Abstract
Glioma is one of the most common intracranial tumors, and the prognosis is poor though more and more treatments are employed. ERK/MAPK signaling has been reported to be associated with glioma. In the present study, we aimed to investigate hirudin as an antineoplastic drug inhibits ERK/MAPK signaling in glioma growth in vitro. The cell proliferation and apoptosis rate were detected using MTS and Annexin V staining assay, cell cycle distribution was detected using flowcytometry assay. Furthermore, the relevant molecules of ERK/MAPK signaling were examined using Western blot analysis and immunofluorescence staining assay. We provide the first evidence that hirudin increase inactivation state of ERK1/2, down-regulate the expression of canonical ERK/MAPK signaling pathway and establish an important role for hirudin in the treatment of glioma cells.
Keywords: Hirudin, ERK/MAPK signaling, glioma, cell growth
Introduction
Glioma is one of the most frequent types of brain tumor. Standard treatments include surgery, radiotherapy and chemotherapy [1-4]. Prognosis is dismal with an average survival of approximately 12 months and the poor prognosis of patients with glioma has been remained during the past decades [2-5].
The mechanism of the development and progression depend on many factors and high-grade gliomas are heterogeneous tumors in both cytology and genetics signatures [5]. Up-regulation of the ERK/MAPK signaling pathway has been proven to take part in the amplification of mitogenic stimuli and promotion of cellular proliferation of malignant gliomas [6-8]. Investigators indicate that the majority of gliomas display upregulated ERK/MAPK signaling pathway, therefore down-regulation of the ERK/MAPK signaling pathway may represent appropriate alternate therapy for glioma patients [9].
Hirudin is the generic name for a family of closely related homologous peptides that are all found in the cranial salivary glands of the medicinal leech (Hirudo medicinalis). Hirudin had been used in medical therapies since ancient times [10,11]. Investigators indicated that hirudin may act as an anti-inflammatory/edema mediator in lung protection and ICH, exert antitumor effects in Hep-2 human laryngeal cancer cells [12-14].
In this study, we demonstrated that hirudin suppressed ERK1/2 nuclear translocation and pERK1/2 expression using western blot analysis. These results are consistent with our hypothesis that hirudin may down-regulate the ERK/MAPK signaling pathway in glioma cell lines.
We provide the first evidence that hirudin increase inactivation state of ERK1/2, down-regulate the canonical ERK/MAPK signaling pathway and establish an important role for hirudin in the treatment of gliomas. This thus enhances the potential use to release patients suffering from gliomas.
Materials and methods
Cell culture, culture conditions and reagents
The human LN229 and U251 cell lines were purchased from the Institute of Biochemistry and Cell Biology, Chinese Academy of Science. All cells were maintained in a 37°C, 5% carbon dioxide incubator in Dulbecco’s modified Eagle’s medium (DMEM; Gibco) supplemented with 10% fetal-bovine serum (Invitrogen).
Hirudin was purchased from Sigma and maintained in -20°C (St. Louis, MO, USA).
Cell proliferation assay/MTS assay
Cells were plated in 96-well plates at a density of 5000 cells/well. Cells were allowed to attach overnight in growth medium. After 24 hours, cells were treated with hirudin. After incubation for 72 hours, cellular proliferation was measured using the MTS assay and absorbance was measured at 490 nm. Proliferation data are presented as means ± SD.
Cell cycle assay
For cell cycle analysis by flow cytometry, hirudin-treated and negative control (NC) cells in the log phase of growth were harvested, washed with phosphate-buffered saline, fixed with 90% ethanol overnight at 4°C, and then incubated with RNase at 37°C for 30 min. Nuclei of cells were stained with propidium iodide for an additional 30 min. A total of 20 000 nuclei were examined in a FACS Calibur flow cytometer (Becton-Dickinson), and DNA histograms were analyzed using Modifit software. Three independent experiments were performed and the data are presented as the mean ± SD.
Apoptosis assay
48 hours after presentation of hirudin and PBS, apoptosis in cultured cells was evaluated using annexin V labeling. For the annexin V assay, an annexin V-FITC labeled Apoptosis Detection Kit (Abcam) was used according to the manufacturer’s protocol. Three independent experiments were performed and the data are presented as the mean ± SD.
