Abstract
Objective: To identify high risk factors in chronic hepatitis B (CHB) patients for suboptimal response to adefovir dipivoxil (ADV) monotherapy, and to assess the efficacy of optimized therapy combining ADV with lamivudine (LAM), telbivudine (LdT), or entecavir (ETV) in patients with a suboptimal response to ADV alone. Methods: Suboptimal response to ADV monotherapy was defined as having a decline in serum hepatitis B virus (HBV) DNA level of more than 1 log compared to baseline, but with viremia still detectable (HBV DNA ≥ 100 IU/mL), after 48 weeks of therapy. All patients who received ADV monotherapy in our clinic were analyzed retrospectively. Both univariate and multivariate logistic regression models were applied for risk factor analysis. Patients who showed suboptimal response completed at least 12 months of optimized combination therapy consisting of ADV plus LAM, ADV plus LdT, ADV plus ETV, or continuous ADV monotherapy. The primary outcome measurement was complete viral suppression, indicated by a reduction of HBV DNA to undetectable levels (CVS, with HBV DNA < 100 IU/mL). Secondary outcome measures were HBeAg seroconversion for HBeAg-positive patients, HBsAg loss, alanine aminotransferase (ALT) normalization and virological breakthrough rates. Results: Of 521 patients who received ADV monotherapy, 170 showed a suboptimal response. These were grouped for continued therapy as follows: 34 in group A (continuous ADV monotherapy), 55 in group B (ADV plus LAM), 38 in group C (ADV plus LdT), and 43 in group D (ADV plus ETV). Using a logistic model, five conditions were identified as high risk factors for suboptimal response: presence of the tyrosine-methionine-aspartate-aspartate (YMDD) HBV DNA polymerase mutation; being HBeAg positive; having a high baseline level of HBV DNA; having a primary virological non-response to ADV; and [initial virological response] to ADV. After 48 weeks of ADV monotherapy, there were no withdrawn patients who had experienced side effects. The median HBV DNA levels were (4.4±1.3) log, (4.6±1.3) log, (4.7±1.4) log, and (4.5±1.5) log in groups A, B, C, D, respectively (F = 0.228, P = 0.876). After 48-weeks of continued ADV monotherapy or optimized combination therapy, the CVS rates were 23.5% (8/34), 60% (33/55), 52.6% (20/38), and 58.1% (25/43), respectively (χ2 = 12.952, P = 0.005). The median HBV DNA declines were (0.5±1.7) log, (2.0±1.3) log, (1.8±1.6) log, and (1.8±1.5) log, respectively (F = 6.775, P < 0.001). Virologic breakthrough rates were 26.5% (9/34), 7.3% (4/55), 10.5% (4/38), and 9.3% (4/43), respectively (χ2 = 8.057, P = 0.045). Conclusion: Optimized combination therapies consisting of ADV plus LAM, LdT, or ETV may be reasonable choices for hepatitis B patients with a suboptimal response to ADV monotherapy.
Keywords: Hepatitis B, adefovir dipivoxil, suboptimal response, optimal combination therapy
Introduction
Chronic hepatitis B virus (HBV) infection is a leading cause globally of chronic hepatitis, cirrhosis and hepatocellular carcinoma, particularly in Asian areas where it is endemic, such as China. Treatment of chronic hepatitis B (CHB) has improved greatly with the availability of nucleos(t)ide analogues (NA). The sustained suppression of serum HBV DNA to very low or undetectable levels by these drugs had been shown to be associated with the prevention of progression of liver disease and inhibition of hepatocellular carcinoma [1,2].
