Abstract
Aim: To evaluate the role of 2,940 nm erbium: YAG laser in hair growth in C57BL/6 mice. Methods: Anagen was experimentally induced by depilation. Healthy C57BL/6 mice (n=22) were randomly divided into four groups, with treatment of laser or minoxidil, or with combined laser and minoxidil treatments. The skin color of each mouse was observed each day. The time from telogen (pink skin color) to anagen (black coloration) phase and from anagen (black coloration) to catagen (all hairs grew out of the depilated skin) have been recorded. Hematoxylin and eosin (H&E) assay was done at fifteen days after the first treatment for each group to observe hair follicles and hair cycle score. Western blot analysis was utilized to detect the expression levels of Wnt 10-b and β-catenin. Results: Black pigmentation started significantly earlier both in the laser and combination group than in the control group. Moreover, the time from anagen to catagen in the laser, minoxidil and combination groups were all significantly shorter from the control group. Histopathology with H&E staining showed an obvious increase in the number of hair follicles in the anagen phase caused by the treatment of 2,940 nm erbium: YAG laser and minoxidil. Similarly, the percentage of hair follicles in anagen VI accounted for 19.5%, 37.5%, 41.5% and 44% in control, laser, minoxidil, and combination group, respectively. Western blot analysis showed that both the levels of Wnt 10b and β-catenin were significantly increased by the treatment of 2,940 nm erbium: YAG laser. Conclusion: Our findings showed that 2,940-nm Er: YAG laser could promote hair growth by inducing hair cycle transition from telogen to anagen phases in C57BL/6 mice through up regulating Wnt 10b and β-catenin. These results suggest that 2,940-nm Er: YAG laser may be a potential therapy for hair loss.
Keywords: Hair loss, erbium, YAG laser, hair growth, hair cycle, Wnt 10b, β-catenin
Introduction
Hair loss is generally considered not to be a life-threatening event. However, it often has impact on social interactions and people’s psychological well-being. Hair is considered as one of the accessory structures of integument which include sebaceous glands, nails and sweet glands. The growth of hair is cyclic with three different phases, including phases of growth (anagen), involution (catagen) and rest (telogen) [1,2].
Though the number of patients suffering from hearing loss has increased dramatically, only two drugs-finasteride and minoxidil-have been approved to be used in the treatment of hair loss so far. Minoxidil is widely used for androgenic alopecia patients to promote hair growth through inducing stage transition for hair follicles from telogen stages into anagen stages [3]. However, the adverse dermatological effects, such as scaling, dryness and dermatitis, would also be caused by minoxidil administration [4,5]. Therefore, it is urgent to develop novel treatments.
Laser and light sources have been widely used medically and non-medically for many years. It has been well known that low-level laser therapy (LLLT) seems to be effective and safe in the treatment of hair loss. Various LLLT have been reported to treat hair loss, such as He-Ne laser, excimer laser, and PUVA, and some successful cases have been reported [6-8]. Besides LLLT, high-energy lasers have also been tried in the treatment of hair loss. Lee et al. reported a 1550 nm fractional erbium-glass laser in the treatment of female pattern hair loss [9]. Their results suggest that high-energy laser 1550 nm fractional erbium-glass laser may be an effective and safe treatment option for women with female pattern hair loss. Although the mechanism of laser induced hair growth has not been well elucidated, the great potential of various types of lasers in the treatment hair loss has been verified. In this study, we aimed to evaluate the role of 2,940-nm erbium: YAG laser treatment in the hair growth in mice.
Materials and methods
Animals
Healthy C57BL/6 mice (6-8 weeks old, weighted 20-30 g) were obtained from Laboratory Animals Center of Wuhan University. Mice 6-8 weeks old mice were used for subsequent experiments since mice at this stage are in the telogen stage of the hair cycle [10]. Mice were given rodent chow and water ad libitum, with humidity (35-60%), temperature (23±2°C), and 12 h light and 12 h darkness cycles. The animal care and experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publication Number 80-23) and institutional ethical guidelines for animal experiment of Wuhan University.
