Abstract
Background: Our research aims to investigate the associations between education level and osteoporosis (OP) in Chinese postmenopausal women. Methods: A large-scale, community-based, cross-sectional study was conducted to examine the associations between education level and OP. A self-reported questionnaire was used to access the demographical information and medical history of the participants. A total of 1905 postmenopausal women were available for data analysis in this study. Multiple regression models controlling for confounding factors to include education level were performed to investigate the relationship with OP. Results: The prevalence of OP was 28.29% in our study sample. Multivariate linear regression analyses adjusted for relevant potential confounding factors detected significant associations between education level and T-score (β = 0.025, P-value = 0.095, 95% CI: -0.004-0.055 for model 1; and β = 0.092, P-value = 0.032, 95% CI: 0.008-0.175 for model 2). Multivariate logistic regression analyses detected significant associations between education level and OP in model 1 (P-value = 0.070 for model 1, Table 5), while no significant associations was reported in model 2 (P value = 0.131). In participants with high education levels, the OR for OP was 0.914 (95% CI: 0.830-1.007). Conclusion: The findings indicated that education level was independently and significantly associated with OP. The prevalence of OP was more frequent in Chinese postmenopausal women with low educational status.
Keywords: Educational level, osteoporosis, Chinese postmenopausal women
Introduction
Osteoporosis (OP) is a systemic skeletal disorder, characterized by decreased bone mass and deterioration of the microarchitecture of bone tissue, which leads to a higher risk of fragility fractures [1]. The disease usually remains undiagnosed until the first fracture is exhibited, and the fracture frequently involves the spine, hip, or wrist [1,2]. Among these, hip fractures have mortality rates as high as 15-30% [3]. The prevalence rate of hip fractures is ~1.66 million in the world annually, which has been predicted to increase four-fold by 2050 due to the rapid aging of the population globally [4]. It is well known that low levels of estrogen in postmenopausal females lead to increased bone loss and thus a higher prevalence of OP [5]. Consequently, OP has progressively become a worldwide public health problem, especially in older women, which generates a substantial burden of morbidity and economic cost to society [6].
OP is a complicated condition resultant from various determinants affecting all races and ethnicities. Risk factors include both genetic components and environmental influences, which are implicated in considerable variation in the quality of the bone [7,8]. For instance, high age, low body mass, female gender, lack of physical activity, smoking, low calcium/vitamin D intake, and excessive alcohol intake are several frequently reported risk factors of OP [9-11]. Additionally, OP may be complicated by a number of diseases, such as kidney-related diseases [12] and chronic inflammatory disease [13]. Moreover, a low educational status and inadequate health knowledge have been reported as possible contributing factors to several diseases, including rheumatoid arthritis [14] and OP [15-17].
However, the association between education level and OP remains controversial. A higher education level has been shown to be significantly associated with better health literacy about OP in Salvadorian women [18] and Chinese women in Singapore [19]. In Moroccan postmenopausal women [20] and Korean breast cancer survivors [21], a low education level was significantly correlated with low bone density. However, some studies have argued that knowledge about OP does not necessarily result in OP-preventive lifestyles, such as regular exercise and sufficient intake of calcium and vitamin D supplements [18,22]. Therefore, more studies about the relationship between education level and the prevalence of OP will provide a better insight into the contributory factors of OP and designing efficient interventional programs. A self-reported questionnaire is an accessible, reliable, and economical tool to conduct a large-scale comprehensive survey and to estimate the potential risk factors and causes for common diseases [23]. Previous reports have shown that patient self-report outcomes could provide customary clinical care for many diseases, such as OP [24].
Using this convenient approach, together with an association analysis, the present study aims to examine whether OP prevalence is related to education level in a large number of Chinese postmenopausal women.
Methods
Study population
We performed a risk-factor study for OP using a random sample of the Chinese population. Participants were recruited from rural and urban communities in Shanghai. Participants aged 30-90 years were included in this study. More than 2,000 postmenopausal women were invited to a screening visit between 2011 and 2014. Written consent was obtained from all patients before the study, which was performed in accordance with the ethical standards in the Declaration of Helsinki, and approved by the Medicine Ethical Committee of Shanghai Tongji Hosptial.
Some participants with chronic diseases and conditions that might potentially affect bone mass, structure, or metabolism were excluded. Briefly, the exclusion criteria were as follows: a history of 1) serious residual effects of cerebral vascular disease; 2) serious chronic renal disease (Glomerular filtration rate-GFR < 30 mL/min/1.73 m2); 3) serious chronic liver disease or alcoholism; 4) significant chronic lung disease; 5) corticosteroid therapy at pharmacologic levels; 6) evidence of other metabolic or inherited bone disease, such as hyper- or hypoparathyroidism, Paget disease, osteomalacia, or osteogenesis imperfecta; 7) recent (within the past year) major gastrointestinal disease, such as peptic ulcer, malabsorption, chronic ulcerative colitis, regional enteritis, or significant chronic diarrhea; 8) Cushing syndrome; 9) hyperthyroidism; and 10) any neurologic or musculoskeletal condition that would be a non-genetic cause of low bone mass. A total of 1905 individuals were available to the data analysis in this study.