Western blotting
Extraction of proteins from cultured cells was followed by immunoblotting with the relevant antibodies (primary antibodies: mouse anti-human monoclonal antibodies, 1:1000; secondary antibody: rabbit anti-mouse polyclonal antibody, 1:2000). Each experiment was repeated at least three times.
Immunofluorescence staining assay
Immunofluorescence staining was conducted with LN229 and U251 cells cultured on cover slips. The cells were fixed in 4% paraformaldehyde and permeabilized for 10 min in buffer containing 0.1% Triton X-100. The relevant antibodies were then added at the dilutions recommended by the manufacturers (primary antibodies: mouse anti-human monoclonal antibodies, 1:1000; secondary antibody labeled with fluorescence: rabbit anti-mouse polyclonal antibody, 1:2000). DAPI reagent was used to stain the glioma cell nuclei, and the cells were visualized using FV-1000 laser-scanning confocal microscopes and analyzed using IPP5.1 (Olympus).
Statistical analysis
Quantitative variables were expressed as means ± SD and analyzed by one-way ANOVA and Student’s t-test. All differences were considered to be statistically significant at the level of P<0.05. Statistics were performed using the SPSS Graduate Pack, version 11.0, statistical software (SPSS).
Results
Hirudin suppressed growth of LN229 and U251 cell lines
In the study, the LN229 and U251 cells were exposed to 0, 3.75, 7.5, 15, 30, 60 and 120 mM hirudin and PBS for 48 hours. The results indicated that the cellular viability decreased with the increased hirudin concentration (Figure 1). The 50% growth inhibition (IC50) of hirudin was 30 mM for LN229 and 15 mM for U251 after 48 hours, respectively.
Figure 1.
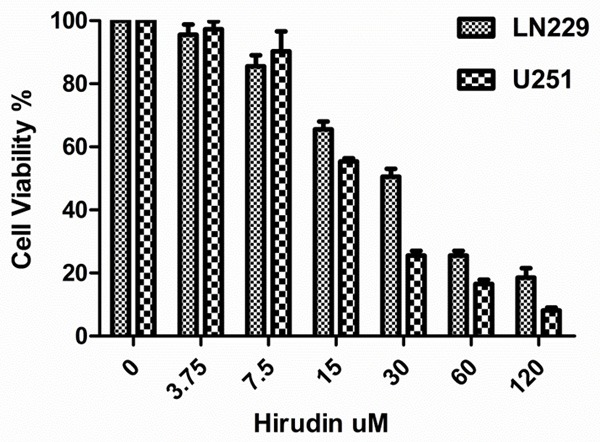
Proliferation effects in LN229 and U251 cells. Cells were treated with hirudin (3.75-120 mM) and PBS, incubated for 48 hours. Proliferation was assessed by MTS assay. Experiments were performed independently at least 3 times. Data are the means ± SD.
Hirudin increased cell apoptosis in LN229 and U251 cell lines
Hirudin also inhibited glioma cell survival. As shown in Figure 2A, compared with NC group (2.6% ± 4.1% and 4.0% ± 0.2%) in LN229 and U251 cells, the treatment of hirudin caused a significant increase in (30.0% ± 1.0% and 39.00% ± 3.3%) apoptotic death (P<0.05).
Figure 2.
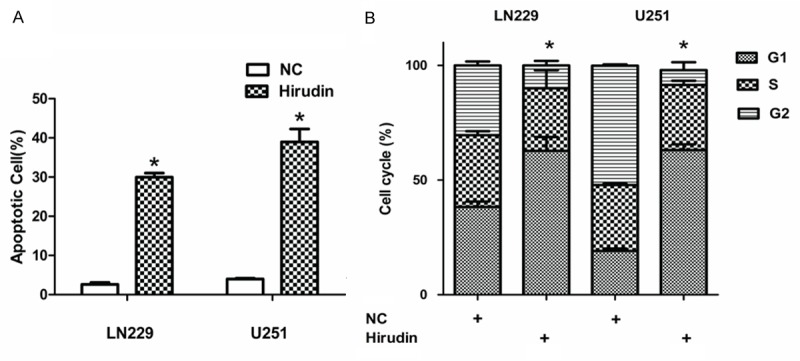
Hirudin promotes apoptosis and arrest cycle. A and B: Hirudin significantly led to cell apoptosis and G2 arrest in both LN229 and U251 cells (*P<0.05), relative to the NC cells.
