Abstract
Objectives: The aim of this study was to assess the effects of FibroScan, aspartate aminotransferase and alanine aminotransferase ratio (AAR), aspartate aminotransferase to platelet ratio index (APRI), fibrosis index based on the 4 factor (FIB-4) and their combinations on liver fibrosis in patients with hepatitis B. Methods: 406 hospitalized patients with chronic hepatitis B (CHB) and cirrhosis in our hospital were analyzed retrospectively and collected patients clinical indicators, including liver stiffness (LS), AAR, APRI and FIB-4, and then compared the differences of these indicators between CHB group and hepatitis B with cirrhosis group. Receiver operating curve (ROC) was used to evaluate the differentiating capacity of these indicators on CHB and liver cirrhosis. Results: Four indicators related to liver cirrhosis had a statistical significance between two groups (P < 0.01); the under ROC curve areas of LS, AAR, APRI and FIB-4 for differential diagnosis of CHB and liver cirrhosis were 0.866, 0.772, 0.632 and 0.885, respectively. The under ROC curve areas of LS, AAR, APRI and FIB-4 for differential diagnosis of liver cirrhosis at compensatory stage and de-compensatory stage were 0.627, 0.666, 0.795 and 0.820, respectively. Conclusion: LS, AAR, APRI and FIB-4 were good indicators as clinical diagnosis and differential diagnosis on hepatitis B related cirrhosis.
Keywords: Chronic hepatitis B, liver fibrosis, cirrhosis, transient elastography
Introduction
Liver fibrosis results from chronic damage to the liver in conjunction with the accumulation of extracellular matrix (ECM) proteins, which is a characteristic of most types of chronic liver diseases [1]. Hepatitis B virus (HBV) infection is a major cause of chronic liver diseases, especially in Asia-Pacific area including China. Nearly 20% of patients with chronic hepatitis B can evolve to cirrhosis, with hepatic insufficiency and portal hypertension being the most serious consequences [2]. Clinically, cirrhosis has been regarded as an end-stage disease that invariably leads to death, unless liver transplantation is done, and the only preventive strategies have been screening for oesophageal varices and hepatocellular carcinoma [3].
Liver biopsy is still the gold standard to evaluate hepatic fibrosis, while its extensive application in the clinical practice is obstacle by the fact that sampling errors and interpreter biases may reduce diagnostic accuracy of liver biopsy, and invasive procedure with certain unavoidable risks and complications is not practical to follow-up disease progression and treatment response [4,5]. Therefore, the growing need for alternative approaches to the assessment of liver disease severity has driven the development of several non-invasive methods in order to overcome the limitations of liver biopsy.
Serum markers for liver fibrosis offer a cost-effective alternative to liver biopsy being less invasive and theoretically without complications. Serum markers like procollagen peptide, matrix metalloproteinases (MMPs), tissue inhibitors of matrix metalloproteinases (TIMPs), Laminins, Transforming growth factor beta 1 (TGF-β1), Connective tissue growth factor (CTGF), human cartilage glycoprotein-39 (HC gp-39), human microfibril-associated protein 4 (MFAP-4), and Cytokeratin-18 fragments, are correlated with molecules which derive directly from the ECM or are produced by activated hepatic stellate cells (HSC). Thus, the elevation of these serum markers suggests the activation of fibrogenesis [6,7].
A major limitation of biological liver fibrosis markers is their continual quantitative nature, which does not necessarily reflect the complexity of the fibrotic process. They all discriminate the presence of extreme stages (F0 and F4), but greater overlapping for intermediate stages makes their discrimination difficult [8]. Four indices resulting from the combination of serum markers of liver fibrosis are commercially available: FibrotestTM, FibrometerTM, HepascoreTM, ELFTM test, and transient elastography, FibroscanTM are well validated in chronic liver diseases of various etiologies by US, European and Asian studies and have been recognized as having the best diagnostic performance for discriminating significant fibrosis or severe fibrosis and for assessing cirrhosis (reviewed by Guéchot J) [9].
A retrospective study included 1168 severe CHB patients conducted by Ma J and his colleagues showed that FIB-4 and Lok’s model are the most effective models for distinguishing significant and extensive fibrosis, whereas APRI, FIB-4, and Lok’s model are suitable for staging fibrosis in CHB patients [10]. Meta-analysis showed that FibroTest has excellent diagnostic accuracy for identification of HBV-related significant fibrosis and cirrhosis, and FIB-4 has modest benefits and may be suitable for wider scope implementation [11].
