Abstract
To observe the effects of different concentrations of sevoflurane on synaptotagmin 1 (Syt1) expression, synaptic long term depression (LTD), and paired pulse depression (PPD) in the rat hippocampus as well as to investigate the association between these effects and the cognitive function of rats. A total of 24 male Sprague-Dawley (SD) rats were selected and randomly divided into 3 groups: the control group (group A), which inhaled air; group B, which inhaled 0.65 minimum alveolar concentration (MAC) sevoflurane for 2 h; and group C, which inhaled 1.30 MAC sevoflurane for 2 h. The subsequent experiments were performed after one day. (1) Y maze tests were performed, and the expression of Syt1 in hippocampal tissues was detected using western blot. (2) The changes in LTD and PPD in rat hippocampal slices were examined using electrophysiological techniques. Compared to the control group, the cognitive function was decreased and Syt1 expression in the hippocampus was significantly decreased in rats in the 1.30 MAC sevoflurane inhalation group. After 60 min of low frequency stimulation, the amplitudes of population spike (PS) potentials in rat hippocampal slices were significantly decreased. After induction of PPD, the P2/P1 ratio was significantly increased. No indicators in the 0.65 MAC sevoflurane inhalation group showed any significant changes. Inhalation of high concentrations of sevoflurane significantly reduced Syt1 protein levels in the rat hippocampus, significantly inhibited the release of presynaptic neurotransmitters, and reduced the efficiency of synaptic transmission, thus causing memory impairment.
Keywords: Sevoflurane, hippocampus, synaptotagmin 1 (Syt1), cognitive function, synaptic transmission
Introduction
Sevoflurane is the most extensively applied inhalational anesthetic. Its advantages include a low blood-gas partition coefficient, rapid anesthesia induction, ease of depth control, and fast and complete recovery [1,2]. Some studies have shown that sevoflurane causes early cognitive impairment of patients after surgery, albeit the degree was milder than other systemic fluorinated inhalation anesthetics. Some animal studies have shown that young rats that inhaled a certain concentration of sevoflurane developed cognitive impairment in adulthood [3,4]. Studies of the decline of learning and memory caused by sevoflurane inhalation have received increasing amounts of attention.
The synaptic circuits and their integration in the hippocampal structure are very important for all types of learning and memory functions [5]. Brain functions such as learning and memory are closely associated with synaptic plasticity, which requires normal synaptic transmission and normal release of neurotransmitters [6]. The release of neurotransmitters involved in the formation of synaptic plasticity requires the involvement of many synaptic proteins. The synaptotagmin proteins, which are closely associated with the release of neurotransmitters, have received widespread attention [7]. The synaptotagmin (Syt) family members are currently the most important Ca2+ sensors in the regulation of membrane fusion; of which, syt-1 is considered a major rapid phase Ca2+ sensor during the process of synaptic vesicle exocytosis and is an important synaptic protein associated with the decline of learning and memory abilities [8].
Syt1 is an important synaptic protein that is associated with the decline of learning and memory abilities; however, whether sevoflurane inhalation can affect Syt1 expression to cause changes in the efficiency of synaptic transmission that result in cognitive function impairment has not been reported. Therefore, this study used the concentrations and times commonly used in clinical situations to observe the effect of sevoflurane on the cognitive function, degree of inhibition of hippocampal synaptic transmission, and changes in Syt1 expression in rats to investigate the possible mechanism underlying the development of postoperative cognitive dysfunction (POCD).
Materials and methods
Experimental animals and grouping
After the experimental protocol was approved by the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication No. 85-23, revised 1985) and approval by the Ethics Committee of our hospital was obtained, a total of 24 clean-grade 8-week-old male Sprague-Dawley (SD) rats with a body weight of 300-350 g were selected. The rats were provided by the Shanghai SLAC Laboratory Animal Co., Ltd., and the animal license number was SCXK (Hu) 2012-0002. The rats were randomly divided into three groups: the control group (group A), the 0.65 minimum alveolar concentration (MAC) sevoflurane group (group B), and the 1.30 MAC sevoflurane group (group C). Each group consisted of eight animals. The rats were first adapted in the animal room for one week and had access to food and water ad libitum. The light/dark cycle was 10/14 h (lighting starting at 7:30 am), the temperature was 22°C, and the humidity was 60%-80%.