The intrinsic pathway of apoptosis is controlled by Bcl-2 family proteins, and cell death depend on the balance between pro-apoptotic (for example, Bax) and anti-apoptotic (for example, Bcl-2, Bcl-xl, Mcl-1 and so on) proteins. Bcl-2 protein level decreased in hirudin-treated cell lines, as shown using western blotting (Figure 3A and 3B). Together, this analysis demonstrates that hirudin induce cell apoptosis, down-regulate of Bcl-2 through apoptosis pathways as reported previously.
Figure 3.
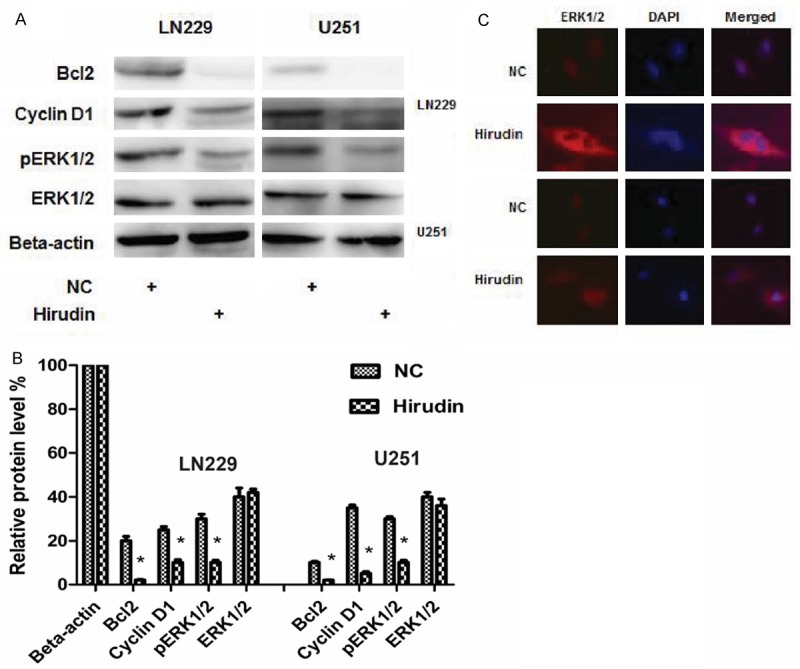
Expression of related protein and immunofluorescence assay after presentation of PBS and hirudin. A and B: Expression of related protein changed in LN229 and U251 cells. Relative protein levels were determined with Western blot analysis using beta-actin as an internal control. C: Immunofluorescence assay for ERK1/2. The location of ERK1/2 in cells shifted from nucleus to cytoplasm when treated by hirudin, compared with NC group.
Hirudin increased cell cycle arrest at the G1 phase in LN229 and U251 cell lines
To further characterize the growth arrest, LN229 and U251 cell lines were treated with 30 mM and 15 mM hirudin, and after 48 hours they were subjected to FACS analysis of propidium-iodide-stained cells. Treatment with hirudin resulted in accumulation of cells in the G1 phase, with approximately 62.7% ± 6.1% LN229 and 63.1% ± 2.5% U251 for hirudin-treated cells in the G1 phase compared with 38.3% ± 2.3% LN229 and 19.1% ± 1.0% U251 for PBS-treated cells (Figure 2A) (P<0.05). Cyclin D1 level decreased in cell lines, as shown using western blotting (Figure 3A and 3B). These analyses indicate that hirudin can induce cell cycle at arrest G1 phase.
