharmacokinetic comparisons of puerarin, daidzin and the glucuronide metabolite of puerarin after administration of total flavonoid from Gegen alone and total flavonoid from Gegen combined with total saponin from Sanqi in rats under different physiological states. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;931:127–133. doi: 10.1016/j.jchromb.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Chen Y, Shan Y, Wang D, Zhu C, Xu Y. Effects of puerarin on cholinergic enzymes in the brain of ovariectomized guinea pigs. Int J Neurosci. 2013;123:783–791. doi: 10.3109/00207454.2013.803103. [DOI] [PubMed] [Google Scholar]
- 25.van Lessen M, Nakayama M, Kato K, Kim JM, Kaibuchi K, Adams RH. Regulation of Vascular Endothelial Growth Factor Receptor Function in Angiogenesis by Numb and Numb-Like. Arterioscler Thromb Vasc Biol. 2015;35:1815–1825. doi: 10.1161/ATVBAHA.115.305473. [DOI] [PubMed] [Google Scholar]