Major instruments and reagents
The BX45 microscope and imaging system were obtained from Olympus. The anesthesia machine was obtained from Drager. The S/5 multi-functional monitor was obtained from Datex-Ohmeda. Sevoflurane was obtained from Abbott (USA). The Y maze was obtained from Zhangjiagang Biomedical Instruments. Rabbit anti-mouse Syt-1 and bovine serum albumin were obtained from Sigma Company. Secondary antibodies, NBT/BCIP chromogenic substrate and BCA protein analytical reagent were obtained from Nantong Biyuntian Company. The MEZ-8301 microelectrode amplifier, SEN-3301 stimulator, SS-202J isolator, ZQP-86 vibrating microtome, and HXD-2000 electric signal processing analysis software were provided by Beijing Huaxiang Company.
Anesthetic management
Rats in group A inhaled air, and rats in other groups were placed in a homemade acrylic glass anesthesia box. An anesthesia machine was used to provide sevoflurane for inhalation by the rats. The oxygen flow was 4 l/min. After the level of sevoflurane in the box was stabilized at 1.5% or 3%, as detected by a monitoring machine, the rats were anesthetized in the anesthesia box for two hours. During the inhalation process, the sevoflurane concentration and oxygen saturation in rats (maintained at 99%-100%) were monitored using a multi-functional monitor. All rats in group B awoke within two minutes, and all rats in group C awoke within five minutes; all rats resumed drinking and eating within one hour.
Behavioral tests
One day after anesthesia, rats in groups B and C received learning and memory training in a Y maze [8], and rats in group A received learning and memory training at the same time as the other groups. The learning and memory training was tested using a Y maze in a quiet dark room. The bottom of the maze box consisted of a copper grid, and one arm contained a signal light. The copper grid at the bottom of the box and the arm with a light did not have current. The second and the third arms without a light were connected to an electric current generator. Before the test, the rats were adapted in the maze for five minutes. During the test, the stimulation voltage was adjusted to ensure that rats escaped and ran within ten seconds. A correct reaction was recorded when the rats ran directly to the safe area after an electric shock was administered; otherwise the response was considered an incorrect reaction. Each time that a rat ran to the safe area, the light was turned on continuously for 15 s, followed by turning off the light for 30 s before the next test. The location in which the light was present varied according to the order I→II→III→I. The learning standard was nine correct reactions in ten continuous tests. The observations included ① the total number of times that the learning standard was achieved and ② the total training time needed to achieve the learning standard.
Specimen collection
After training, the rats in each group were anesthetized by peritoneal injection of 10% chloral hydrate. The rats were decapitated, and the whole brains were collected via a craniotomy. The brains were placed in artificial cerebrospinal fluid (ACSF), and the bilateral hippocampi were dissected on frozen ACSF. The samples stored in a -80°C refrigerator for future usage.
Western blot
Brain tissues were weighed on a balance, and approximately 60 mg of the tissue samples was transferred to Eppendorf tubes. Radioimmunoprecipitation assay (RIPA) lysis buffer was added to the tissue samples at a ratio of 1 mg sample/10 µL lysis buffer. The tissues were homogenized with a tissue homogenizer and lysed in an ice bath for 45 min. Subsequently, the lysates were centrifuged at 12,000 rpm for 10 min. The resulting supernatant was collected, and the total protein concentration in the supernatant was determined using the bicinchoninic acid (BCA) protein assay. The protein samples were mixed thoroughly with an equal volume of 2× sample loading buffer, boiled for 3 min, and stored at -80°C for future assays.