Data collection
All study subjects underwent complete clinical baseline characteristics evaluation, which included a physical examination and response to a structured, nurse-assisted, self-administrated questionnaire to collect information on age, gender, residential region, visit date, family history, lifestyle, dietary habits, physical activity level during leisure time, use of vitamins and medications, smoking, alcohol consumption, and self-reported medical history.
Body weight and height were measured according to a standard protocol. Smoking and alcohol consumption were categorized as never, current (smoking or consuming alcohol regularly in the past 6 months), or ever (cessation of smoking or alcohol consumption for more than 6 months). Regular exercise was defined as any kind of physical activity 3 or more times per week. Self-reported medical and therapy history was categorized as “no” or “yes.” HTN was defined as blood pressure ≥ 140/90 mmHg, or a history of hypertension medication. Diabetes mellitus (DM) was defined by oral glucose tolerance test (OGTT) and either HbAlc ≥ 6.5% or the use of insulin or hypoglycemic medications. Dietary habits, including consumption of meat, fish and potato food was evaluated by a semi-quantitative food frequency questionnaire (group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always). For instance, to determine frequency of meat food preference, the participants were asked, “How often you eat meat food”? The possible answers were: “seldom”, “once or twice per week”, “once per 2 days”, or “always”, and the answers were taken as a subjective assessment. To answer the question, the participants were required to decide two issues based on their impressions: 1) whether or not the consumed food were actually meat; and 2) the frequency with which they consumed meat foods.
Education was commonly divided into five stages: illiteracy, primary school, junior high school, senior high school and group. To determine education level, the participants were asked, “How about your education level”? The possible answers were: “illiteracy”, “primary school”, “junior high school”, “senior high school”, or “college”, and the answers were taken as a subjective assessment. To answer the question, the participants were required to decide one issues based on their impressions: whether or not the education experience was actually approved by authority.
The study outcomes
The bone mineral density (BMD g/cm2) was measured at calcaneus by standardized quantitative ultrasound (QUS, Hologic Inc., Bedford, MA, USA) utilizing T-scores based on WHO criteria [25], which were obtained from the automated equipment. T-score refers to the ratio between patient’s BMD and that of young adult population of same sex and ethnicity. T-score of > -1 was taken as normal, between -1 and -2.5 osteopenic and < -2.5 as osteoporotic. Daily calibration was performed during the entire study period by a trained technician. The coefficients of variation of the accuracy of the QUS measurement were 0.9%. The QUS technology is less expensive, portable and also has the advantage of not using ionizing radiation, so it is safer than dual-energy X-ray absorptiometry (DEXA).
Statistical analysis
Continuous variables were analyzed to determine whether they followed normal distributions, using the Kolmogorov-Smirnov Test. Variables that were not normally distributed were log-transformed to approximate a normal distribution for analysis. Results are described as mean ± SD or median, unless stated otherwise. Differences in variables among subjects grouped by education level were determined by one way analysis of variance. Among groups, differences in properties were detected by χ2 analysis.
Two models were considered to develop for data analysis in this study. In model 1, education level was categorized by group 1: illiteracy, group 2: primary school, group 3: junior high school, group 4: senior high school and group 5: college. In model 2: education level was categorized education level were categorized by group 1: illiteracy & primary school group and group 2: high school & college group.
Univariate regression analysis was performed to determine variables associated with outcomes (T-score or OP), and to estimate confounding factors possibly disturbing the relation of frequency of fish food intake to outcomes (T-score or OP). For the associations analysis, there model have been developed. Tests were two-sided, and a P-value of < 0.05 was considered significant. Multivariable regression (MR) was performed to control potential confounding factors and determine the independent contribution of variables to outcomes (T-score or OP). Under MR models, tests were two-sided, and a P-value of < 0.1 was considered significant. Results were analyzed using the Statistical Package for Social Sciences for Windows, version 16.0 (SPSS, Chicago, IL, USA). Odds ratios (OR) with 95% confidence intervals (CI) were calculated for the relative risk of frequency of fish food intake with the outcome of OP.
Results
Clinical characteristics of subjects
The clinical baseline characteristics of the 1905 Chinese postmenopausal women are listed in Table 1. In the total sample, the mean age was 62.39 years. There was significant difference in age among groups according to education level. The minority proportions of subjects having current smoking and alcohol habits were reported (0.79% and 2.10% for smoking and drink intake, respectively). The prevalence of HTN, coronary artery disease (CAD), DM and Rheumatoid arthritis (RA) were 45.57%, 10.03%, 11.47%, and 5.71%, respectively. There were significant differences in exercise, smoking, medical and therapy history, and most of dietary habits among groups according to education level (P value < 0.05 for all).