In this study, FibroScan was compared with fibrosis score systems aspartate aminotransferase and alanine aminotransferase ratio (AAR), aspartate aminotransferase to platelet ratio index (APRI), fibrosis index based on the 4 factor (FIB-4) in the assessment of liver fibrosis in patients with hepatitis B. The accuracies, sensitivities, and specificities of different combinations of FibroScan with the fibrosis score systems were investigated in detail.
Material and methods
General data
406 patients diagnosed as chronic hepatitis B (CHB) and cirrhosis in our hospital during July, 2012 to December, 2012 were studies, including 297 males and 109 females, aged from 9 to 86 years, and the mean age was 42.17±13.75 years old. The diagnosis standard of CHB and cirrhosis complied with EASL clinical practice guidelines: management of chronic hepatitis B virus infection (2012 version) [12]. Meanwhile, patients who suffered from hepatitis A, C-E virus infections, HIV infection, CMV infection, EBV infection, alcoholic liver disease, autoimmune liver disease, drug induced liver injury or fatty liver were exclude. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Hubei Medicine University. Written informed consent was obtained from all participants.
Serum biochemical detection and fibrosis diagnosis model calculation
LC-170CRP automatic blood cell analyzer from Horiba, Ltd (Japan) and COBA INTEGRA 800 automatic biochemistry analyzer from Roche Company were used to conduct blood routine examination and liver function detection. The biochemical indexes were from that day or yesterday results that patients received liver protective and anti-virus therapy. AAR [13] = AST(U/L)/ALT(U/L), APRI [14] = AST(U/L)/PLT (×109/L), FIB-4 index [15] = [Age(years)×AST(U/L)]/[PLT(×109/L)×ALT(U/L)].
FibroScan test [16]
According to standard operation of FibroScan instrument (ECHOSENS Company, Paris, France), liver fibrosis elasticity was measured. When measured, patients kept supine position, taking head with right hand, then the couplant agent was smeared at the 7th, 8th and 9th intercostals position of right side. Next, probes were connected with skin and kept vertical direction. The measurement should be succeeded continuously for 10 times, and the mediate value of the effective measurements represented liver stiffness (LS) (Kpa). If range interquartile was fewer than 30% of median and the success rate was more than 60%, this value was effective.
Statistical analysis
Statistical analysis was performed by SPSS17.0 software and measurement data were represented as mean ± SD, LSD-t test was used for two samples comparisons from different groups by homogeneity test of variance and normal distribution test, and the correlation was analyzed by Pearson test. Receiver operating curve (ROC) was used to evaluate the effectiveness and compare the differences of four indexes on chronic hepatitis B and cirrhosis. P < 0.05 denoted a significant statistical difference.
Results
General data
406 patients included 227 chronic hepatitis B, 179 cirrhosis caused by hepatitis B, meanwhile, there were 61 cases with cirrhosis at compensatory stage and 118 at de-compensatory stage. There were statistically significant differences of age, ALT level, AST level and PLT level between cirrhosis caused by hepatitis B group and chronic hepatitis B group (all P < 0.05). All patients’ age and biochemical indicators were as follows (Table 1).
Table 1.
General data in all patients
| N | Age (year) | ALT (U/L) | AST (U/L) | PLT (×109/L) | |
|---|---|---|---|---|---|
| CHB | 227 | 36.7±12.66 | 208.1±328.3 | 139.5±209.3 | 168.0±57.3 |
| Cirrhosis | 179 | 49.1±11.4 | 80.1±133.0 | 84.3±101.5 | 95.1±59.4 |
| t | --- | -10.150 | 4.904 | 3.240 | 12.465 |
| P | --- | < 0.001 | < 0.001 | 0.001 | < 0.001 |
Comparisons of four indexes between CHB group and cirrhosis group
Table 2 showed that except APRI, other three indexes had significant differences to diagnose liver fibrosis between CHB group and cirrhosis group (P < 0.05).
Table 2.