The obtained protein samples were subjected to western blot analysis according to the instruction manual of the reagents. The gels have been run under the same experimental conditions. The procedure is briefly summarized below. The separating gel and stacking gel were prepared. The protein samples were diluted by adding an equal volume of 2× sample loading buffer and placed in boiling water for 10 min (the prestained marker was boiled for 5 min). Sample loading: Approximately 10-20 μL of protein samples was added to each well of the gel. Electrophoresis: The gel was placed in the electrophoresis tank, and the protein samples were electrophoresed for 45 min at a constant voltage of 130 v. The gel plate was removed from the electrophoresis tank, and the gel was trimmed with a cutting knife. The gel was then immersed and equilibrated in the transfer buffer. The transfer sandwich was assembled with the materials listed below in the following order: black side of the transfer cartridge→sponge→filter paper→gel→nitrocellulose (NC) membrane→filter paper→sponge. The transfer stack was then placed in a transfer apparatus (black side of the cartridge was placed toward the black side of apparatus). Ice and transfer buffer were added to the transfer apparatus. After the electrodes were correctly connected, the proteins were transferred for 90 min at a constant current of 250 mA. Blocking: The membrane was blocked with 5% nonfat dry milk in Tris-Buffered Saline/Tween 20 (TBST) at room temperature for 1 h on a shaker. The blocking solution was then removed, and the membrane was incubated with the primary antibodies (rabbit anti-mouse Syt-1; 1:200 dilution) at 4°C overnight on a shaker. The primary antibodies were collected, and the membrane was washed three times in TBST (10 min each wash). The membrane was then incubated with the secondary antibodies at room temperature for 1 h on a shaker. The secondary antibodies were removed, and the membrane was washed three times in TBST (10 min each wash). The membrane was then incubated with the chemiluminescence solution for 5 min. The membrane was placed in a film exposure box and exposed to light-sensitive films in a darkroom. The film was then developed. β-Actin was used as an internal reference (experimental procedure was the same as described above). The relative optical density (ROD) values of the Syt1 protein bands in each lane were determined using Gel-Pro Analyzer 4.0.
Preparation of in vitro hippocampal slices
One day after anesthesia was administered, the rats were decapitated, and the brains were collected. The samples were placed in ACSF saturated with 95% O2-5% CO2 at 0-4°C. The blood was washed out, and the temperature was reduced to remove the hippocampi. Three to four hippocampal slices were sectioned perpendicularly to the long axis of the hippocampus using a vibrating microtome at a thickness of 400 μm. Hippocampal slices were incubated in ACSF at 32.0±0.5°C for two to three hours, and 95% O2-5% CO2 was continuously supplied. After incubation, the brain slices were completely immersed in a 32.0±0.5°C thermostat perfusion tank. The liquid surface was 2 mm higher than the surface of the slices, and ACSF saturated with 95% O2-5% CO2 was continuously supplied (gas flow 200 ml·min-1). The perfusion speed of ACSF was 1.5-2 ml·min-1. The components of the ACSF included (mmol·l-1) NaCl (124), KCl (3.3), NaH2PO4 (1.24), MgSO4 (2.4), NaHCO3 (25.7), CaCl2 (2.4), and glucose (10.0) at pH 7.35-7.45.
Electrophysiology
Under a dissection microscope, a bipolar stimulating electrode was placed on a Schaffer collateral from the CA3 pyramidal cell region, and a glass microelectrode (filled with 2 mmol·l-1 NaCl solution with an impedance of 2-10 MΩ) was placed in the CA1 pyramidal cell layer to record the population spike (PS) potentials induced by electrical stimulation. The stimulation was first tested before the experiment. The stimulation parameter was a single pulse with a 0.15-ms pulse width and a stimulation strength that could induce approximately 60% of the maximum response of the PS (the stimulation strength that induced the maximum re-sponse of the PS was the maximum stimulation strength). When the PS had been stabilized for 10-15 min, low frequency stimulation (LFS, 900 pulse stimulation, 1 Hz) was used to induce long term depression (LTD). The recording time was at least 60 min for the entire experimental process. After the potential was amplified by a microelectrode amplifier, the results were stored on a computer, and analysis and processing were performed. Paired pulse depression (PPD) was measured by the ratio of the second PS amplitude (P2) to the first PS amplitude (P1) after administration of a paired pulse at a certain interval (20-150 ms); an induced PS amplitude ratio (P2/P1) after stimulation smaller than 1 was considered PPD.
Statistical analysis
All analyses were performed with SPSS19.0 statistical software. Experimental data are presented as X̅ ± s. The indicators of the cognitive function of each group, changes in PS amplitude, and the expression of Syt1 proteins were analyzed using one-way analysis of variance (ANOVA). Multiple analyses were performed using least-significant difference for post hoc analysis. A value of P<0.05 indicated a statistically significant difference.
Results
The oxygen saturation (SpO2) and heart rate of each group did not show obvious variations (Table 1).
Table 1.