Table 1.
The baseline characteristics of participants
| Variable | Total sample | Education level | P-value | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | |||
| N | 1905 | 224 | 264 | 716 | 447 | 254 | |
| Age | 62.39±8.99 | 71.73±6.09 | 67.53±7.49 | 59.28±7.98 | 59.29±8.79 | 63.04±7.13 | < 0.001 |
| Height | 156.35±5.71 | 152.4±4.51 | 156.22±6.76 | 157.77±4.49 | 157.56±6.28 | 162.33±6.81 | 0.002 |
| Weight | 58.85±8.47 | 57.3±5.17 | 60.33±8.16 | 58.36±9.59 | 59.39±10.3 | 64.67±9.07 | 0.602 |
| Exercise | 1364 (71.6%) | 144 (64.29%) | 187 (70.83%) | 501 (69.97%) | 341 (76.29%) | 191 (75.2%) | 0.010 |
| Smoking | 15 (0.79%) | 2 (0.89%) | 7 (2.65%) | 4 (0.56%) | 1 (0.22%) | 1 (0.39%) | 0.001 |
| Drink | 40 (2.1%) | 4 (1.79%) | 8 (3.03%) | 16 (2.23%) | 8 (1.79%) | 4 (1.57%) | 0.427 |
| HTN | 837 (44.57%) | 129 (58.37%) | 144 (55.81%) | 286 (40.4%) | 175 (39.68%) | 103 (41.2%) | < 0.001 |
| CAD | 183 (10.03%) | 20 (9.22%) | 39 (15.48%) | 55 (7.93%) | 42 (10.05%) | 27 (11.07%) | 0.016 |
| DM | 214 (11.47%) | 39 (17.89%) | 43 (16.6%) | 75 (10.7%) | 39 (8.99%) | 18 (7.11%) | < 0.001 |
| RA | 105 (5.71%) | 12 (5.53%) | 22 (8.66%) | 49 (7.05%) | 11 (2.59%) | 11 (4.45%) | 0.005 |
| Vitamin C | 247 (12.97%) | 9 (4.02%) | 32 (12.12%) | 90 (12.57%) | 71 (15.88%) | 45 (17.72%) | < 0.001 |
| Coffee | 95 (5.23%) | 1 (0.49%) | 10 (4.07%) | 35 (5.16%) | 33 (7.53%) | 16 (6.37%) | 0.004 |
| Meat | 849 (44.57%) | 78 (34.82%) | 101 (38.26%) | 339 (47.35%) | 216 (48.32%) | 115 (45.28%) | 0.001 |
| Potato | 264 (13.86%) | 34 (15.18%) | 33 (12.5%) | 106 (14.8%) | 56 (12.53%) | 35 (13.78%) | 0.748 |
| Oil | 19.14±9.05 | 20.16±8.31 | 19.63±8.79 | 20.19±9.55 | 18.35±9.15 | 16.16±7.44 | < 0.001 |
| T-score | -1.86±0.74 | -2.13±0.78 | -2.08±0.69 | -1.77±0.74 | -1.76±0.7 | -1.85±0.74 | < 0.001 |
| OP | 539 (28.29%) | 101 (45.09%) | 97 (36.74%) | 168 (23.46%) | 104 (23.27%) | 69 (27.17%) | < 0.001 |
Note: Education level were categorized by group 1: illiteracy, group 2: primary school, group 3: junior high school, group 4: senior high school and group 5: college; HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA-Rheumatoid arthritis.
An average T-score of -1.86 was reported, in addition, the prevalence of OP was 28.29% in our study sample. Significant differences in T-Score and the prevalence of OP among the five groups were reported (P value < 0.001 for both outcomes).
Association analysis for T-score
Univariate linear regression analyses were developed to include demographical information, medical history, and lifestyle to estimate the association of various clinical factors and T-score (Table 2). The variables age, Vitamin C supplement, meat food preference and education were significantly associated with the T-score (P < 0.05 for all). The results of comparison of T-score among groups according to Model 1 showed that the mean T-score was -2.13, -2.08, -1.77, -1.76 and -1.85 in the five groups, respectively (Figure 1A). There were significantly differences among the five groups (P value < 0.001). Additionally, there were significant differences among groups according to model 2 (Figure 1B, P value < 0.001), univariate analysis demonstrated a positive correlation between education level and T-score.
Table 2.