Adefovir dipivoxil (ADV), the second oral drug approved for treatment of CHB, is an oral prodrug of adefovir, a phosphonate acyclic nucleotide analogue of adenosine monophosphate [3]. ADV which is effective drug without cross-resistant for other NAs had been indicated with a low rate of resistance. For these reasons, ADV has been widely used in the initial treatment of CHB patients, especially in China [4,5]. However, as ADV monotherapy has been used over time, it has become clear that it demonstrates a relatively high rate of suboptimal response, defined as a decline in HBV DNA greater than 1 log IU/mL from baseline, but with a detectable viral load, at week 48 of treatment [6]. Such suboptimal responses are probably due to the dose restriction needed to avoid renal toxicity [7]. Persistent viremia has been identified as a risk factor for poor outcomes and is associated with increased risk of resistance [8,9]. In these cases it is recommended to switch to or to add a more potent drug with a complementary cross-resistance profile [6,7,10]. Currently, combination therapy may be the best strategy for optimizing antiviral treatment, especially when tenofovir (TDF) is not available. Unfortunately, existing data on patients with suboptimal response to ADV and their subsequent management are very limited.
Nucleoside analogues lack cross-resistance with ADV and are superior to it in their ability to inhibit hepatitis B virus replication. These include lamivudine (LAM), which has a well-established safety and efficacy profile, but also has the highest incidence of resistant mutations compared with other nucleoside/nucleotide analogs. Telbivudine (LdT) is effective and has a higher seroconversion rate than ADV. Entecavir (ETV), a cyclopentyl guanosine analogue, is known for its potency with low rate of resistance and high cost [11].
Therefore, despite the lack of clinical data on combination therapies, utilizing ADV in combination with LAM, LdT, or ETV as an optimized therapy for patients who have suboptimal response to ADV monotherapy may have additional benefits compared to using ADV alone.
The purpose of this cohort study was to identify factors that can negatively influence the response of patients receiving ADV monotherapy and to determine the clinical efficacy of adding LAM, LdT or ETV to ADV in suboptimal responders.
Materials and methods
Study population
This was a retrospective cohort study among patients who received ADV monotherapy for at least 12 months, demonstrated a suboptimal response, and subsequently received optimized therapy with ADV plus LAM, Ldt, or ETV. Under local reimbursement policy, most patients had to pay for their anti-viral drugs, which had already influenced their compliance. For this reason a portion of patients had a partial response to ADV and needed to continue with ADV monotherapy. All patients attending the hepatitis clinic of the Third Affiliated Hospital of Sun Yat-sen University from January 2008 to September 2011 were screened. Since the study aimed to investigate the response to ADV optimized therapy, only patients who had received ADV monotherapy for 12 months or more were included for analysis. Further eligibility criteria were: patient age 18-65 years; having detectable HBsAg for 6 months; HBV DNA > 2000 IU/mL; ALT > twice the upper limit of normal (ULN). Patients were excluded from studies if they were co-infected with hepatitis C, hepatitis D, or human immunodeficiency viruses; were pregnant or lactating; were transplant recipients; lacked HBV DNA data before the commencement of adefovir and/or within 12 months of adefovir therapy, or showed evidence of liver decompensation (alcoholic hepatitis, autommune heptatitis, or drug-induced liver disease). The study protocol conformed to the ethical guidelines of the Declaration of Helsinki Ethical Committee. Informed consent was obtained from each patient enrolled in the study.
Study design
Subjects received adefovir dipivoxil 10 mg/day monotherapy for 48 weeks. Patients with suboptimal response to ADV monotherapy were eligible for optimized therapy, and were allocated to one of four treatment groups as described below under Results. Routine hematologic analysis, hepatobiliary enzymes, HBV DNA, and serologic analysis, hepatic synthetic function, creatine kinase, blood urea nitrogen, creatinine, and blood lactate were assayed at base line and every 3-6 months thereafter, as described below under “Assay Methods”. A 2 mL blood sample was collected at each follow-up for future assessment.
Endpoint
The primary outcome measurement was complete viral suppression (CVS, with HBV DNA < 100 IU/mL) and the reduction of HBV DNA. Secondary outcome measures were HBeAg seroconversion for HBeAg-positive patients, HBsAg loss, alanine aminotransferase (ALT) normalization and virological breakthrough rates.