The oxygen saturation (SpO2) and heart rate of each group (X̅±s)
| Group | Number of cases | SpO2 | Heart rate |
|---|---|---|---|
| A | 8 | 98.3±1.6 | 383.8±16.4 |
| B | 8 | 97.8±2.1 | 379.5±14.7 |
| C | 8 | 97.4±2.3 | 372.4±15.3 |
Behavioral results
The learning and memory training results in the Y maze showed that in group C, the time to reach the correct location and the training times were increased compared to those in the group A, and the differences were statistically significant (P<0.01). There were not significantly different between the groups B and A (P>0.05), but the different was also significantly between the groups B and C (P<0.01) (Table 2).
Table 2.
Comparison of cognitive function and Syt1 expression in each group (X̅±s)
| Group | Number of cases | Training time (s) | Number of training sessions | Syt1 expression (relative density, %) |
|---|---|---|---|---|
| A | 8 | 2294.4±40.8 | 33.6±4.1 | 0.73±0.17 |
| B | 8 | 2373.1±12.9 | 34.6±3.5 | 0..69±0.15 |
| C | 8 | 2928.8±60.9**,## | 41.9±5.7**,## | 0.54±0.12*,# |
P<0.05 compared with group A;
P<0.01 compared with group A.
P<0.05 compared with group B;
P<0.05 compared with group B.
Western blot results
The protein band optical density analysis showed that Syt1 expression in the 3.0% sevoflurane group (group C) was significantly decreased compared to group A (P<0.05). Moreover the different was also significantly between the groups B and C (P<0.01). Syt1 expression in the hippocampi of group B and A rats were not significantly different (P>0.05). (Table 2 and Figure 1).
Figure 1.
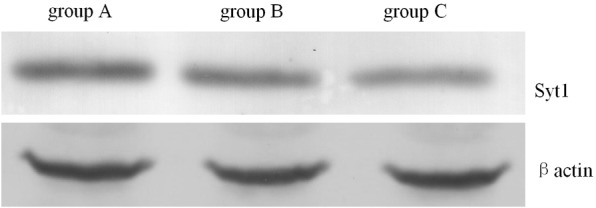
Expression of Syt-1 in each group determined by western blot.
LTD in hippocampal slices
The PS amplitude of brain slices before LFS was 100%. The PS amplitude after LFS in group A was significantly reduced; at 60 min, the amplitude was 78.46±13.5% of that before stimulation, which constituted LTD. At 60 min after LFS, the PS amplitude in group C was significantly reduced and was significantly different from that in group A (P<0.05). The PS amplitude in group B was not significantly different from that in group A or C (P>0.05) (Table 3 and Figure 2).
Table 3.
Comparison of electrophysiological changes in hippocampal slices in each group (X̅±s)
| Group | Number of cases | PS changes (%) | |
|---|---|---|---|
|
| |||
| PS amplitude after LTD induction | P2/P1 ratio after PPD induction | ||
| Group A | 8 | 78.46±13.5 | 60.82±3.4 |
| Group B | 8 | 72.92±11.1 | 64.35±4.2 |
| Group C | 8 | 61.15±12.3*,# | 72.92±6.6**,## |
P<0.05 compared with group A;
P<0.01 compared with group A.
P<0.05 compared with group B;
P<0.05 compared with group B.
Figure 2.
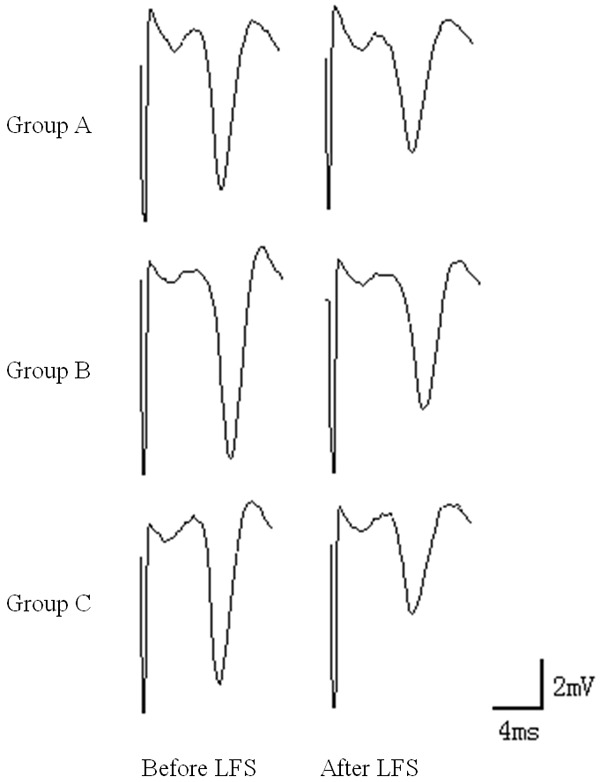
LTD in hippocampal slices. The PS amplitude after LFS in group A was significantly reduced; at 60 min, the amplitude was 78.46±13.5% of that before stimulation, which constituted LTD. At 60 min after LFS, the PS amplitude in group C was significantly reduced and was significantly different from that in group A (P<0.05).