Univariate linear regression analysis for associations among variables and T-score
| Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|
| Age | -0.033 | 0.002 | < 0.001 | -0.036-0.029 |
| Height | 0.019 | 0.015 | 0.195 | -0.01-0.048 |
| Weight | -0.002 | 0.010 | 0.836 | -0.022-0.018 |
| Exercise | 0.063 | 0.038 | 0.096 | -0.011-0.137 |
| Smoking | -0.09 | 0.093 | 0.333 | -0.273-0.093 |
| Drink | 0.031 | 0.059 | 0.592 | -0.084-0.147 |
| HTN | -0.045 | 0.035 | 0.196 | -0.112-0.023 |
| CAD | -0.116 | 0.058 | 0.048 | -0.23-0.001 |
| DM | 0.039 | 0.054 | 0.467 | -0.067-0.146 |
| RA | -0.133 | 0.075 | 0.075 | -0.279-0.013 |
| Vitamin C | -0.114 | 0.051 | 0.024 | -0.213-0.015 |
| Meat intake | 0.120 | 0.034 | < 0.001 | 0.053-0.187 |
| Education | 0.088 | 0.014 | < 0.001 | 0.060-0.116 |
Note: HTN-hypertension, CAD-coronary artery disease, DMdiabetes mellitus, RA-Rheumatoid arthritis.
Figure 1.
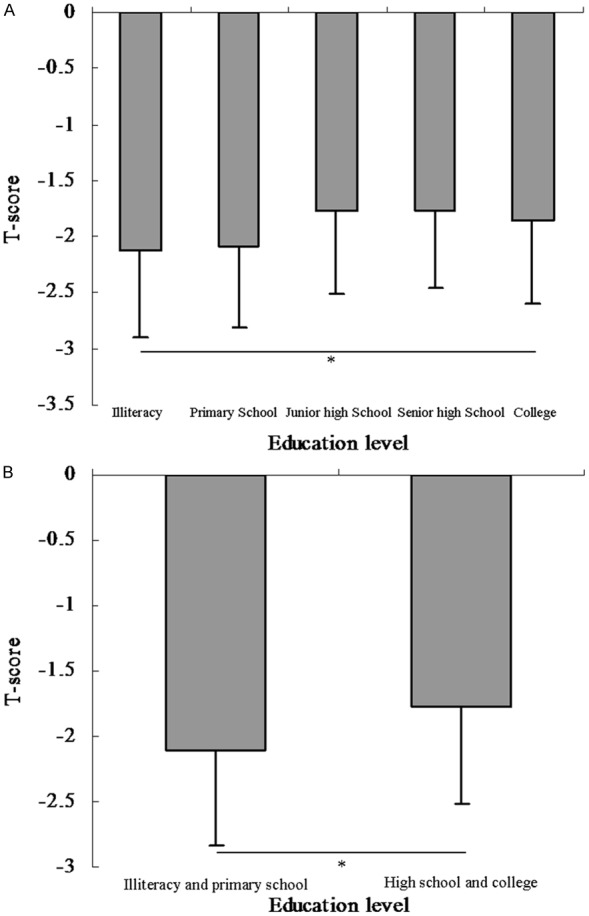
Comparison of T score among groups according to education level in Chinese postmenopausal women. A: The results of comparison of T-score among groups according to Model 1 (Model 1: education level were categorized by group 1: illiteracy, group 2: primary school, group 3: junior high school, group 4: senior high school and group 5: college). The mean T-score was -2.13, -2.08, -1.77, -1.76 and -1.85 in the five groups, respectively. There were significantly differences among the five groups (P value < 0.001). B: The results of comparison of T-score between groups according to Model 2 (Model 2: education level was categorized by illiteracy & primary school group and high school & college group). The mean T-score was -2.10 and -1.78 in the two groups, respectively. There were no significantly differences between the two groups (P value < 0.001).
Multivariate linear regression analyses were developed to include education level and the outcome of T-score. After adjustment for relevant potential confounding factors, the multivariate linear regression analyses detected significant associations (β = 0.025, P-value = 0.095, 95% CI: -0.004-0.055 for model 1; and β = 0.092, P-value = 0.043, 95% CI: 0.008-0.175 for model 2, Table 4).
Table 4.
Multiple variables linear regression analysis for the associations between frequency of meat food intake and T score
| Model | Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|---|
| Model 1 | Education level | 0.025 | 0.015 | 0.095 | -0.004-0.055 |
| Model 2 | Education level | 0.092 | 0.043 | 0.032 | 0.008-0.175 |
Note: Model 1: education level were categorized by group 1: illiteracy, group 2: primary school, group 3: junior high school, group 4: senior high school and group 5: college; Model 2: education level were categorized by illiteracy & primary school group and high school & college group; and all models adjusted for age, smoking, alcohol intake, education, exercise, medical and therapy history.
Multiple variable analyses for T-score and OP
Univariate logistic analyses were performed to evaluate associations with OP. The results indicate that age, HTN, CAD, RA, Vitamin C, Vitamin D, frequency of meat intake and education were significantly associated with OP (P value < 0.05 for all, Table 3). The comparison of prevalence of OP among groups according to model 1 reported that the prevalence of osteoporosis was 45.09%, 36.74%, 23.46%, 23.27% and 27.17% in the five groups, respectively (Figure 2A). There were significant differences among the four groups (P value < 0.001). Significant differences among groups according to model 2 were also reported (Figure 2B, P value < 0.001 for model 2). Univariate analysis demonstrates a negative correlation between education level and OP.