Anagen induction
As previously described [10], anagen was experimentally induced by depilation. Briefly, all mice were anesthetized by intraperitoneal injection of 2.5% chloral hydrate at a dose of 0.1 ml/10 g body weight of mice. All hair follicles were indicated in telogen by the pink back skin color [11]. A wax/rosin mixture was applied to dorsal skin (about 4 cm × 4 cm), and all hair shafts were removed through peeling off the wax/rosin mixture. It has been reported that removal of all hair shafts immediately induces a highly synchronized hair growth [12,13].
Treatment
Mice were randomly divided into four groups (n=22) to study the hair promoting activity. Dorsal skin was cleaned before treatment for all groups. The control group did not receive any treatment. In the group of laser treatment, treatment was carried out using a 2,940-nm Er: YAG laser (Pixel, Alma Lasers Ltd, Caesarea, Israel) with a microlens aligned in a matrix of 9 × 9 (81) dots (pixels) at a setting of 1200 mJ/cm2 and a spot area of 1.5 cm × 4.0 cm. Two laser passes were performed during each treatment session. Each mouse received 3 treatments at one-week interval. In the group of minoxidil treatment, 5% Minoxidil Tincture (Wanma Group, Zhejiang, China) was treated each day and lasting for 3 weeks. In the group combined laser treatment with minoxidil, the number of passes, matrix size, and the level of energy used for 2,940-nm Er: YAG laser treatment was the same as the laser treatment group; in the meantime, 5% Minoxidil Tincture (Wanma Group, China) was treated each day and lasting for 3 weeks. The skin color of each mouse was observed each day. Anagen was indicated by the black coloration. In our study, we used black coloration covering about half of the depilated skin area as the sign of anagen. The time interval between telogen (treatment beginning) and anagen has been recorded. Moreover, we had also recorded the time interval between anagen and catagen (all hairs grew out of the depilated skin).
Hematoxylin and eosin (H&E) assay
Fifteen days after the first treatments for each group, four mice from each group were sacrificed and dorsal skin was excised. Dorsal skin was fixed in 4% paraformaldehyde at 4°C for at least 24 hours and embedded in paraffin blocks to obtain longitudinal and transverse section. Stain the sliced sections with hematoxylin and eosin. Representative areas were selected from digital photomicrographs at a fixed magnification of 400 ×.
Hair cycle score
The H&E stained slides were photographed using a digital photomicrograph to evaluate hair cycles. Fifty hair follicles identified on sections were graded for each mouse. Hair cycle score was calculated as following: hair follicles in anagen VI were attributed a sore of 100, in early catagen (catagen I-catagen III) a score of 200, in mid-catagen (catagen IV-catagen V) a score of 300, and in late catagen (catagen VI-VIII) a score of 400. The percentage of each hair cycle stage was also calculated.
Western blotting
Methods for western blot were as previously described [14]. Briefly, proteins were extracted from tissue samples at 24 hours after the first treatments for each group, and quantified by Bradford assay [15]. Equal amounts of protein were subjected to SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The primary antibodies used for western blot were biotin polyclonal rabbit anti-Wnt 10b antibody (bs-3662r; 1:1000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and monoclonal rabbit anti-β-catenin (#9582; 1:1000 dilution; Cell Signaling Technology, Boston, MA, USA). All experiments were repeated three times.
Statistical analysis
Results are expressed as mean ± SD. Data was analyzed by SPSS software (version 17.0). Statistical evaluation of the data was performed using One-way Analysis-of-variance (ANOVA) test, followed by Bonferroni’s post hoc test. The values P<0.05 was considered as statistically significant.