PPD results in hippocampal slices
After PPD was induced by paired pulses in group A, the P2 amplitude was significantly lower than the P1 amplitude, and the P2/P1 ratio was 60.82±3.4%. After PPD induction in group C, the P2/P1 ratio was significantly increased and was significantly different from that in group A (P<0.01). The difference between groups B and A was not statistically significant (P>0.05), but the different was significantly between the groups B and C (P<0.01) (Table 3 and Figure 3).
Figure 3.
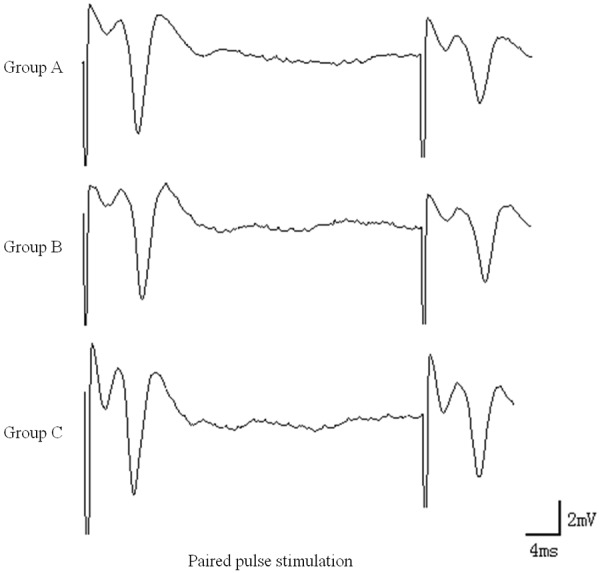
PPD results in hippocampal slices. After PPD induction in group C, the P2/P1 ratio was significantly increased and was significantly different from that in group A (P<0.01). The different was significantly between the groups B and C (P<0.01).
Discussion
Our study showed that the inhalation of 3.0% sevoflurane significantly decreased Syt1 protein levels in the rat hippocampus, inhibited the release of presynaptic transmitters, and decreased the efficiency of synaptic transmission, thus impairing memory function.
Studies have shown that the MAC of sevoflurane in rats is approximately 2.4% [9]. For routine clinical anesthesia, the 99% effective dose (ED99) is approximately 1.30 MAC, while 0.65 MAC is the commonly used dose [10]. Therefore, in this study, the selected values of the depth of sevoflurane were 1.5% and 3.0% [11]. The early effect of general anesthesia on cognitive function is mainly observed by the changes that occur on the first day after anesthesia. One day after the administration of sevoflurane anesthesia, the rats completed a spatial cognition task that they had learned before anesthesia. The results showed that rats that inhaled 1.30 MAC sevoflurane required more time to complete the task; however, 0.65 MAC sevoflurane inhalation had little effect on the cognitive function of rats. These results indicated that higher concentrations of sevoflurane had larger effects on the memory of rats and caused memory impairment.
The hippocampus is the anatomical center of learning and memory and is an important structure in the memory circuits of the brain; it is also an ideal model for examining the learning and memory functions of the brain [12]. LTD and long term potentiation (LTP) in the hippocampus are indispensable for learning and memory formation [13,14]. The induction of LTP can enhance learning and memory abilities, while LTD is generally associated with the integration and abolishment of learning and memory. The function of LTD is to prevent the oversaturation of LTP and to maintain the memory at a certain volume. However, facilitation of LTD will cause a reduction of the efficiency of synaptic transmission and impair memory functions [15]. In this study, after inhalation of 1.30 MAC sevoflurane, the PS amplitude of rat hippocampal brain slices was significantly reduced after LFS induction, suggesting that inhalation of high concentrations of sevoflurane could cause LTD facilitation in the rat hippocampus that resulted in the impairment of learning and memory function.