Hirudin inhibits ERK/MAPK signaling through nuclear ERK1/2 loss
ERK1/2, target proteins and their expression in many cell lines were descripted. ERK/MAPK signaling has been proved to be associated with various disease pathologies. Hirudin was reported merely to act as a negative regulator of signaling. Western blotting of total protein extracts from LN229 and U251 cells revealed that total ERK1/2 content remained, but pERK1/2 content reduced after treatment of hirudin (Figure 3A and 3B). Level of the protein Cyclin D1, a known Wnt downstream target, was also significantly reduced (Figure 3A and 3B). Immunofluorescence assays in the LN229 and U251 cell lines revealed the changed nuclear location of ERK1/2. After the treatment of hirudin, ERK1/2 was mainly located in the cytoplasm, while ERK1/2 was mainly located in the nucleus in NC cells (Figure 3C). It shows that the location of ERK1/2 in cells shifts from nucleus to cytoplasm when the cells are treated with hirudin.
Discussion
Glioma is one of the most frequent types of brain tumor and it is invariably associated with a poor prognosis. Standard treatments include surgery, radiotherapy and chemotherapy [1-4]. Prognosis is dismal with an average survival of approximately 12 months despite the rapid progress of new insights and technology in therapy and nursing care, and the poor prognosis of patients with glioma have been remained during the past decades [2-5].
The mechanism of the development and progression depend on many factors and high-grade gliomas are heterogeneous tumors in both cytology and genetics signatures [5]. It is reported that ERK/MAPK signaling is closely associated with glioma.
ERK/MAPK signaling pathway is an essential serine/threonine kinase constituent of the mitogen-activated protein kinase (MAPK) pathway. Upon activation by growth factors, serum, cytokines, and osmotic stresses, ERK can phosphorylate and regulate multiple substrates such as cytoskeletal proteins, kinases, and transcription factors. These events in turn result in gene expression changes and regulate many fundamental cellular functions such as cell growth, proliferation, and apoptosis. The up-regulation of the ERK/MAPK signaling pathway has been proven to take part in the amplification of mitogenic stimuli and promotion of cellular proliferation of malignant gliomas [6-8]. Investigators indicate that the majority of gliomas display upregulated ERK/MAPK signaling pathway, therefore down-regulation of the ERK/MAPK signaling pathway may represent appropriate alternate therapy for glioma patients [9].
Hirudin is the generic name for a family of closely related homologous peptides that are all found in the cranial salivary glands of the medicinal leech (Hirudo medicinalis). Hirudin is the most potent natural direct thrombin inhibitor currently known and it is capable of inhibiting fluid phase and clot-bound thrombin [10]. Because of their high capacity for blood removal, leeches have been used in medical therapies since ancient times [11]. Investigators indicated that hirudin may inhibit the inflammation and fibrosis play a role in lung protection as an anti-inflammatory mediator, and hirudin may be helpful in the treatment of edema at the acute stage of ICH [12,13].
It is reported that hirudin followed by giving stealthy liposomal vinblastine may be beneficial for inhibiting the growth and metastasis of melanoma in vivo and exerts antitumor effects in Hep-2 human laryngeal cancer cells [14,15].
These papers suggest that hirudin may have therapeutic potential in cancer. The real role in glioma and relative mechanism of hirudin has not been reported previously. Herein, we hypothesize that hirudin may be used as an effective anti-glioma agents via suppressing the activity of ERK/MAPK signaling pathway.
In this study, we demonstrated that the pERK1/2 expression decreased in hirudin-treated cells. We also showed that hirudin suppressed nuclear translocation and expression of ERK1/2 using western blot analysis. These results are consistent with our hypothesis that hirudin may down-regulate the ERK/MAPK signaling pathway in glioma cell lines.
We provide the first evidence that hirudin increase inactivation state of ERK1/2, down-regulate the canonical ERK/MAPK signaling pathway and establish an important role for hirudin in the treatment of gliomas. This thus enhances the potential use to release patients suffering from gliomas.
Disclosure of conflict of interest
None.