Results
Effect of 2,940-nm Er: YAG laser on hair growth
Mice aged at 6-8 weeks old were synchronized by depilation. The transition of hair follicles from telogen to anagen phase was indicated by the black pigmentation. Our results showed that time length for the beginning of black pigmentation was significantly different in four groups (Table 1; Figure 1, left). As shown in the Figure 1, black pigmentation started significantly earlier in the laser and minoxidil group than in the control group, suggesting that 2,940-nm Er: YAG laser induced black coloration for C57BL/6 mice. In the combination group (with both laser treatments and minoxidil administration), the time took from telogen to anagen phase was significantly shorter than that in the control group. Moreover, the time from anagen (black skin color) to catagen (all hairs grew out of the depilated skin) was recorded for each group. Our results showed that the time from anagen to catagen in the laser, minoxidil and combination groups were all significantly shorter from the control group (Table 1; Figure 1, right). The time from anagen to catagen in the combination group was significantly shorter than that in the minoxidil group, suggesting combined treatment of 2,940-nm Er: YAG laser and minoxidil could further stimulated hair growth.
Table 1.
Effect of 2,940-nm Er: YAG laser on hair growth
| No. of mice (n) | Time length from telogen to anagen (days, mean ± SD) | Time length from anagen to catagen (days, mean ± SD) | |
|---|---|---|---|
| Control | 15 | 12.07±1.28 | 10.53±1.73 |
| Laser | 15 | 9.27±1.33*** | 6.47±1.13*** |
| Minoxidil | 15 | 9.07±1.16*** | 6.13±1.19*** |
| Combination | 15 | 8.27±2.31*** | 4.27±1.44***,## |
compare to the laser treatment group, P<0.001;
compare to the laser treatment, P<0.01 group.
Figure 1.
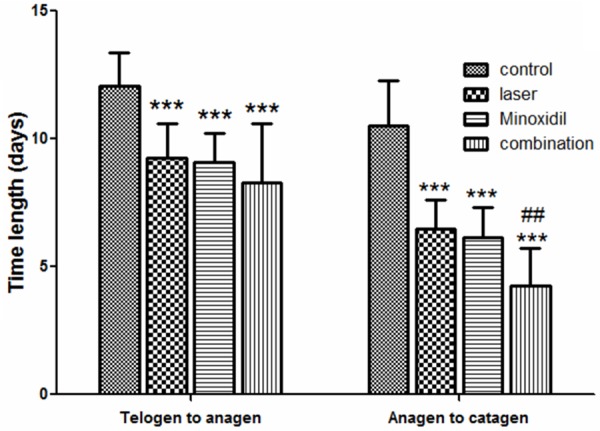
Effect of 2,940-nm Er: YAG laser on hair growth. The time took from telogen to anagen phase was shown on the left panel, while time from anagen (black skin color) to catagen (all hairs grew out of the depilated skin) was shown on the right panel.
Effect of 2,940-nm Er: YAG laser on hair cycle
Histopathology with H&E staining was performed 15 days after the first treatment for each group. Our results showed an obvious increase in the number of hair follicles in the anagen phase in the laser, minoxidil and combination group compared with that in the control group (Figure 2). The anagen hair bulbs were larger in groups with treatment (laser, minoxidil, and combination group) compared to the control group with the majority of the hair follicles in telogen. Hair cycle score for the laser group was 171, for minoxidil was 165 and for combination was 158, suggesting the majority of hair follicles was in anagen phase or early catagen phase. Whereas, hair cycle score for the laser group was 336, suggesting the majority of hair follicles was in mid-catagen phase or late catagen phase. Through calculating the percentage of hair follicles in each hair cycle stage, we found that follicles in anagen VI accounted for 19.5%, 37.5%, 41.5% and 44% in control, laser, minoxidil and combination group, respectively (Table 2).
Figure 2.