Syt1 is a membrane protein specific to small synaptic vesicles and large dense core vesicles in the brain. It is a key factor for membrane fusion during the neurotransmitter release induced by Ca2+. Syt1 binding with Ca2+ and regulation of membrane fusion play important roles in the release of presynaptic neurotransmitters [16,17]. Reports have shown that Syt1 influences synaptic plasticity via the regulation of neurotransmitter release, thus further influencing learning and memory [18]. The present study showed that inhalation of 1.30 MAC sevoflurane caused significant reduction of Syt1 expression in the rat hippocampus, suggesting that high concentrations of sevoflurane could reduce cognitive function via the inhibition of Syt1 expression in the hippocampus and the inhibition of presynaptic neurotransmitter release.
Paired pulse stimulation is one of the most commonly used methods to examine synaptic transmission. When the interval between two stimulations is short, synapses that have high probabilities of releasing neurotransmitters are prone to develop PPD [19,20]. In this study, we found that after the inhalation of 1.30 MAC sevoflurane, the P2/P1 amplitude ratio, which was inhibited by paired pulses in the CA1 region of the rat hippocampus under control conditions, was significantly increased. The P2/P1 ratio exhibited a negative correlation with the probability of transmitter release, demonstrating that when the release of the presynaptic transmitter vesicles decreases, the P2/P1 value increases. These results suggest that sevoflurane might directly decrease the stimulation-induced release of presynaptic membrane vesicles in the CA1 region, thus reducing the efficiency of synaptic transmission, which further confirms that sevoflurane could inhibit presynaptic transmitter release.
Due to the different stages of the physiological cycle in female rats, they exhibit more variable responses to the experimental conditions, which may allow changes in body weight and hormone levels to directly affect the reliability and repeatability of the experiments. Therefore, we used male SD rats for model establishment to eliminate sex effects. The preparation of hippocampal brain slices relied on incubation in ACSF to simulate the in vivo environment and reduce the influence of ischemia and hypoxia.
There were some limitations in this study. First, the sample size was small. Only eight rats were used in each group. Next, we only observed the effects of 1.5% and 3.0% sevoflurane on the cognitive function and did not observe the effects of other concentrations. Therefore, the lowest concentration of sevoflurane that could influence cognitive function was not determined. Finally, the sevoflurane exposure time was not further examined, and therefore, the effects of exposure time on the cognitive function were not determined.
Conclusion
Overall, the results of this study showed that inhalation of high concentrations of sevoflurane significantly reduced Syt1 protein levels in the rat hippocampus, inhibited presynaptic neurotransmitter release, and decreased the efficiency of synaptic transmission, thus resulting in memory impairment. The results also suggested that the reasonable selection of the inhalation concentration of sevoflurane in clinical anesthesia could reduce the incidence of POCD.
Disclosure of conflict of interest
None.
References
- 1.Andersen Y, Johansen JD, Garvey LH, Thyssen JP. Occupational airborne contact dermatitis caused by sevoflurane. Contact Dermatitis. 2015;72:241–243. doi: 10.1111/cod.12361. [DOI] [PubMed] [Google Scholar]
- 2.Green MS, Green P, Neubert L, Voralu K, Saththasivam P, Mychaskiw G. Recovery following desflurane versus sevoflurane anesthesia for outpatient urologic surgery in elderly females. Anesth Pain Med. 2015;5:e22271. doi: 10.5812/aapm.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fang F, Xue Z, Cang J. Sevoflurane exposure in 7-day-old rats affects neurogenesis, neurodegeneration and neurocognitive function. Neurosci Bull. 2012;28:499–508. doi: 10.1007/s12264-012-1260-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu B, Yu Z, You S, Zheng Y, Liu J, Gao Y, Lin H, Lian Q. Physiological disturbance may contribute to neurodegeneration induced by isoflurane or sevoflurane in 14 day old rats. PLoS One. 2014;9:e84622. doi: 10.1371/journal.pone.0084622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fortress AM, Frick KM. Hippocampal Wnt Signaling: Memory Regulation and Hormone Interactions. Neuroscientist. 