Abstract
Objectives: Both chronic kidney disease (CKD) and hemodialysis (HD) are reported to elevate oxidative stress. Available evidence for oxidative stress is indirect measurement of oxidative stress as accumulation of byproducts by reactive oxygen species (ROS). We aimed to examine the effect of CKD and HD on ROS levels in circulating leukocytes and to compare those with conventional oxidative stress marker, F2-isoprostane, in HD patients. Methods: Using flowcytometry techniques, ROS levels in circulating leukocytes can be directly measured in 16 HD patients and 12 healthy volunteers. We also measured circulating F2-isoprostanes levels in both groups. Results: HD patients demonstrated a significant increase in serum levels of F2-isoprostanes. The direct measurement of ROS levels in leukocytes showed increase in HD patients compared to the control; 1.91-fold in polymorphonuclear leukocytes (PMN), 1.06-fold in lymphocytes, and 1.35-fold in monocytes. Significant difference between the two groups could be observed only in PMN. The ROS levels in all three fractions of leukocytes showed negative correlations with serum F2-isoprostane levels but the ROS levels only in PMN showed significant correlation (r2 = 0.774, P = 0.001). Conclusions: Our results indicate that direct measurement of the ROS levels in circulating leukocytes by flowcytometry is a useful method to examine oxidative stress during HD procedure. The ROS levels in circulating leukocytes showed negative correlation with serum F2-isoprostane levels.
Keywords: Dialysis, oxidative stress, flowcytometry, reactive oxygen sepsis, F2-isoprostane
Introduction
Chronic kidney disease (CKD) increases oxidative stress compared with healthy matched controls, and this is postulated as contributing to the high levels of cardiovascular disease (CVD) morbidity and mortality in CKD group [1]. Hemodialysis (HD) therapy is widely used for removal of excessive toxins, metabolic products, and blood components from patients with CKD. However, HD itself may further contribute to progress atherosclerosis by enhancing oxidative stress, cytokine stimulation, and other events [2,3]. Possible mechanism of increasing oxidative stress by HD procedure includes loss of antioxidants by HD, interactions between blood and dialysis membrane, transfer of bacterial products in the dialysate to circulation crossing the dialysis membrane, and malnutrition decreasing the uptake of dietary antioxidants [4]. Reactive oxygen species (ROS) formed within cells can oxidize various molecules, leading to cell death and tissue injury [5].
The markers of oxidative stress shown to increase in HD therapy include F2-isoprostanes, lipid hydroperoxides, oxidized anti-LDL antibodies, the oxidizability of LDL, free sulfhydryl groups, carbonyl groups, 3-chlorotyrosine, and advanced oxidation protein products [6-13]. Free radicals have extremely short half-lives, so that in most case oxidative stress is measured by specific end-products of the process. The end-products can be measured in urine, serum, tissue, cell culture or other biological products but serum may be the most common for HD population. Some researchers consider that isoprostanes are the best available biomarker of lipid peroxidation and have been investigated in the pathogenesis of CKD [14,15]. One of their limitations as a biomarker of oxidative stress is that they are rapidly metabolized and any increase in serum isoprostane level may be due not only to their increased formation from lipid peroxidation, but also to a slower metabolism [16]. However, this limitation is characteristic not only for isoprostane but other markers such as malondialdehyde, 8-hydroxy deoxyguanosine, etc [17].
Using flowcytometry techniques, ROS levels in circulating blood cells can be directly measured. The aim of the present study was to examine the effect of HD procedure on ROS levels in leukocytes. The second aim was to compare ROS levels in circulating leukocytes with serum F2-isoprostane levels in HD patients. We could consider the usefulness of direct measurement of ROS levels in leukocytes.