Assay methods
Liver function and other biochemical index assays were measured using automated techniques. Serum HBV DNA levels were measured using a quantitative real-time PCR assay (DAAN Gene Co., Ltd., Guangzhou, China), with a lower limit of detection of 100 IU/mL. HBsAg, HBeAg, and anti-HBe (antibody against hepatitis B e antigen) were measured using commercially available chemiluminescence assay kits (Roche Diagnotistic Systems). Detection of HBV polymerase gene mutations was determined by direct sequencing PCR (BGI-Shenzhen, Shenzhen, China).
Definitions
Virological response (VR) was defined by a decline in HBV DNA levels during therapy. Different profiles of response could be distinguished, as follows: Primary virological non-response (PVNR) was defined as any reduction in viral load less than 1 log IU/mL or no reduction by week 12. Initial virological response (IVR) was characterized by a decrease of at least 1 log IU/mL in viral load by week 12. Early virological response (EVR) was defined as undetectable HBV DNA at 6 months. Maintained virological response (MVR) was defined by undetectable HBV DNA until the last follow-up. Suboptimal response (SOR) to ADV monotherapy was defined by a decline in serum HBV DNA level of more than 1 log compared to baseline, but with HBV DNA still detectable (≥ 100 IU/mL) at 48 weeks. Virological breakthrough (VBT) was defined by an increase of at least 1 log IU/mL compared to the lowest value during treatment, and was confirmed by a second test in a treatment-compliant patient. Lamivudine resistance was defined as > 1 log increase of HBV DNA from nadir in those who had an initial virological response.
Statistical analysis
Statistical analysis was performed using SPSS v.17 software (SPSS, Chicago, IL, USA). Continuous variables were presented as mean ± standard deviation or median (interquartile range), and compared using unpaired t-test or Mann-Whitney U-test as appropriate. Categorical variables were presented as frequency (percentage) and compared using the Chi-squared test or Fisher exact test as appropriate. The HBV DNA levels were logarithmically transformed for analysis. All statistical tests were two-sided and a P-value of < 0.05 was considered statistically significant.
Results
Patient disposition
A total of 521 patients received ADV 10 mg daily for 48 weeks. At week 12, 12 of the 521 patients that had levels of HBV DNA that decreased by < 1 log were switched to other therapies while the other 509 continued to receive ADV monotherapy. At week 48 of ADV monotherapy, 170 of the 509 patients still had HBV DNA levels > 103 IU/mL; these 170 patients were included in this study. Among the 170 patients with a suboptimal response, 34 received ADV monotherapy for economic reasons. 55 were treated with ADV plus LAM, 38 with ADV plus LdT, and 43 with ADV plus ETV. Figure 1 depicted the selection process. There were no significant differences in baseline characteristics among these four groups (Table 1).
Figure 1.

Study flow.
Table 1.
Baseline characteristics of CHB patients before optimized therapy
| Variables | Group A (n = 34) | Group B (n = 55) | Group C (n = 38) | Group D (n = 43) | P-value** |
|---|---|---|---|---|---|
| Male (%) | 26 (76.5) | 41 (74.5) | 35 (92.1)* | 27 (62.8) | 0.023 |
| Age, median (years) | 38.9 (21~70) | 40.6 (24~70) | 32.6 (21~54) | 40.5 (26~69) | 0.308 |
| ALT, median (U/L) | 42.3 (10~63) | 52.3 (14~239) | 49.1 (14~200) | 46.4 (12~217) | 0.069 |
| TBil, mean ± sd (mg/dl) | 14.8±7.0 | 19.1±12.1 | 16.0±6.8 | 16.6±6.7 | 0.228 |
| ALB, mean ± sd (g/dl) | 46.0±4.1 | 46.2±4.5 | 46.3±2.5 | 47.0±3.1 | 0.729 |
| HBeAg positivity (%) | 22 (64.7) | 36 (65.5) | 35 (92.1)* | 38 (88.4)* | 0.002 |
| HBV DNA, mean ± sd (log10 IU/mL) | 4.4±1.3 | 4.6±1.3 | 4.7±1.4 | 4.5±1.5 | 0.876 |
| YMDD mutation (%) | 9/18 (50.0) | 9/24 (37.5) | 15/26 (57.8) | 17/27 (63.0) | 0.298 |
Compared with group A P < 0.05;
Comparison of group A, B, C and D.