2015 doi: 10.1177/1073858415574728. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Massobrio P, Tessadori J, Chiappalone M, Ghirardi M. Studies of Neuronal Networks and Synaptic Plasticity in Invertebrates and in Mammals Using Multielectrode Arrays. Neural Plast. 2015;2015:196195. doi: 10.1155/2015/196195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishimoto T, Kadoyama K, Taniguchi T, Takano M, Otani M, Nakamura-Hirota T, Lu Y, Matsumoto A, Matsuyama S. Synaptotagmin1 synthesis induced by synaptic plasticity in mouse hippocampus through activation of nicotinic acetylcholine receptors. Neurosci Lett. 2011;489:25–9. doi: 10.1016/j.neulet.2010.11.059. [DOI] [PubMed] [Google Scholar]
- 8.Liu CL, Xu YX, Zhan Y, Hu HL, Jia XM, Chen GH, Zhu DF. Effect of thyroxine on synaptotagmin 1 and SNAP-25 expression in dorsal hippocampus of adult-onset hypothyroid rats. J Endocrinol Invest. 2011;34:280–286. doi: 10.1007/BF03347086. [DOI] [PubMed] [Google Scholar]
- 9.Abreu M, Aguado D, Benito J, Gómez de Segura IA. Reduction of the sevoflurane minimum alveolar concentration induced by methadone, tramadol, butorphanol and morphine in rats. Lab Anim. 2012;46:200–206. doi: 10.1258/la.2012.010066. [DOI] [PubMed] [Google Scholar]
- 10.Gupta M, Shri I, Sakia P, Govil D. Comparison of equi-minimum alveolar concentration of sevoflurane and isoflurane on bispectral index values during both wash in and wash out phases: A prospective randomised study. Indian J Anaesth. 2015;59:79–84. doi: 10.4103/0019-5049.151363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng D, Fahimi J, Hern HG. Sevoflurane administration initiated out of the ED for life-threatening status asthmaticus. Am J Emerg Med. 2015;33:1110, e3–6. doi: 10.1016/j.ajem.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 12.Ryals AJ, Wang JX, Polnaszek KL, Voss JL. Hippocampal contribution to implicit configuration memory expressed via eye movements during scene exploration. Hippocampus. 2015;25:1028–41. doi: 10.1002/hipo.22425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nabavi S, Fox R, Proulx CD, Lin JY, Tsien RY, Malinow R. Engineering a memory with LTD and LTP. Nature. 2014;511:348–352. doi: 10.1038/nature13294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Antion MD, Nomura T, Kraniotis S, Zhu Y, Contractor A. Hippocampal metaplasticity is required for the formation of temporal associative memories. J Neurosci. 2014;34:16762–16773. doi: 10.1523/JNEUROSCI.2869-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JM, Jung SC, Eun SY. Long-term Synaptic Plasticity: Circuit Perturbation and Stabilization. Korean J Physiol Pharmacol. 2014;18:457–60. doi: 10.4196/kjpp.2014.18.6.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hueting R, Tavare R, Dilworth JR, Mullen GE. Copper-64 radiolabelling of the C2A domain of synaptotagmin I using a functionalised bis(thiosemicarbazone): A pre- and post-labelling comparison. J Inorg Biochem. 2013;128:108–111. doi: 10.1016/j.jinorgbio.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Y, Wang J, Wang Y, Ding M. Synaptotagmin 1 directs repetitive release by coupling vesicle exocytosis to the Rab3 cycle. Elife. 2015:4. doi: 10.7554/eLife.05118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue Y, Takayanagi M, Sugiyama H. Presynaptic protein synaptotagmin1 regulates the activity-induced remodeling of synaptic structures in cultured hippocampal neurons. J Neurosci Res. 2013;91:882–889. doi: 10.1002/jnr.23215. [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Jackson MF, MacDonald JF. Calciummediated paired pulse depression in juvenile rat dorsal striatum. Neural Regen Res. 2012;7:772–777. doi: 10.3969/j.issn.1673-5374.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirischuk S, Clements JD, Grantyn R. Presynaptic and postsynaptic mechanisms underlie paired pulse depression at single GABAergic boutons in rat collicular cultures. J Physiol. 2002;15:99–116. doi: 10.1113/jphysiol.2002.021576. [DOI] [PMC free article] [PubMed] [Google Scholar]