Comparisons of four indexes between CHB group and cirrhosis group
| N | AAR | APRI | FIB-4 | LS | |
|---|---|---|---|---|---|
| CHB | 227 | 0.90±0.68 | 1.02±1.68 | 0.32±0.95 | 10.6±10.2 |
| Cirrhosis | 179 | 1.59±1.38 | 1.33±2.06 | 1.32±1.95 | 30.0±21.2 |
| t | --- | -6.579 | -1.617 | -6.739 | -12.116 |
| P | --- | < 0.001 | 0.107 | < 0.001 | < 0.001 |
Assessment capacity of ROC curve on differential diagnosis of chronic hepatitis B and cirrhosis caused by hepatitis B
Four liver fibrosis indexes and related indexes were as test variables and diagnosed as liver cirrhosis was as condition variables, then ROC curve was drawn. According to ROC curve (Figure 1), we found that the under ROC curve areas of FIB-4, LS, AAR and APRI indexes that differentiated chronic hepatitis B and cirrhosis were 0.885, 0.866, 0.772, 0.632, respectively. Table 3 showed the corresponding references for differential diagnosis of chronic hepatitis B and cirrhosis caused by hepatitis B.
Figure 1.
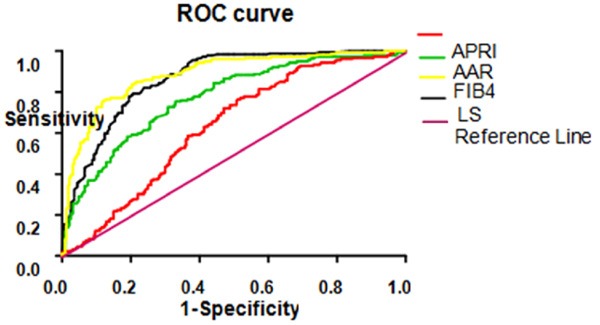
ROC curve of 4 liver fibrosis indexes for differentiation of chronic hepatitis B and cirrhosis.
Table 3.
Related parameters of four indexes differentiating chronic hepatitis B and cirrhosis
| Index | AUC | Optimal critical value | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy | Youden index | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|---|---|---|---|---|---|---|---|
| AAR | 0.772 | 0.934 | 0.754 | 0.670 | 0.36 | 0.97 | 0.66 | 0.348 | 2.328244 | 0.528455 |
| APRI | 0.632 | 0.317 | 0.765 | 0.489 | 0.28 | 0.93 | 0.54 | 0.543 | 3.072519 | 0.264228 |
| FIB-4 | 0.885 | 0.416 | 0.760 | 0.881 | 0.39 | 0.98 | 0.71 | 0.584 | 3.53913 | 0.241558 |
| LS | 0.866 | 12.6 | 0.793 | 0.793 | 0.34 | 0.96 | 0.68 | 0.266 | 1.736842 | 0.583725 |
Assessment capacity of ROC curve of four indexes for differential diagnosis of cirrhosis at compensatory stage and de-compensatory stage
Four liver fibrosis indexes and related indexes were as test variables and diagnosed as liver cirrhosis at de-compensatory stage was as condition variables, then ROC curve was drawn. According to ROC curve (Figure 2). The under ROC curve areas of FIB-4, APRI, AAR and LS indexes that differentiated liver cirrhosis at compensatory stage and de-compensatory stage were 0.820, 0.795, 0.666, 0.627, respectively. Table 4 showed the corresponding parameters for differential diagnosis of cirrhosis at compensatory stage and de-compensatory stage.
Figure 2.

ROC curve of 4 liver fibrosis indexes for differentiation of cirrhosis at compensatory stage and de-compensatory stage.
Table 4.
Related parameters of four indexes differentiating cirrhosis at compensatory stage and de-compensatory stage
| Index | AUC | Optimal critical value | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Accuracy |
|---|---|---|---|---|---|---|---|
| AAR | 0.666 | 1.246 | 0.610 | 0.738 | 0.35 | 0.94 | 0.77 |
| APRI | 0.795 | 0.465 | 0.805 | 0.738 | 0.33 | 0.96 | 0.65 |
| FIB-4 | 0.820 | 0.587 | 0.814 | 0.770 | 0.41 | 0.98 | 0.82 |
| LS | 0.627 | 21.7 | 0.627 | 0.639 | 0.31 | 0.89 | 0.61 |
Analysis of AAR, APRI and FIB-4 correlated with LS
We conducted Pearson correlation analysis between LS and AAR, APRI and FIB-4 and the related coefficient was 0.313, 0.265, 0.156, respectively (all P < 0.05), which was a statistical significance. Table 5 showed the related coefficient value and Figure 3 showed the correlation scatter graph.