ALT, alanine aminotransferase; TBil, total bilirubin; ALB, albumin; HBeAg, hepatitis B e antigen.
Factors associated with SOR to ADV monotherapy at 48 weeks
Potential predictive factors included in univariate analyses were age, gender, body mass index (BMI), baseline serum ALT level (U/L), baseline serum HBV DNA level (log IU/mL), baseline HBeAg positivity, presence of a YMDD mutation, and PVNR, EVR, or IVR to ADV monotherapy. This analysis showed that age, baseline HBeAg positivity, YMDD mutation, high baseline serum HBV DNA level, PVR, and absence of IVR to ADV monotherapy were significantly associated with SOR. Because some of these variables were mutually correlated, multivariate analysis was also performed, which eliminated age as an associated factor. Five independent factors predictive of SOR to ADV monotherapy were thus identified: baseline HBeAg positivity, YMDD mutation, baseline serum HBV DNA level, PVR, and IVR to ADV monotherapy.
Patient characteristics at the initiation of combination therapy
Patient characteristics at the initiation of optimized therapy were shown in Table 1. All biochemical, serological, and virological parameters were similar among the four groups, except for a higher percentage of HBeAg positivity in Groups C and D, despite which seroconversion rates were similar across all four groups after treatment (Table 2).
Table 2.
Virological and serological outcomes of optimized combination therapies for CHB patients with a suboptimal response to ADV
| Outcome | Duration (Weeks) | Group A n = 34 | Group B n = 55 | Group C n = 38 | Group D n = 43 | P-value |
|---|---|---|---|---|---|---|
| CVS (%) | 12 | 8 (23.5) | 25 (45.5) | 12 (31.6) | 18 (41.9) | 0.155 |
| 24 | 11 (32.4) | 29 (52.7) | 16 (42.1) | 24 (55.8) | 0.147 | |
| 48 | 8 (23.5) | 33 (60.0) | 20 (52.6) | 25 (58.1) | 0.005** | |
| HBV DNA decline, mean ± s.d (log10 IU/mL) | 12 | 0.5±1.3 | 1.5±1.1 | 1.2±1.3 | 1.2±1.2 | 0.010** |
| 24 | 0.4±1.3 | 1.7±1.3 | 1.4±1.4 | 1.7±1.2 | 9.3E-3 | |
| 48 | 0.5±1.7 | 2.0±1.3 | 1.8±1.6 | 1.8±1.5 | 4.7E-3 | |
| VB (%) | 12 | 2 (5.9) | 1 (1.8) | 1 (2.6) | 2 (4.7) | 0.735 |
| 24 | 4 (11.8) | 0 (0.0)* | 0 (0.0)* | 0 (0.0)* | 0.001** | |
| 48 | 9 (26.5) | 4 (7.3)* | 4 (10.5) | 4 (9.3)* | 0.045** | |
| HBeAg/HBeAb conversion rate (%) | 12 | 1/22 (4.5) | 1/36 (2.8) | 0/35 (0.0) | 1/38 (2.6) | 0.711 |
| 24 | 1/22 (4.5) | 2/36 (5.6) | 0/35 (0.0) | 1/38 (2.6) | 0.562 | |
| 48 | 2/22 (9.0) | 3/35 (8.3) | 1/35 (2.9) | 1/38 (2.6) | 0.527 |
Compared with group A P < 0.05;
Comparison of Group A, B, C and D.
CVS, Complete viral suppression; VB, virological breakthrough rate; Groups were defined in the text.