Materials and methods
Patients
We measured serum levels of F2-isoprostane and ROS levels in circulating leukocytes in 16 HD patients and 12 healthy volunteers. Cause of uremia were chronic glomerulonephritis (n = 5), renal screlosis (n = 4), and diabetes mellitus (n = 7). We excluded HD patients with malignancy, active inflammatory disease, CVD within 1 year because they may affect severity of oxidative stress imbalance. Medication and clinical histories were obtained from clinical records. All the participants, have no history of smoking and of taking anti-oxidative medication such as vitamin C or E. Maintenance HD were carried out for 4 h every session, 3 times a week with blood flow of 200-300 mL/min and dialysate flow rate of 500 ml/min containing Na+ (140 mEq/l), K+ (2.0 mEq/l), Cl- (110 mEq/l), Ca2+ (3.0 mEq/l), Mg2+ (1.0 mEq/l), HCO3 - (30 mEq/l) and CH3COO- (10-15 mEq/l). All the participants gave their informed consent to the measurement.
Blood samples and biochemical analysis
Hematological and biochemical blood samples were collected after at least 10 h of fasting just before the first HD session of the week and tested at the laboratory at SRL, Inc. (Tokyo, Japan). Samples from the control were obtained in the morning after at least 10 h fasting. The spKt/V was evaluated according to the procedure of Daugirdas [18]. Serum concentrations of F2-isoprostanes were measured by competitive ELISA, using polyclonal antibody specific for F2-isoprostane coated on a micro-plate specific for mouse (Northwest Life Science Specialities, Vancouver, WA, USA). Subsequent TMB substrate addition resulted in a blue color development and addition of an acid stop solution caused a color change to yellow where absorbance was read at 450 nM.
Preparation of leukocytes
Blood samples for flowcytometric analysis were obtained at the same day of other laboratory test and at middle of HD session, using a syringe, applying gentle aspiration to minimize shear stress ex vivo. To avoid in vitro activation/modification, blood samples were processed immediately without being manipulated. The red blood cells were lysed using FACS lysis solution. Cells were washed twice with phosphate-buffered saline (PBS). Total ROS detection kit (Enzo Life Science, Plymouth Meeting, PA, USA) was used for the following procedure. Resuspended cells were treated with pyocyanin as the ROS inducer for about 20 min. Final concentration of pyocyanin was 200 M. After centrifugation, the cells were washed twice with PBS and resuspended with the ROS detection solution, following 30 min incubation at 37 degrees Celsius in the dark. Then, the cells were assayed with flowcytometry.
Flowcytometry
The cells were gated for lymphocytes, monocytes, and polymorphonuclear (PMN) leukocytes using a FACSort flow cytometer (Becton-Dickinson, San Jose, CA, USA). For each sample, data from 10,000 cells were collected and analyzed. The cells with increased levels of oxidative stress demonstrate bright green fluorescence in presence of the ROS detection solution and were detected using FL1 channel.
Statistical analysis
Clinical parameters are expressed as mean ± standard deviation. Two-sided Mann-Whitney U test was used to analyze data, which were thought as non-parametric since sample size was relatively small. Pearson correlation was used to examine the relationship between serum F2-isoprostanes levels and ROS levels in leukocytes. Statistical analyses were performed with JMP 11.2.0 from SAS (Cary, NC, USA). P < 0.05 was considered to indicate a statistically significant difference.
Results
The characteristics of healthy controls and HD patients are shown in Table 1. The two groups differ significantly in terms of serum hemoglobin levels, serum albumin levels, blood urea nitrogen levels, and serum creatinine levels. All the characteristics with significant difference are thought to be caused by CKD. The blood samples obtained from HD patients demonstrated a significant increase in serum levels of F2-isoprostanes (Figure 1). Consistent with many previous studies, imbalance of oxidative stress was augmented by CKD and maintenance HD therapy. Next, we measured the ROS levels in circulating leukocytes in the two groups. Figure 2 summarizes the results of the ROS levels in PMN, lymphocytes, and monocytes. HD patients have a tendency to show higher ROS levels in all the three fractions of leukocytes compared to the healthy controls. The increases of ROS levels in HD patients are 1.73-fold in PMN, 1.14-fold in lymphocytes, and 1.31-fold in monocytes compared to the control. However, significant difference between the two groups was observed only in PMN. Next, we examined correlation between serum F2-isoprostanes levels and ROS levels in circulating leukocytes in HD patients (Figure 3). ROS levels in all three fractions of circulating leukocytes showed negative correlations with serum F2-isoprostane levels in HD patients. The ROS levels in circulating PMN showed significant correlation (r2 = 0.774, P = 0.001). These indicated that both serum end-products of imbalance of oxidative stress and direct measurement of ROS levels in circulating leukocytes are increased by CKD and HD, but that these levels do not show positive correlation.