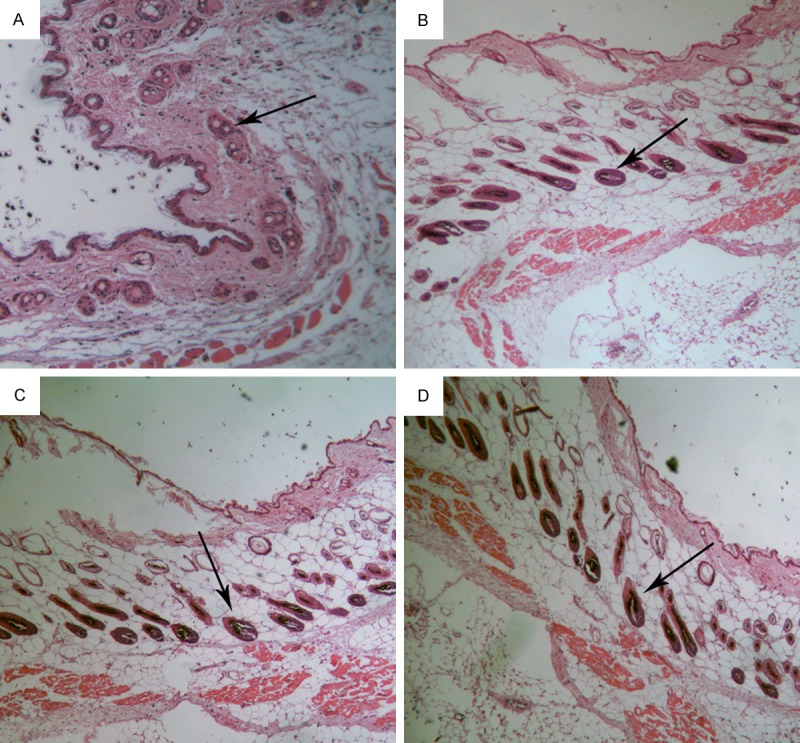
Histopathology with H&E staining of hair follicles in the control (A), laser (B), minoxidil (C) and combination group (D). Arrow indicates hair follicles.
Table 2.
The percentage of hair follicles at different stage
| Control | Laser | Minoxidil | Combination | |||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Hair Follicles (n) | Ratio (%) | Hair Follicles (n) | Ratio (%) | Hair Follicles (n) | Ratio (%) | Hair Follicles (n) | Ratio (%) | |
| Anagen VI | 9.75±0.98 | 19.5 | 18.75±0.5* | 37.5 | 20.75±0.96* | 41.5 | 22±0.82* | 44 |
| Early catagen | 9.25±0.96 | 18.5 | 21.75±0.96* | 43.5 | 18±0.82* | 36 | 16.5±1* | 33 |
| Mid-catagen | 16.25±0.5 | 32.5 | 7.75±1.26* | 15.5 | 8.5±0.58* | 17 | 8.5±0.58* | 17 |
| Late-catagen | 14.75±0.5 | 29.5 | 1.75±0.96* | 3.5 | 2.75±0.96* | 5.5 | 3±0.82* | 6 |
P<0.05 VS control group.
2,940-nm Er: YAG laser treatment induced the expression of Wnt 10b and β-catenin
The mechanism underlying transition of hair cycle growth from telogen to anagen has not been well understood. However, many signaling pathways have been reported to be involved in this transition, such as Wnt, BMP, Shh, and FGF [16-18]. β-catenin has been reported that could induce the hair growth cycle transition from the telogen to anagen phases [19-21]. It has also been report that Wnt 10b may induce the transition from telogen to anagen via a canonical Wnt signaling pathways to promote hair follicle growth [22]. In this study, in order to elucidate the mechanism of 2,940-nm Er: YAG laser treatment in the hair growth, western blot analysis was used to detect expression level of Wnt 10b and β-catenin. In our study, we found that the levels of Wnt 10b were significantly higher in the laser, minoxidil and combination groups than in control group. Moreover, levels of β-catenin were higher in the laser and combination groups than in control group. While, no difference was observed in the minoxidil group compared with control group (Figure 3). Taking together, these data indicated that 2,940-nm Er: YAG laser treatment promoted hair growth partly through upregulating Wnt 10b and β-catenin.
Figure 3.