Outcomes of combination optimized therapies
Table 2 showed the major virological and serological outcomes after 12, 24, and 48 weeks of therapy in suboptimal responders to ADV monotherapy, including those who continued on ADV (Group A) or who were treated with ADV plus LAM (Group B), LdT (Group C), or ETV (Group D). The combination therapies provided clear improvements in CVS, HBV DNA decline, and VB compared to continued ADV therapy, but seroconversion rates were unchanged. Figure 2 depicted the large decreases in HBV DNA induced by combination therapies relative to ADV alone. Changes in biochemical measures, including ALT, ALB, and TBIL, as well as normalization rates, were shown in Table 3. There were no significant differences between the group that continued ADV monotherapy and the 3 optimized therapy groups. Means of ALT in 3 optimized therapy groups were (41.4±61.0), (35.4±14.7), (37.1±27.1) U/L, respectively. Means of ALB were (46.9±3.6), (45.6±9.9), (47.1±2.6) g/L, respectively. Means of TBIL were (18.1±10.0), (14.9±4.0), (16.4±6.6) μmol/L, respectively. Normalization rates were 42.9% (15/35), 61.1% (11/18), 34.8% (8/23) (Table 3). Results for the ADV monotherapy group were (47.0±43.2) U/L, (47.1±4.4) g/L, (18.4±8.8) μmol/L and a 41.6% (5/12) normalization rate (Figure 2).
Figure 2.
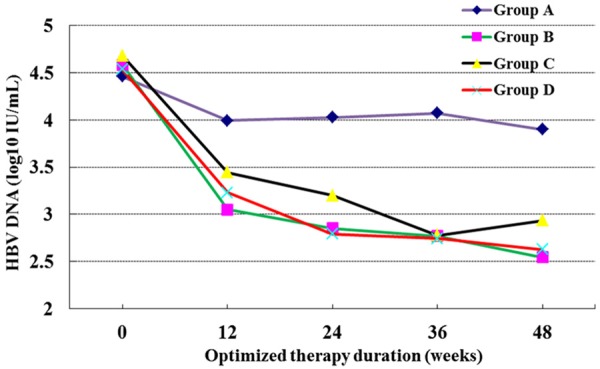
HBV DNA level of CHB patients received optimized therapy at 0, 12, 24, 36 and 48 weeks.
Table 3.
Biochemical measurements of optimized combination therapies for CHB patients with a suboptimal response to ADV within 48 weeks
| Date (wks) | Group | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Measurement | A | B | C | D | F/χ2 | P | |
| ALT mean (U/L) | 12 | 47.0±43.2 | 47.9±77.6 | 75.0±11.8 | 43.8±33.0 | 0.796 | 0.506 |
| 24 | 38.0±33.8 | 46.2±61.0 | 38.6±16.4 | 37.2±21.8 | 0.498 | 0.705 | |
| 48 | 34.2±16.2 | 41.1±20.9 | 35.4±14.7 | 37.1±27.1 | 0.645 | 0.587 | |
| ALT normalized rates | 12 | 25.0% (3/12) | 20.0% (7/35) | 38.9% (7/18) | 26.1% (6/23) | 2.207 | 0.531 |
| 24 | 25.0% (3/12) | 37.1% (13/35) | 38.9% (7/18) | 21.7% (5/23) | 2.206 | 0.531 | |
| 48 | 41.6% (5/12) | 42.9% (15/35) | 61.1% (11/18) | 34.8% (8/23) | 3.496 | 0.321 | |
| ALB mean (g/L) | 12 | 46.1±2.8 | 46.5±3.6 | 47.6±2.5 | 47.5±3.0 | 1.581 | 0.198 |
| 24 | 45.7±3.0 | 45.8±5.4 | 47.6±2.2 | 45.1±9.0 | 0.888 | 0.450 | |
| 48 | 47.1±4.4 | 46.9±3.6 | 45.6±9.9 | 47.1±2.6 | 0.352 | 0.788 | |
| TBIL mean (μmol/L) | 12 | 17.4±7.9 | 20.3±14.0 | 16.4±8.6 | 16.8±7.7 | 1.030 | 0.308 |
| 24 | 17.2±5.7 | 17.9±12.4 | 15.2±6.3 | 13.7±5.8 | 1.636 | 0.185 | |
| 48 | 18.4±8.8 | 18.1±10.0 | 14.9±4.0 | 16.4±6.6 | 1.146 | 0.344 | |
Note: F represented ANOVA test; χ2 represented χ2 test.