Table 3.
Univariate logistic regression analysis for associations among variables and osteoporosis
| Variable | β | S.E. | P value | OR | 95.0% CI |
|---|---|---|---|---|---|
| Age | 0.099 | 0.007 | < 0.001 | 1.104 | 1.09-1.119 |
| Height | 0.008 | 0.05 | 0.871 | 1.008 | 0.914-1.112 |
| Weight | 0.005 | 0.033 | 0.886 | 1.005 | 0.941-1.073 |
| Exercise | -0.236 | 0.111 | 0.033 | 0.79 | 0.636-0.981 |
| Smoking | 0.030 | 0.276 | 0.913 | 1.031 | 0.601-1.769 |
| Drink | -0.193 | 0.192 | 0.315 | 0.824 | 0.565-1.202 |
| HTN | 0.310 | 0.103 | 0.003 | 1.364 | 1.114-1.668 |
| CAD | 0.498 | 0.162 | 0.002 | 1.646 | 1.198-2.26 |
| DM | 0.178 | 0.157 | 0.255 | 1.195 | 0.879-1.625 |
| RA | 0.480 | 0.208 | 0.021 | 1.616 | 1.075-2.429 |
| Vitamin C | 0.314 | 0.145 | 0.030 | 1.369 | 1.03-1.817 |
| Meat intake | -0.256 | 0.103 | 0.013 | 0.774 | 0.632-0.948 |
| Education | -0.240 | 0.044 | < 0.001 | 0.787 | 0.722-0.858 |
Note: HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA- Rheumatoid arthritis.
Figure 2.
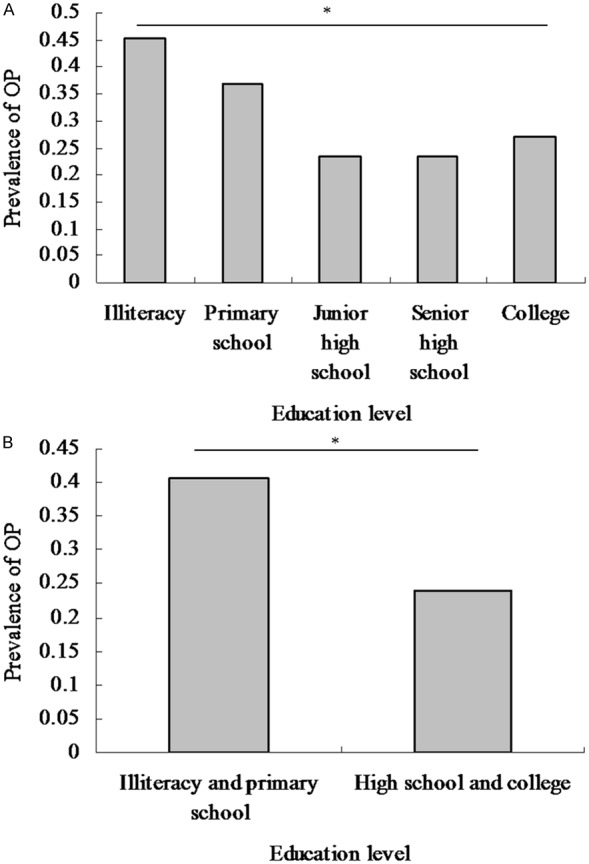
Comparison of prevalence of osteoporosis among groups according to education level in Chinese postmenopausal women. A: The results of comparison of prevalence of osteoporosis among groups according to Model 1 (Model 1: education level were categorized by group 1: illiteracy, group 2: primary school, group 3: junior high school, group 4: senior high school and group 5: college). The prevalence of osteoporosis was 45.09%, 36.74%, 23.46%, 23.27% and 27.17% in the five groups, respectively. There were significantly differences among the five groups (P value < 0.001). B: The results of comparison of prevalence of osteoporosis between groups according to Model 2 (Model 2: education level was categorized by illiteracy & primary school group and high school & college group). The prevalence of osteoporosis was 40.57% and 24.06% between the two groups, respectively. There were significantly differences between the two groups (P value < 0.001).
Multivariate logistic regression analyses were employed to evaluate the association between frequency of meat food intake and the OP outcome. After adjustment for relevant potential confounding factors, the multivariate logistic regression analyses detected significant associations between education level and OP in model 1 (P-value = 0.070 for model 1, Table 5), while no significant associations was reported in model 2 (P value = 0.131). In participants with high education level, the OR for OP was 0.914 (95% CI: 0.830-1.007).
Table 5.