Table 5.
Pearson correlation analysis between LS and AAR, APRI and FIB-4
| Index | r value | P value |
|---|---|---|
| LS and AAR | 0.313 | < 0.001 |
| LS and APRI | 0.265 | < 0.001 |
| LS and FIB-4 | 0.156 | 0.002 |
Figure 3.
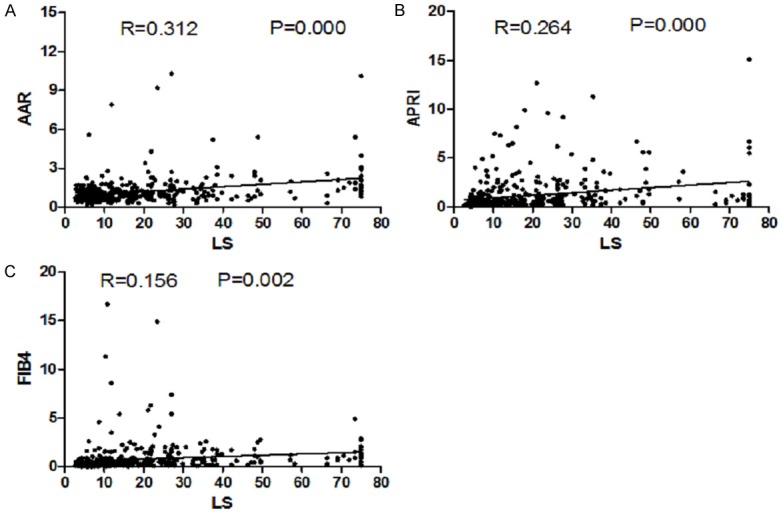
Scatter graph between LS and AAR, APRI and FIB-4. A. Scatter graph between LS and AAR. B. Scatter graph between LS and APRI. C. Scatter graph between LS and FIB-4.
Discussion
Liver fibrosis is a repaired response on chronic liver injury and pathophysiologic basis of many chronic liver diseases, meanwhile, liver fibrosis is a necessary step to develop from liver diseases to cirrhosis and liver cancer. Therefore, to accurately understand liver fibrosis degree has an important significance for assessment of chronic liver diseases conditions, prediction of prognosis and therapy [17,18]. Liver biopsy is still the gold standard to evaluate hepatic fibrosis and diagnose cirrhosis, but it has some disadvantages, such invasive procedure with certain unavoidable risks, poor repetitiveness, which limit its clinical applications for patients with end-stage liver diseases [19]. Recent years, many liver fibrosis diagnosis methods and model designs bring liver fibrosis diagnosis into a non-invasive period. Although these diagnosis techniques were not replaced by liver biopsy, they have non-trauma, convenience, good repetition advantages, moreover, these techniques have high accurate rate of diagnosis on liver fibrosis to avoid receiving liver biopsy for some patients [20,21].
Serum markers like AAR, APRI and FIB-4 for non-invasive liver fibrosis diagnosis were used to assess chronic hepatitis C and other alcoholic liver diseases related to liver fibrosis in some European-American countries [22-24]. Whereas, there were few studies to assess hepatitis B related cirrhosis and it was controversial whether these serum liver fibrosis diagnosis models were adapted to predict the degree of hepatitis B related cirrhosis [25-27]. FibroScan uses transient elastography to test liver hardness for non-invasive, subjective and quantitative assessments due to high accuracy and sensitivity, which becomes popular technique to evaluate liver fibrosis in recent years [28]. This study assessed the effects of FibroScan, AAR, APRI and FIB-4 indexes on clinical and differential diagnosis of hepatitis related to cirrhosis. Our results showed that except APRI, other three indexes had differential diagnosis values for chronic hepatitis B and liver cirrhosis caused by hepatitis B, and the minimum and maximum of corresponding under ROC curve area were 0.632 (APRI) and 0.885 (FIB-4 index), which indicated that FIB-4 was the optimal index to predict the degree of hepatitis B related to cirrhosis, and the best critical value of differential diagnosis was 0.416 and the accuracy value was 0.76. Furthermore, we compared the difference between cirrhosis patients at compensatory stage and de-compensatory stage and implied that four indexes had good differential diagnosis value, in addition, the minimum and maximum of the corresponding under ROC curve area were 0.627 (LS) and 0.820 (FIB-4 index), which also indicated that FIB-4 was the optimal index to distinguish cirrhosis patients at compensatory stage or de-compensatory stage, and LS value was low due to some patients at de-compensatory stage with ascites. Additionally, AAR, APRI and FIB-4 indexes had positive correlation with LS through Pearson analysis (all P < 0.05), which suggested that four indexes had some reference value for assessment of the degree of hepatitis B and liver fibrosis of cirrhosis caused by hepatitis B.