Table 1.
Clinical and laboratory characteristics of the participants
| HD (n = 16) | Control (n = 12) | P | |
|---|---|---|---|
| Age (years) | 70.3 ± 10.5 | 64.7 ± 10.3 | 0.658 |
| Gender (M:F) | 5:11 | 4:8 | 0.926 |
| Dialysis vintage (months) | 70.3 ± 10.5 | NA | |
| No. of hypertension | 7 | 2 | 0.204 |
| No. of diabetes mellitus | 7 | 1 | 0.114 |
| No. of current smoker | 0 | 1 | 0.663 |
| Hemoglobin (g/dL) | 10.3 ± 1.1 | 13.8 ± 1.4 | 0.008 |
| White blood cell (/mm3) | 4433 ± 1216 | 5132 ± 989 | 0.682 |
| Albumin (g/dL) | 3.4 ± 0.4 | 4.6 ± 0.2 | 0.007 |
| Blood urea nitrogen (mg/dL) | 61.5 ± 18.0 | 14.5 ± 0.3 | 0.001 |
| Creatinin (mg/dL) | 8.8 ± 1.7 | 0.9 ± 0.2 | 0.001 |
| Calcium (mg/dL) | 9.8 ± 1.9 | 9.8 ± 1.2 | 0.796 |
| Phosphorus (mg/dL) | 4.5 ± 0.6 | 4.6 ± 0.3 | 0.631 |
| Total cholesterol (mg/dL) | 152.6 ± 16.4 | 163.6 ± 37.2 | 0.744 |
| Triglyceride (mg/dL) | 129.3 ± 70.6 | 77.6 ± 31.8 | 0.497 |
| C-reactive protein (mg/dL) | 0.3 ± 0.2 | 0.2 ± 0.2 | 0.448 |
| spKt/V | 1.4 ± 0.2 | NA | |
| No. of oral statin | 0 | 0 | NA |
| No. of oral calcium channel blocker | 3 | 2 | 0.926 |
| No. of oral RAS inhibitors | 7 | 2 | 0.227 |
| No. of oral cinacalcet | 0 | 0 | NA |
Data are expressed as mean ± SD or total numbers. NA, not applicable.
Figure 1.
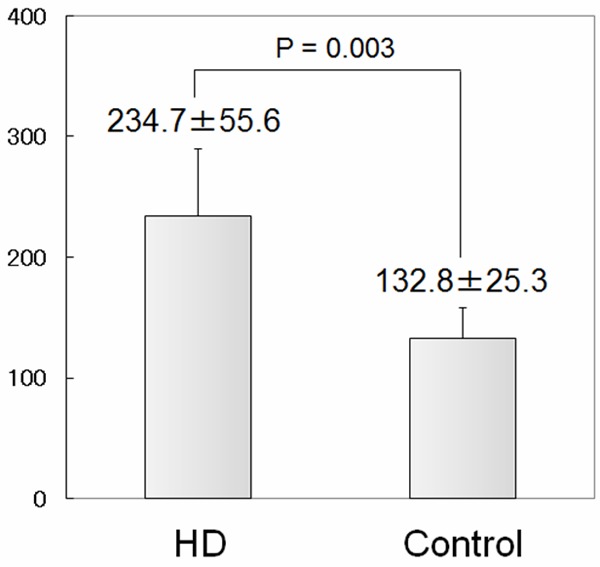
Serum levels of F2-isoprostanes in healthy controls and stable hemodialysis (HD) patients. Blood samples were collected just before the first HD session of the week. Serum levels of F2-isoprostanes were measured by competitive ELISA. HD group shows a significant increase of the marker compared to the control. Brackets indicate SD.
Figure 2.