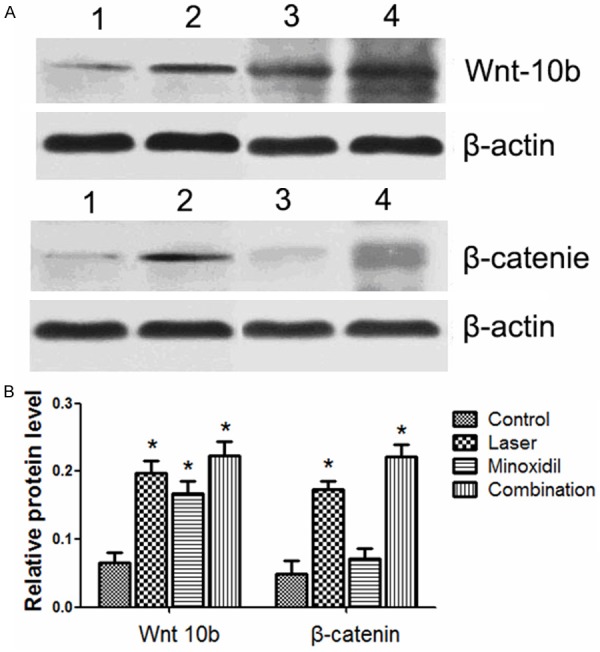
The expression levels of Wnt 10b and β-catenin after 2,940-nm Er: YAG laser treatment. A. Western blot analysis was used to detect levels of Wnt 10b and β-catenin in control (lane 1), laser (lane 2), minoxidil (lane 3), and combination groups (lane 4). B. Relative protein level of Wnt 10b and β-catenin.
Discussion
Hair loss is one of the most common chronic dermatological problems worldwide. Multiple reasons may cause hair loss, such as genetic heredity, levels of local androgen, sleep, and sebum secretion. Though not a life-threatening event, hair loss often may often have significant psycho-social consequences especially in younger people. Minoxidil is a widely used drug approved by FDA (US Food and Drug Administration) to treat androgenic alopecia through inducing hair cycle transition from telogen to anagen phases [23]. However, adverse dermatogogical effects such as dryness, local irritation and dermatitis may be also caused by minoxidil treatment [4].
Laser therapy seems to be effective and safe in the treatment of hair loss, though the mechanism underlying is not well elucidated. LLLT, such as He-Ne laser, excimer laser, and PUVA, have been reported to be effective in the treatment of hair loss [6-8]. Unlike LLLT, use of high-energy lasers in the treatment of hail loss has been relatively less reported. Lee et al. reported a 1550 nm fractional erbium-glass laser in the treatment of female pattern hair loss and found that the treatment may be an effective and safe treatment option for women with female pattern hair loss [9]. Ablative fractional Er: YAG laser (2,940 nm) has been reported to be a promising option for skin resurface and traumatic scars reduction [24,25]. However, the role of 2,940-nm Er: YAG laser in hair growth has not been elucidated. In this study, we found that 2,940-nm Er: YAG laser could significantly promote hair growth via inducing hair cycle transition from telogen to anagen phases in C57BL/6 mice.
C57BL/6 mice are useful models used for the screening of hair growth promoting agents. The black pigment is only produced during anagen, making the skin color as a visible indicator for hair cycles. Our results showed that black pigmentation started significantly earlier in the laser group than in the control group, suggesting that 2,940-nm Er: YAG laser induced transition telogen to anagen in C57BL/6 mice. In the group treated with both 2,940-nm Er: YAG laser and minoxidil, the time took from telogen to anagen phase was significantly shorter than both control and laser groups. These results may be partially caused by the increased absorption of minoxidil after ablative fractional Er: YAG laser (2,940 nm) treatment. It has been reported that Er: YAG laser treatment could enhance absorption of transdermal peptides delivery for 3-140 folds compared with intact skin [26]. Further, histopathology with H&E staining at 15 days after the first treatment for each group showed an obvious increase in the number of hair follicles in the anagen phase in the laser, minoxidil and combination group compared with that in the control group. The anagen hair bulbs were larger in groups with treatment (laser, minoxidil, and combination group) compared to the control group. Our results suggest that 2,940-nm Er: YAG laser treatment promotes hair growth through inducing hair cycle transition from telogen to anagen phases in C57BL/6 mice.