Multiple variables logistic regression analysis for associations between education level and osteoporosis
| Model | Variable | β | S.E. | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | Education level | -0.090 | 0.049 | 0.070 | 0.914 | 0.830-1.007 |
| Model 2 | Education level | -0.207 | 0.137 | 0.131 | 0.813 | 0.622-1.063 |
Note: Model 1: education level were categorized by group 1: illiteracy, group 2: primary school, group 3: junior high school, group 4: senior high school and group 5: college; Model 2: education level were categorized by illiteracy & primary school group and high school & college group; and all models adjusted for age, smoking, alcohol intake, education, exercise, medical and therapy history.
Discussion
The present study in a large group of general Chinese postmenopausal women has shown a significant association between education level and OP: the population with a higher education level had a significantly lower prevalence of OP compared to that with a lower education level (Figure 2; Tables 3, 5). Additionally, a significant association between education level and T-score was also reported, with a lower education level linked to more a negative T-score (Figure 1; Tables 2, 4). In summary, the participants with higher education levels were associated with significantly higher T-scores but a lower prevalence of OP.
Previous studies also reported an inverse relationship between education level and the prevalence of OP [15-17,26]. Varenna et al. evaluated whether the prevalence of OP, and related risk factors might be influenced by the level of education in a cohort consisting of 6,160 postmenopausal women, suggesting differences in the prevalence of OP among educational classes and the protective role played by increases in formal education [15]. A study in northern Iran showed that education level is associated with bone health in this population of postmenopausal women, with a significantly higher prevalence of OP found in lower social groups, suggesting that women with a low social level should be carefully evaluated for signs of OP during routine physical examinations [16]. Ho et al. performed a population-based cross-sectional study consisting of 685 postmenopausal Chinese women to examine the association of education level with BMD and with the OP prevalence, indicating that a higher education level is independently associated with better BMDs and a lower prevalence of OP among postmenopausal Chinese women [17]. In the population of Salvadorian women [18] and Chinese women in Singapore [19], a higher education level is related to greater health literacy about OP. Correspondingly, a low bone density was frequently observed among poorly educated Moroccan postmenopausal women [20] and Korean breast cancer survivors [21]. However, in some studies, a higher education level is not actually linked to OP-preventive lifestyles, such as regular exercise and sufficient intake of calcium and vitamin D supplements, although, people with a higher educational status have better health literacy about OP [18,22]. Therefore, the higher educated women from the two studies in Salvador [18] and Iran [22] are still at risk of low bone mass and OP. In contrast, our findings have shown that higher vitamin D supplement intake and a lower prevalence of OP were observed in the people with higher education levels, suggesting a protective role of education in skeletal status.
In the present study, we reported a lower T-score and a higher OP prevalence in the people with a lower educational status. The potential protective role of education in OP could be due to several indirect causes, which may include socioeconomic status, health literacy, lifestyles, and access to early medical screening. Lifestyles including dietary intake, insufficiency of trace minerals, sedentariness, number of pregnancies, and smoking are related to changes in bone mass and the prevalence of OP. In general, a higher educational level may be associated with higher household incomes, healthier diet, sufficient calcium intake, frequent annual physical examinations, and more health consciousness about various diseases, including OP [27]. For instance, bone mass density has been shown to be associated with nutritional condition, educational status, and physical exercise [28]. Therefore, the protective role provided by education could be resultant from a better nutritional status, more positive medical adherence, more regular physical exercise, more access to health care resources, and so on [28-30]. It was reported that there was a significant difference in body weight, BMI, and dietary intake of energy, calcium, and protein among various educational groups [28]. Our study also shows a higher vitamin D supplement intake in more educated people, which is consistent with some previous reports [31,32]. Moreover, a higher educational status is also correlated with efficient osteoporotic treatment initiation [33] and combined therapy [34]. An OP-specific educational program has been shown to increase the participants’ knowledge about OP and better adherence to treatment [26]. Therefore, the availability of well-designed educational programs to increase the health literacy of the public will be beneficial in reducing the prevalence of OP and health care costs.
Our study has enabled a better understanding of the underlying determinants of OP, which could provide more clinical and therapeutic guidance for the disease. However, several limitations remain, which could be addressed in future studies. The sample of participants in our study contains a large population of postmenopausal women from rural and urban communities in Shanghai, ranging from 45-90 years in age; however, data in the male population and greater geographic representations would provide a more comprehensive illustration about the association between education level and OP. In addition, the subjective self-reported questionnaire used in current research could provide some exaggerated and inaccurate answers due to the respondent’s personal bias. Another limitation is that the exact protective role of education in OP remains to be further investigated, but this could be rather complicated due to a widespread influence of education on numerous risk factors affecting OP.
Our findings suggested that education level was independently and significantly associated with T-score and OP in the investigated population. The prevalence of OP was less frequent in Chinese postmenopausal women with higher education levels. Accordingly, we suggest that women with low education levels be carefully assessed for signs of OP during regular physical examinations. Providing adequate and efficient OP-related educational programs to increase the health literacy of the public may be rational.
Acknowledgements
We thank the grant from Shanghai Tongji Hospital to support the study. Grants from the Clinical Medicine Foundation of Shanghai Tongji Hospital. Clinical Trials. Gov Identifier: NCT02451397.