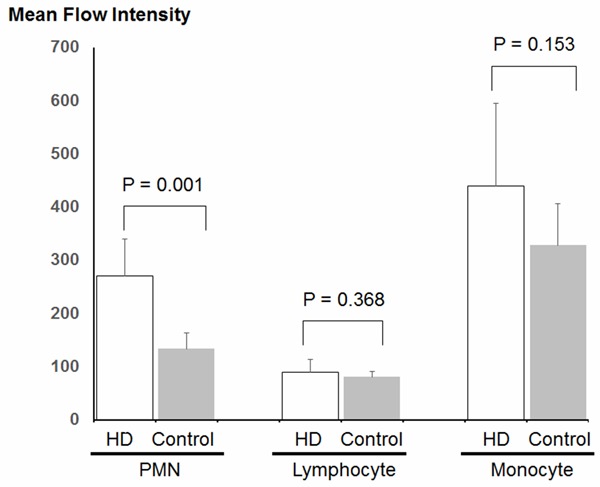
Flowcytometric analysis of reactive oxygen species (ROS) production in circulating leukocytes during HD. Blood samples were obtained at middle of HD session in HD group. After lysing red blood cells, leukocytes were treated using total ROS detection kit from Enzo Life Science. Resuspended cells were assayed with flowcytometry. Significant difference is observed only in polymorphonuclear leukocytes (PMN) subtype. Brackets indicate SD.
Figure 3.
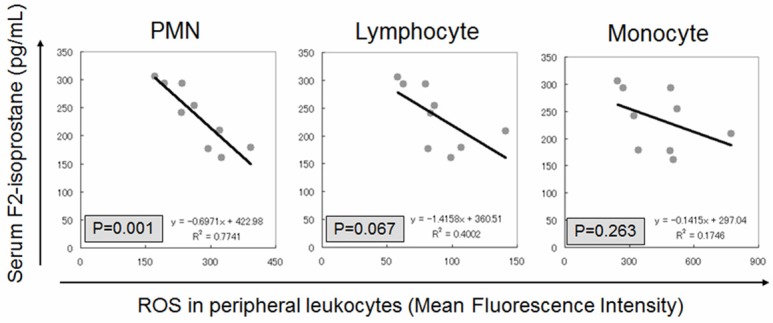
Correlation between serum levels of F2-isoprostanes and reactive oxygen species (ROS) production in circulating leukocytes in HD patients. All the fractions of leukocytes show negative correlation but only PMN subtype has significant correlation.
Discussion
Using flowcytometry analysis, we showed that different subtypes of circulating leukocytes undergo oxidative stress during HD therapy. The main finding of the present study is the direct demonstration of increased levels of ROS in circulating PMN during HD procedure. This suggests that circulating PMN have an important role as a pathogenesis of HD-related oxidative stress. Tarng et al. showed that phorbol-12-myristate-13-acetate (PMA)-induced ROS levels in circulating leukocytes of peritoneal dialysis patients was significantly higher than that obtained from healthy controls or nondialyzed patients and extensive increase was observed only in PMN fraction [19]. Our result is partially consistent with their result. It is not well studied why ROS levels in circulating PMN is stronger than other subtypes of leukocytes.
Another important finding in the present study is that there is a negative correlation between serum F2-isoprostanes levels and the ROS levels in circulating leukocytes in HD patients. One possible explanation is that the inverse correlation is resulted from different characteristics of the targets. Serum F2-isoprostanes are one of the end products of lipid metabolism during imbalance of oxidative stress. On the other hand, the measurement of intracellular ROS levels in leukocytes may reflect direct response of circulating blood cells against oxidative stress during HD procedure. Oxidative stress due to HD procedure may affect quality and longevity of circulating leukocytes. Consistent with our results, cell viability and p-cresyl sulfate-induced ROS levels have a significant negative correlation in in vitro study [20]. Oxidative stress has deleterious effects on granulocyte, resulting in failure to generate oxidative burst-an intracellular mechanism of bacteriolysis [21]. Serum concentration of lipid peroxidation product, malondialdehyde (MDA), is elevated in samples from post-dialyzed patients compared to those from non-dialyzed patients, while the ROS levels in circulating granulocytes remained in the same levels [22]. Another possible explanation is that negative correlation is caused from decrease of leukocyte viability by enhanced oxidative stress in CKD and HD. Apoptosis of monocytes are enhanced in uremic patients [23]. In vitro study, PMNs are injured by accelerated oxidative stress with methylglyoxal and H2O2, which are resemble to uremic milieu [24]. High levels of serum F2-isoprostane suggest augmented imbalance of oxidative stress, which may decrease viability of circulating leukocytes with high ROS levels in circulating blood cells. This may result in negative correlation between serum F2-isoprostane levels and ROS levels in PMN. There was negative but no significant correlation between these two factors in healthy control (data not shown). This suggests that imbalance of oxidative stress is a result of the quite compound cause.