Recent years, there were more and more studies to focus on non-invasive diagnosis methods for liver fibrosis and the accuracy was higher and higher, which not only was benefit for evaluating the degree of liver fibrosis, but had important directive significance to assess diseases and predict prognosis and so on [29]. This study confirmed that LS, AAR, APRI and FIB-4 indexes were the good indexes to diagnose the cirrhosis, furthermore, FibroScan and FIB-4 index were better for differential diagnosis of chronic hepatitis B and cirrhosis than AAR and APRI indexes. Meanwhile, the reference value of ARPI and FIB-4 indexes was better in the differential process of liver cirrhosis at compensatory stage and de-compensatory stage than AAR and FibroScan test. Combinations of these diagnosis methods might improve the accuracy and effect of differential diagnosis, but there was still to be studied further.
Acknowledgements
This work was partly supported by the National Key Program for Infectious Disease of China (2013ZX10002-001), the Natural Science Foundation of Hubei Province of China (2011BCB030, 2014CFB645), and the Key Discipline Project of Hubei Province (2014XKJSSJ00).
Disclosure of conflict of interest
None.
References
- 1.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganem D, Prince AM. Hepatitis B virus infection--natural history and clinical consequences. N Engl J Med. 2004;350:1118–1129. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- 3.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749–1761. doi: 10.1016/S0140-6736(14)60121-5. [DOI] [PubMed] [Google Scholar]
- 4.Bedossa P, Carrat F. Liver biopsy: the best, not the gold standard. J Hepatol. 2009;50:1–3. doi: 10.1016/j.jhep.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Sanai FM, Keeffe EB. Liver biopsy for histological assessment: The case against. Saudi J Gastroenterol. 2010;16:124–132. doi: 10.4103/1319-3767.61244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soresi M, Giannitrapani L, Cervello M, Licata A, Montalto G. Non invasive tools for the diagnosis of liver cirrhosis. World J Gastroenterol. 2014;20:18131–18150. doi: 10.3748/wjg.v20.i48.18131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–117. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin-Gabriel JC, Solis-Herruzo JA. Noninvasive assessment of liver fibrosis. Serum markers and transient elastography (FibroScan) Rev Esp Enferm Dig. 2009;101:787–799. doi: 10.4321/s1130-01082009001100006. [DOI] [PubMed] [Google Scholar]
- 9.Guechot J. Noninvasive evaluation of liver fibrosis: More well-validated tests available for patient management. Liver Int. 2015;35:1643–5. doi: 10.1111/liv.12790. [DOI] [PubMed] [Google Scholar]
- 10.Ma J, Jiang Y, Gong G. Evaluation of seven noninvasive models in staging liver fibrosis in patients with chronic hepatitis B virus infection. Eur J Gastroenterol Hepatol. 2013;25:428–434. doi: 10.1097/MEG.0b013e32835cb5dd. [DOI] [PubMed] [Google Scholar]
- 11.Xu XY, Kong H, Song RX, Zhai YH, Wu XF, Ai WS, Liu HB. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One. 2014;9:e100182. doi: 10.1371/journal.pone.0100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papatheodoridis G, Buti M, Cornberg M, Janssen HL, Mutimer D, Pol S, Raimondo G, Dusheiko G, Lok A, Marcellin P. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Imperiale TF, Born LJ. Clinical utility of the AST/ALT ratio in chronic hepatitis C. Am J Gastroenterol. 2001;96:919–920. doi: 10.1111/j.1572-0241.2001.03647.x. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Gordon SC, Rupp LB, Zhang T, Boscarino JA, Vijayadeva V, Schmidt MA, Lu M. The validity of serum markers for fibrosis staging in chronic hepatitis B and C. J Viral Hepat. 2014;21:930–937. doi: 10.1111/jvh.12224. [DOI] [PubMed] [Google Scholar]