Disclosure of conflict of interest
None.
Abbreviations
- BMD
Bone mineral density
- BM-MNC
Bone marrow-derived mononuclear cell
- BMI
Coronary artery disease
- CAD
Body mass index
- CI
Confidence intervals
- DM
Diabetes
- DXA
Dualenergy X-ray
- HTN
Hypertension
- GFR
Glomerular filtration rate
- OR
Odds ratios
- OP
Osteoporosis
- QUS
Quantitative ultrasound
- RA
Rheumatoid arthritis
References
- 1.Dequeker J, Geusens P. Osteoporosis and arthritis. Ann Rheum Dis. 1990;49:276–280. doi: 10.1136/ard.49.5.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vestergaard P, Rejnmark L, Mosekilde L. Osteoporosis is markedly underdiagnosed: a nationwide study from Denmark. Osteoporos Int. 2005;16:134–141. doi: 10.1007/s00198-004-1680-8. [DOI] [PubMed] [Google Scholar]
- 3.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307:1248–1250. doi: 10.1136/bmj.307.6914.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper C, Campion G, Melton LJ 3rd. Hip fractures in the elderly: a world-wide projection. Osteoporos Int. 1992;2:285–289. doi: 10.1007/BF01623184. [DOI] [PubMed] [Google Scholar]
- 5.Pinkerton JV, Thomas S, Dalkin AC. Osteoporosis treatment and prevention for postmenopausal women: current and future therapeutic options. Clin Obstet Gynecol. 2013;56:711–721. doi: 10.1097/GRF.0b013e3182a9fb02. [DOI] [PubMed] [Google Scholar]
- 6.Cotte FE, Cortet B, Lafuma A, Avouac B, Hasnaoui AE, Fardellone P, Pouchain D, Roux C, Gaudin AF. A model of the public health impact of improved treatment persistence in postmenopausal osteoporosis in France. Joint Bone Spine. 2008;75:201–208. doi: 10.1016/j.jbspin.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Krall EA, Dawson-Hughes B. Heritable and lifestyle determinants of bone mineral density. J Bone Miner Res. 1993;8:1–9. doi: 10.1002/jbmr.5650080102. [DOI] [PubMed] [Google Scholar]
- 8.Jouanny P, Guillemin F, Kuntz C, Jeandel C, Pourel J. Environmental and genetic factors affecting bone mass. Similarity of bone density among members of healthy families. Arthritis Rheum. 1995;38:61–67. doi: 10.1002/art.1780380110. [DOI] [PubMed] [Google Scholar]
- 9.Vis M, Guler-Yuksel M, Lems WF. Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int. 2013;24:2541–2553. doi: 10.1007/s00198-013-2334-5. [DOI] [PubMed] [Google Scholar]
- 10.Ibanez M, Ortiz AM, Castrejon I, Garcia-Vadillo JA, Carvajal I, Castañeda S, González-Alvaro I. A rational use of glucocorticoids in patients with early arthritis has a minimal impact on bone mass. Arthritis Res Ther. 2010;12:R50. doi: 10.1186/ar2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kling JM, Clarke BL, Sandhu NP. Osteoporosis prevention, screening, and treatment: a review. J Womens Health (Larchmt) 2014;23:563–572. doi: 10.1089/jwh.2013.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ersoy FF. Osteoporosis in the elderly with chronic kidney disease. Int Urol Nephrol. 2007;39:321–331. doi: 10.1007/s11255-006-9109-2. [DOI] [PubMed] [Google Scholar]
- 13.Montalcini T, Romeo S, Ferro Y, Migliaccio V, Gazzaruso C, Pujia A. Osteoporosis in chronic inflammatory disease: the role of malnutrition. Endocrine. 2013;43:59–64. doi: 10.1007/s12020-012-9813-x. [DOI] [PubMed] [Google Scholar]
- 14.Joplin S, van der Zwan R, Joshua F, Wong PK. Medication Adherence in Patients with Rheumatoid Arthritis: The Effect of Patient Education, Health Literacy, and Musculoskeletal Ultrasound. Biomed Res Int. 2015;2015:150658. doi: 10.1155/2015/150658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varenna M, Binelli L, Zucchi F, Ghiringhelli D, Gallazzi M, Sinigaglia L. Prevalence of osteoporosis by educational level in a cohort of postmenopausal women. Osteoporos Int. 1999;9:236–241. doi: 10.1007/s001980050143. [DOI] [PubMed] [Google Scholar]
- 16.Maddah M, Sharami SH, Karandish M. Educational difference in the prevalence of osteoporosis in postmenopausal women: a study in northern Iran. BMC Public Health. 2011;11:845. doi: 10.1186/1471-2458-11-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho SC, Chen YM, Woo JL. Educational level and osteoporosis risk in postmenopausal Chinese women. Am J Epidemiol. 2005;161:680–690. doi: 10.1093/aje/kwi047. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Rauda R, Martinez-Garcia S. Osteoporosis-related life habits and knowledge about osteoporosis among women in El Salvador: a cross-sectional study. BMC Musculoskelet Disord. 2004;5:29. doi: 10.1186/1471-2474-5-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro V, Blakeley J, Laryea M. Women’s knowledge and practices regarding the prevention and treatment of osteoporosis. Health Care Women Int. 2000;21:347–353. doi: 10.1080/073993300245195. [DOI] [PubMed] [Google Scholar]