Authors’ contribution
L. Z. conceived and designed the study, performed experiments, analyzeddata, and finally prepared the initialmanuscript.
Abbreviation
NC, Negative control
References
- 1.Chen W, Wu Q, Mo L. Intra-arterial chemotherapy is not superior to intravenous chemotherapy for malignant gliomas: a systematic review and meta-analysis. Eur Neurol. 2013;70:124–132. doi: 10.1159/000346580. [DOI] [PubMed] [Google Scholar]
- 2.Bregy A, Shah A, Diaz M, Pierce H, Ames P, Diaz D, Komotar RJ. The role of gliadel wafers in the treatment of high-grade gliomas. Expert Rev Anticancer Ther. 2013;13:1453–1461. doi: 10.1586/14737140.2013.840090. [DOI] [PubMed] [Google Scholar]
- 3.Yin A, Cai S, Dong Y, Zhang L, Liu B, Cheng J, Zhang X. A meta-analysis of temozolomide versus radiotherapy in elderly glioblastoma patients. J Neurooncol. 2014;116:315–324. doi: 10.1007/s11060-013-1294-0. [DOI] [PubMed] [Google Scholar]
- 4.Yin A, Zhang L, Cheng J, Dong Y, Liu B, Han N, Zhang X. Radiotherapy plus concurrent or sequential temozolomide for glioblastoma in the elderly: a meta-analysis. PLoS One. 2013;8:e74242. doi: 10.1371/journal.pone.0074242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Jiang T. Understanding high grade glioma: molecular mechanism, therapy and comprehensive management. Cancer Lett. 2013;331:139–146. doi: 10.1016/j.canlet.2012.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Gao Q, Lei T, Ye F. Therapeutic targeting of EGFR-activated metabolic pathways in glioblastoma. Expert Opin Investig Drugs. 2013;22:1023–1040. doi: 10.1517/13543784.2013.806484. [DOI] [PubMed] [Google Scholar]
- 7.Kondo Y, Hollingsworth EF, Kondo S. Molecular targeting for malignant gliomas. Int J Oncol. 2004;24:1101–1109. [PubMed] [Google Scholar]
- 8.Wong KK. Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat Anticancer Drug Discov. 2009;4:28–35. doi: 10.2174/157489209787002461. [DOI] [PubMed] [Google Scholar]
- 9.Thompson N, Lyons J. Recent progress in targeting the Raf/MEK/ERK pathway with inhibitors in cancer drug discovery. Curr Opin Pharmacol. 2005;5:350–356. doi: 10.1016/j.coph.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Fischer KG. The role of recombinant hirudins in the management of thrombotic disorders. BioDrugs. 2004;18:235–268. doi: 10.2165/00063030-200418040-00003. [DOI] [PubMed] [Google Scholar]
- 11.Nowak G, Schrör K. Hirudin-the long and stony way from an anticoagulant peptide in the saliva of medicinal leech to a recombinant drug and beyond a historical piece. Thromb Haemost. 2007;98:116–119. [PubMed] [Google Scholar]
- 12.Bao Y, Geng Y, Jing H. Effect of hirudin on the levels of acute lung injury rat tumor necrosis factor-α and matrix metalloproteinase-12. Mol Med Rep. 2012;5:873–875. doi: 10.3892/mmr.2011.739. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Zhao Z, Zhao S, Sheng Y, Zhao Z, Gao C, Li J, Liu X. Recombinant hirudin treatment modulates aquaporin-4 and aquaporin-9 expression after intracerebral hemorrhage in vivo. Mol Biol Rep. 2009;36:1119–1127. doi: 10.1007/s11033-008-9287-3. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Lv M, Xu E, Shao F, Feng Y, Yang J, Shi L. Recombinant hirudin suppresses the viability, adhesion, migration and invasion of Hep-2 human laryngeal cancer cells. Oncol Rep. 2015;33:1358–6134. doi: 10.3892/or.2015.3717. [DOI] [PubMed] [Google Scholar]
- 15.Guo RR, Liu Y, Lu WL, Zhao JH, Wang XQ, Zhang H, Wang JC, Zhang X, Zhang Q. A recombinant peptide, hirudin, potentiates the inhibitory effects of stealthy liposomal vinblastine on the growth and metastasis of melanoma. Biol Pharm Bull. 2008;31:696–702. doi: 10.1248/bpb.31.696. [DOI] [PubMed] [Google Scholar]