- 15.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, Messinger D, Nelson M. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 16.Meng F, Zheng Y, Zhang Q, Mu X, Xu X, Zhang H, Ding L. Noninvasive Evaluation of Liver Fibrosis Using Real-time Tissue Elastography and Transient Elastography (FibroScan) J Ultrasound Med. 2015;34:403–410. doi: 10.7863/ultra.34.3.403. [DOI] [PubMed] [Google Scholar]
- 17.Svegliati-Baroni G, De Minicis S, Marzioni M. Hepatic fibrogenesis in response to chronic liver injury: novel insights on the role of cell-to-cell interaction and transition. Liver Int. 2008;28:1052–1064. doi: 10.1111/j.1478-3231.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 18.Jung KS, Kim SU. Clinical applications of transient elastography. Clin Mol Hepatol. 2012;18:163–173. doi: 10.3350/cmh.2012.18.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung MK, Cho HJ, Lee HC, Park KS, Seo EH, Jeon SW, Cho CM, Tak WY, Kim SK, Choi YH, Kweon YO. Comparison of transient elastography and hepatic fibrosis assessed by histology in chronic liver disease. Korean J Gastroenterol. 2008;51:241–247. [PubMed] [Google Scholar]
- 20.Trifan Anca, Cojocariu C, Sfarti C, Singeap AM, Stanciu C. Non-invasive evaluation of liver fibrosis in chronic hepatitis C. Rev Med Chir Soc Med Nat Iasi. 2012;116:135–138. [PubMed] [Google Scholar]
- 21.Baranova A, Lal P, Birerdinc A, Younossi ZM. Non-invasive markers for hepatic fibrosis. BMC Gastroenterol. 2011;11:91. doi: 10.1186/1471-230X-11-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González Guilabert MI, Hinojosa Mena-Bernal C, del Pozo González J, del Pozo Pérez MA. Retrospective study of FibroScan, APRI, FIB-4 and FORNS indexes compared with liver biopsy in the evaluation of liver fibrosis in patients with chronic hepatitis C monoinfection and HIV coinfection. Gastroenterol Hepatol. 2010;33:425–432. doi: 10.1016/j.gastrohep.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Mueller S, Seitz HK, Rausch V. Non-invasive diagnosis of alcoholic liver disease. World J Gastroenterol. 2014;20:14626–14641. doi: 10.3748/wjg.v20.i40.14626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mallet V, Dhalluin-Venier V, Roussin C, Bourliere M, Pettinelli ME, Giry C, Vallet-Pichard A, Fontaine H, Pol S. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther. 2009;29:409–415. doi: 10.1111/j.1365-2036.2008.03895.x. [DOI] [PubMed] [Google Scholar]
- 25.Abd El Rihim AY, Omar RF, Fathalah W, El Attar I, Hafez HA, Ibrahim W. Role of fibroscan and APRI in detection of liver fibrosis: a systematic review and meta-analysis. Arab J Gastroenterol. 2013;14:44–50. doi: 10.1016/j.ajg.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Vallet-Pichara A, Mallet V, Pol S. FIB-4: a simple, inexpensive and accurate marker of fibrosis in HCV-infected patients. Hepatology. 2006;44:769–770. doi: 10.1002/hep.21334. [DOI] [PubMed] [Google Scholar]
- 27.Han KH, Yoon KT. New diagnostic method for liver fibrosis and cirrhosis. Intervimlogy. 2008;51:11–16. doi: 10.1159/000122594. [DOI] [PubMed] [Google Scholar]
- 28.Schmeltzer PA, Talwalkar JA. Noninvasive tools to assess hepatic fibrosis: ready for prime time. Gastroenterol Clin North Am. 2011;40:507–521. doi: 10.1016/j.gtc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wai CT, Cheng CL, Wee A, Dan YY, Chan E, Chua W, Mak B, Oo AM, Lim SG. Non-invasive models for predicting histology in patients with chronic hepatitis B. Liver Int. 2006;26:666–672. doi: 10.1111/j.1478-3231.2006.01287.x. [DOI] [PubMed] [Google Scholar]