- 20.Allali F, Rostom S, Bennani L, Abouqal R, Hajjaj-Hassouni N. Educational level and osteoporosis risk in postmenopausal Moroccan women: a classification tree analysis. Clin Rheumatol. 2010;29:1269–1275. doi: 10.1007/s10067-010-1535-y. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Cho YU, Kim SJ, Lee JE, Kim JH. Low bone density in breast cancer survivors in Korea: prevalence, risk factors and associations with health-related quality of life. Eur J Oncol Nurs. 2013;17:196–203. doi: 10.1016/j.ejon.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Etemadifar MR, Nourian SM, Fereidan-Esfahani M, Shemshaki H, Nourbakhsh M, Zarezadeh A. Relationship of knowledge about osteoporosis with education level and life habits. World J Orthop. 2013;4:139–143. doi: 10.5312/wjo.v4.i3.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kressin NR, Wang F, Long J, Bokhour BG, Orner MB, Rothendler J, Clark C, Reddy S, Kozak W, Kroupa LP, Berlowitz DR. Hypertensive patients’ race, health beliefs, process of care, and medication adherence. J Gen Intern Med. 2007;22:768–774. doi: 10.1007/s11606-007-0165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Vietri J, Flynn JA, Fujiwara S. Treatment for Osteoporosis among Women in Japan: Associations with Patient Characteristics and Patient-Reported Outcomes in the 2008-2011 Japan National Health and Wellness Surveys. J Osteoporos. 2014;2014:909153. doi: 10.1155/2014/909153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the U S A. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 26.Nielsen D, Ryg J, Nielsen W, Knold B, Nissen N, Brixen K. Patient education in groups increases knowledge of osteoporosis and adherence to treatment: a two-year randomized controlled trial. Patient Educ Couns. 2010;81:155–160. doi: 10.1016/j.pec.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 27.Terrio K, Auld GW. Osteoporosis knowledge, calcium intake, and weight-bearing physical activity in three age groups of women. J Community Health. 2002;27:307–320. doi: 10.1023/a:1019840709367. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Mittal S, Orito S, Ishitani K, Ohta H. Impact of dietary intake, education, and physical activity on bone mineral density among North Indian women. J Bone Miner Metab. 2010;28:192–201. doi: 10.1007/s00774-009-0118-y. [DOI] [PubMed] [Google Scholar]
- 29.Gur A, Sarac AJ, Nas K, Cevik R. The relationship between educational level and bone mineral density in postmenopausal women. BMC Fam Pract. 2004;5:18. doi: 10.1186/1471-2296-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keramat A, Patwardhan B, Larijani B, Chopra A, Mithal A, Chakravarty D, Adibi H, Khosravi A. The assessment of osteoporosis risk factors in Iranian women compared with Indian women. BMC Musculoskelet Disord. 2008;9:28. doi: 10.1186/1471-2474-9-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pluskiewicz W, Adamczyk P, Czekajlo A, Grzeszczak W, Drozdzowska B. Influence of education, marital status, occupation, and the place of living on skeletal status, fracture prevalence, and the course and effectiveness of osteoporotic therapy in women in the RAC-OSTPOL Study. J Bone Miner Metab. 2014;32:89–95. doi: 10.1007/s00774-013-0471-8. [DOI] [PubMed] [Google Scholar]
- 32.Castro-Lionard K, Dargent-Molina P, Fermanian C, Gonthier R, Cassou B. Use of calcium supplements, vitamin D supplements and specific osteoporosis drugs among French women aged 75-85 years: patterns of use and associated factors. Drugs Aging. 2013;30:1029–1038. doi: 10.1007/s40266-013-0121-9. [DOI] [PubMed] [Google Scholar]
- 33.Brennan RM, Wactawski-Wende J, Crespo CJ, Dmochowski J. Factors associated with treatment initiation after osteoporosis screening. Am J Epidemiol. 2004;160:475–483. doi: 10.1093/aje/kwh245. [DOI] [PubMed] [Google Scholar]
- 34.Lucas R, Rocha O, Bastos J, Costa L, Barros H, Lunet N. Pharmacological management of osteoporosis and concomitant calcium supplementation in a Portuguese urban population: the EpiPorto study (2005-2007) Clin Exp Rheumatol. 2009;27:47–53. [PubMed] [Google Scholar]