Many signaling pathways have been reported to be involved in transition of hair cycle growth from telogen to anagen, such as Wnt, BMP, Shh, and FGF [16-18]. It has been report that Wnt 10b may induce the transition from telogen to anagen via a canonical Wnt signaling pathways to promote hair follicle growth [22]. In this study, we found that both the levels of Wnt 10b and β-catenin were significantly higher in the laser, minoxidil and combination groups than in control group. Taking together, these data indicated that 2,940-nm Er: YAG laser treatment promoted hair growth partially through upregulating Wnt 10b and β-catenin.
Conclusion
In this study, we report the role of 2,940-nm Er: YAG laser in hair growth. Our findings suggest that 2,940-nm Er: YAG laser could promote hair growth by inducing hair cycle transition from telogen to anagen phases in C57BL/6 mice through upregulating Wnt 10b and β-catenin. Our results suggest that 2,940-nm Er: YAG laser may be a potential therapy for hair loss.
Acknowledgements
The study was supported by the Natural Science Foundation of Hubei Province (grant number: 303220100480).
Disclosure of conflict of interest
None.
References
- 1.Price VH. Treatment of hair loss. N Engl J Med. 1999;341:964–73. doi: 10.1056/NEJM199909233411307. [DOI] [PubMed] [Google Scholar]
- 2.Wosicka H, Cal K. Targeting to the hair follicles: current status and potential. J Dermatol Sci. 2010;57:83–9. doi: 10.1016/j.jdermsci.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 3.Gregoriou S, Papafragkaki D, Kontochristopoulos G, Rallis E, Kalogeromitros D, Rigopoulos D. Cytokines and other mediators in alopecia areata. Mediators Inflamm. 2010;2010:928030. doi: 10.1155/2010/928030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelfuso GM, Gratieri T, Delgado-Charro MB, Guy RH, Vianna Lopez RF. Iontophoresis-targeted, follicular delivery of minoxidil sulfate for the treatment of alopecia. J Pharm Sci. 2013;102:1488–94. doi: 10.1002/jps.23485. [DOI] [PubMed] [Google Scholar]
- 5.Tarlow JK, Clay FE, Cork MJ, Blakemore AI, McDonagh AJ, Messenger AG, Duff GW. Severity of alopecia areata is associated with a polymorphism in the interleukin-1 receptor antagonist gene. J Invest Dermatol. 1994;103:387–90. doi: 10.1111/1523-1747.ep12395398. [DOI] [PubMed] [Google Scholar]
- 6.Gundogan C, Greve B, Raulin C. Treatment of alopecia areata with the 308-nm xenon chloride excimer laser: case report of two successful treatments with the excimer laser. Lasers Surg Med. 2004;34:86–90. doi: 10.1002/lsm.20002. [DOI] [PubMed] [Google Scholar]
- 7.Shukla S, Sahu K, Verma Y, Rao KD, Dube A, Gupta PK. Effect of helium-neon laser irradiation on hair follicle growth cycle of Swiss albino mice. Skin Pharmacol Physiol. 2010;23:79–85. doi: 10.1159/000265678. [DOI] [PubMed] [Google Scholar]
- 8.Avram MR, Rogers NE. The use of low-level light for hair growth: part I. J Cosmet Laser Ther. 2009;11:110–7. doi: 10.1080/14764170902842531. [DOI] [PubMed] [Google Scholar]
- 9.Lee GY, Lee SJ, Kim WS. The effect of a 1550 nm fractional erbium-glass laser in female pattern hair loss. J Eur Acad Dermatol Venereol. 2011;25:1450–4. doi: 10.1111/j.1468-3083.2011.04183.x. [DOI] [PubMed] [Google Scholar]
- 10.Muller-Rover S, Handjiski B, van der Veen C, Eichmuller S, Foitzik K, McKay IA, Stenn KS, Paus R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J Invest Dermatol. 2001;117:3–15. doi: 10.1046/j.0022-202x.2001.01377.x. [DOI] [PubMed] [Google Scholar]
- 11.Messenger AG, Rundegren J. Minoxidil: mechanisms of action on hair growth. Br J Dermatol. 2004;150:186–94. doi: 10.1111/j.1365-2133.2004.05785.x. [DOI] [PubMed] [Google Scholar]