In conclusion, our results suggest that persistent uremic condition in regular HD patients increases imbalance of oxidative stress, resulting in increase of serum F2-isoprostanes levels and ROS levels in leukocytes but that the ROS levels in circulating leukocytes during HD procedure has negative correlation with F2-isoprostanes, one of indirect oxidative stress markers. The direct measurement of ROS levels in circulating leukocytes by flowcytometry is a useful method to examine condition of oxidative stress during HD procedure. Further study is required for correlation between indirect measurement in serum oxidative stress marker levels and direct measurements of the ROS levels in circulating blood cells.
Disclosure of conflict of interest
None.
References
- 1.Ikizier TA, Morrow JD, Roberts LJ, Evanson JA, Becker B, Hakim RM, Shyr Y, Himmelfarb J. Plasma F2-isoprostane levels are elevated in chronic hemodialysis patients. Clin Nephrol. 2002;58:190–197. doi: 10.5414/cnp58190. [DOI] [PubMed] [Google Scholar]
- 2.Sela S, Shurtz-Swirski R, Shapiro G, Nasser L, Hamzi M, Shasha SM, Kristal B. Oxidative stress during hemodialysis: effect of heparin. Kidney Int Suppl. 2001;78:S159–S163. doi: 10.1046/j.1523-1755.2001.59780159.x. [DOI] [PubMed] [Google Scholar]
- 3.Himmelfarb J, Lazaus JM, Hakim RM. Reactive oxygen species production by monocytes and polymorphonuclear leukocytes during hemodialysis. Am J Kidney Dis. 1991;17:271–276. doi: 10.1016/s0272-6386(12)80473-2. [DOI] [PubMed] [Google Scholar]
- 4.Coombes JS, Fassett RG. Antioxidant therapy in hemodialysis patients: a systemic review. Kidney Int. 2012;81:233–246. doi: 10.1038/ki.2011.341. [DOI] [PubMed] [Google Scholar]
- 5.Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 6.Handelman GJ, Walter MF, Adhikarla R, Gross J, Dallal GE, Levin NW, Blumberg JB. Elevated plasma F2-isoprostanes in patients on longterm hemodialysis. Kidney Int. 2001;59:1960–1966. doi: 10.1046/j.1523-1755.2001.0590051960.x. [DOI] [PubMed] [Google Scholar]
- 7.Chao JC, Yuan MD, Chen PY, Chien SW. Vitamin C and E supplements improve the impaired antioxidant status and decrease plasma lipid peroxides in hemodialysis patients. J Nutr Biochem. 2002;13:653–663. doi: 10.1016/s0955-2863(02)00209-7. [DOI] [PubMed] [Google Scholar]
- 8.Bayes B, Pastor MC, Bonal J, Junca J, Romero R. Homocysteine and lipid peroxidation in haemodialysis: role of folic acid and vitamin E. Nephrol Dial Transplant. 2001;16:2172–2175. doi: 10.1093/ndt/16.11.2172. [DOI] [PubMed] [Google Scholar]
- 9.Ohkawa S, Yoneyama T, Shimoi K, Takita T, Maruyama Y, Kumagai H. Pro-oxidative effect of alpha-tocopherol in the oxidation of LDL isolated from co-antioxidant-depleted non-diabetic hemodialysis patients. Atherosclerosis. 2004;176:411–418. doi: 10.1016/j.atherosclerosis.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 10.Himmelfarb J, McMonagle E, McMenamin E. Plasma protein thiol oxidation and carbonyl formation in chronic renal failure. Kidney Int. 2000;58:2571–2578. doi: 10.1046/j.1523-1755.2000.00443.x. [DOI] [PubMed] [Google Scholar]
- 11.Himmelfarb J, McMenamin ME, Loseto G, Heinecke JW. Myeloperoxidase-catalyzed 3-chlorotyrosine formation in dialysis patients. Free Radic Biol Med. 2001;31:1163–1169. doi: 10.1016/s0891-5849(01)00697-9. [DOI] [PubMed] [Google Scholar]