- 12.Slominski A, Paus R, Plonka P, Chakraborty A, Maurer M, Pruski D, Lukiewicz S. Melanogenesis during the anagen-catagen-telogen transformation of the murine hair cycle. J Invest Dermatol. 1994;102:862–9. doi: 10.1111/1523-1747.ep12382606. [DOI] [PubMed] [Google Scholar]
- 13.Paus R, Stenn KS, Link RE. Telogen skin contains an inhibitor of hair growth. Br J Dermatol. 1990;122:777–84. doi: 10.1111/j.1365-2133.1990.tb06266.x. [DOI] [PubMed] [Google Scholar]
- 14.Kwack MH, Kang BM, Kim MK, Kim JC, Sung YK. Minoxidil activates beta-catenin pathway in human dermal papilla cells: a possible explanation for its anagen prolongation effect. J Dermatol Sci. 2011;62:154–9. doi: 10.1016/j.jdermsci.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim WS, Lee HI, Lee JW, Lim YY, Lee SJ, Kim BJ, Kim MN, Song KY, Park WS. Fractional photothermolysis laser treatment of male pattern hair loss. Dermatol Surg. 2011;37:41–51. doi: 10.1111/j.1524-4725.2010.01833.x. [DOI] [PubMed] [Google Scholar]
- 16.Rendl M, Polak L, Fuchs E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008;22:543–57. doi: 10.1101/gad.1614408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suda T, Arai F. Wnt signaling in the niche. Cell. 2008;132:729–30. doi: 10.1016/j.cell.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, Millar SE. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech Dev. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- 19.Bierie B, Nozawa M, Renou JP, Shillingford JM, Morgan F, Oka T, Taketo MM, Cardiff RD, Miyoshi K, Wagner KU, Robinson GW, Hennighausen L. Activation of beta-catenin in prostate epithelium induces hyperplasias and squamous transdifferentiation. Oncogene. 2003;22:3875–87. doi: 10.1038/sj.onc.1206426. [DOI] [PubMed] [Google Scholar]
- 20.Van Mater D, Kolligs FT, Dlugosz AA, Fearon ER. Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 2003;17:1219–24. doi: 10.1101/gad.1076103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin WH, Xiang LJ, Shi HX, Zhang J, Jiang LP, Cai PT, Lin ZL, Lin BB, Huang Y, Zhang HL, Fu XB, Guo DJ, Li XK, Wang XJ, Xiao J. Fibroblast growth factors stimulate hair growth through beta-catenin and Shh expression in C57BL/6 mice. Biomed Res Int. 2015;2015:730139. doi: 10.1155/2015/730139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YH, Zhang K, Ye JX, Lian XH, Yang T. Wnt 10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin Exp Dermatol. 2011;36:534–40. doi: 10.1111/j.1365-2230.2011.04019.x. [DOI] [PubMed] [Google Scholar]
- 23.Patel M, Harrison S, Sinclair R. Drugs and hair loss. Dermatol Clin. 2013;31:67–73. doi: 10.1016/j.det.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Kim SG, Kim EY, Kim YJ, Lee SI. The Efficacy and Safety of Ablative Fractional Resurfacing Using a 2,940-Nm Er: YAG Laser for Traumatic Scars in the Early Posttraumatic Period. Arch Plast Surg. 2012;39:232–7. doi: 10.5999/aps.2012.39.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lapidoth M, Yagima Odo ME, Odo LM. Novel use of erbium: YAG (2,940-nm) laser for fractional ablative photothermolysis in the treatment of photodamaged facial skin: a pilot study. Dermatol Surg. 2008;34:1048–53. doi: 10.1111/j.1524-4725.2008.34204.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee WR, Pan TL, Wang PW, Zhuo RZ, Huang CM, Fang JY. Erbium: YAG laser enhances transdermal peptide delivery and skin vaccination. J Control Release. 2008;128:200–8. doi: 10.1016/j.jconrel.2008.03.003. [DOI] [PubMed] [Google Scholar]