- 12.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, Nguyen-Khoa T, Nguyen AT, Zingraff J, Jungers P, Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 13.Dounousi F, Papavasiliou E, Makedou A, Ioannou K, Katopodis KP, Tseiepis A, Siamopoulos KC, Tsakiris D. Oxidative stress is progressively enhanced with advancing stages of chronic kidney disease. Nephrol Dial Transplant. 2006;21:385–395. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 14.Karamouzis I, Sarafidis PA, Karamouzis M, Iliadis S, Haidich AB, Sioulis A, Vavatsi-Christaki N, Grekas DM. Increase in oxidative stress but not in antioxidant capacity with advancing stages of chronic kidney disease. Am J Nephrol. 2008;28:397–404. doi: 10.1159/000112413. [DOI] [PubMed] [Google Scholar]
- 15.Basu S. Isoprostanes: novel bioactive products of lipid peroxidation. Free Radic Res. 2004;38:105–122. doi: 10.1080/10715760310001646895. [DOI] [PubMed] [Google Scholar]
- 16.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC. Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology. 2012;17:311–321. doi: 10.1111/j.1440-1797.2012.01572.x. [DOI] [PubMed] [Google Scholar]
- 17.Daugirdas JT. Second generation logarithmic estimates of single pool variable volume Kt/V: an analysis of error. J Am Soc Nephrol. 1993;4:1205–1213. doi: 10.1681/ASN.V451205. [DOI] [PubMed] [Google Scholar]
- 18.Tarng DC, Wen CT, Huang TP, Chen CL, Liu TY, Wei YH. Increased oxidative damage to peritoneal blood leukocyte DNA in chronic peritoneal dialysis patients. J Am Soc Nephrol. 2002;13:1321–1330. doi: 10.1097/01.asn.0000013301.11876.7e. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe H, Miyamoto Y, Honda D, Tanaka H, Wu Q, Endo M, Noguchi T, Kadowaki D, Ishima Y, Kotani S, Nakajima M, Kataoka K, Kim-Mitsuyama S, Tanaka M, Fukagawa M, Otasiri M, Maruyama T. P-Cresyl sulfate causes renal tubular cell damage by inducing oxidative stress by activation of NADPH oxidase. Kidney Int. 2013;83:582–592. doi: 10.1038/ki.2012.448. [DOI] [PubMed] [Google Scholar]
- 20.Amer J, Fibach E. Chronic oxidative stress reduces the respiratory burst response of neutrophils from beta-thalassaemia patients. Br J Haemotol. 2005;129:435–441. doi: 10.1111/j.1365-2141.2005.05463.x. [DOI] [PubMed] [Google Scholar]
- 21.Yoon JW, Pahl MV, Vaziri ND. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 2007;71:167–172. doi: 10.1038/sj.ki.5002019. [DOI] [PubMed] [Google Scholar]
- 22.Heidenreich S, Schmidt M, Bachmann J, Harrach B. Apoptosis of monocytes cultured from long-term hemodialysis patients. Kidney Int. 1996;49:792–799. doi: 10.1038/ki.1996.110. [DOI] [PubMed] [Google Scholar]
- 23.Nakayama M, Nakayama K, Zhu WJ, Shirota Y, Terawaki H, Sato T, Kohno M, Ito S. Polymorphonuclear leukocyte injury by methylglyoxal and hydrogen peroxide: a possible pethological role for enhanced oxidative stress in chronic kidney disease. Nephrol Dial Transplant. 2008;23:3096–3102. doi: 10.1093/ndt/gfn218. [DOI] [PubMed] [Google Scholar]
- 24.Odettl P, Garbaldi S, Gurreri G, Aragno I, Dapino D, Pronzato MA, Marinari UM. Protein oxidation in hemodialysis and kidney transplantation. Metabolism. 1996;45:1319–1322. doi: 10.1016/s0026-0495(96)90108-0. [DOI] [PubMed] [Google Scholar]