Discussion
Adefovir dipivoxil is used widely in China for treatment of hepatitis B infection due to its low cost, low rate of drug resistance, and lack of cross resistance with other nucleoside and nucleotide analogues. Nevertheless, consistent with data reported by others [12,13], we found that 32.6% (170/521) of patients in our study cohort displayed a suboptimal response after 48 weeks of ADV monotherapy. Such patients still had high viral copy number, demonstrating the limited efficacy of therapy with ADV alone. In addition, the risk of acquiring resistance to ADV rose with every year of continuing treatment [14]. In our research, we found that 26.5% (9/34) of suboptimal responders who continued ADV monotherapy suffered virological breakthrough at week 96. ADV resistance may presage the ultimate failure of treatment for hepatits B infection; therefore, it is essential that optimized therapies be found for suboptimal responders to ADV monotherapy.
A current strategy for optimizing treatment of suboptimal responders to ADV monotherapy is to combine ADV with stronger nucleotide analogue drugs that have no cross resistance [6,7,10]. In China, currently available analogues include LAM LdT, and ETV. Sequential therapy is not an ideal method to inhibit viral replication [15,16] and may lead to multidrug resistant HBV [17,18]; however, combination therapy can strongly inhibit HBV replication and reduce the risk of developing resistance even when genetic barriers to resistance are low [19-21]. Consequently, we combined ADV with LMV, LdT, or ETV to treat patients that showed suboptimal responses to ADV alone.
All three combination therapy groups in our study demonstrated improved efficacy at 96 weeks compared to continued ADV monotherapy based on HBV DNA decline, viral suppression, and viral breakthrough rates. Continued monotherapy was just as effective as the combination therapies with respect to serological (Tables 1 and 2) and biochemical (Table 3) measures. Thus our results indicate that all four therapies can improve biochemical and serological parameters in ADV suboptimal responders. Consistent with previous reports [22-24], two of the combination therapies (LdT, Group C and EVT, Group D) were less effective in seroconversion than ADV monotherapy after 48 weeks of combination therapy (96 weeks total therapy). Further observation is required to determine whether, after longer treatment, differences between the optimized therapy groups and the monotherapy group in biochemical response and seroconversion would remain.
Monotherapy is limited in its ability to promote a sustained response and in allowing patients to reach the point of therapy termination (HBeAg serological conversion and HBsAg disappearance). By contrast, combining drugs allows therapy to be optimized and reduces the risk of developing a drug-resistant viremia [6]. Three types of combination therapy are recognized: initial, optimized, and optimized therapies. Evidence-based medicine concentrates primarily on optimized combination therapy for resistance to nucleotide analogue drugs. Many reports have shown that optimized therapy can inhibit HBV replication and significantly lower virological breakthrough rates [9,19,20,25,26]. However, there is a lack of evidence concerning the use of optimized therapies for suboptimal responders. We found that HBV DNA levels in all 3 of the optimized combination therapy groups we tested decreased significantly by week 96 compared to baseline levels (Figure 2; Table 1). At the beginning of combination therapy, the mean HBV DNA levels for groups B, C, and D were comparable (Table 1). After 48 weeks of combination therapy, the differences among the 4 groups in HBV decline amplitudes and virological breakthrough rates were statistically significant (Table 2). These findings indicated that all three optimized therapies inhibit HBV replication and lower virological breakthrough rates in patients that had a suboptimal response to monotherapy, in agreement with work published previously [24].
In addition to comparing combination and monotherapies, we compared the efficacy among the optimized combination therapies of ADV with LAM, LdT, or ETV. Because the three added drugs have different cross-resistance profiles, they might be expected to differ in efficacy when combined with ADV, especially in patients carrying the YMDD mutation in HBV polymerase. We established prior to treatment that there were 9, 15 and 17 patients that had YMDD mutations in therapy groups B, C and D, respectively; Table 4 compared rates of CVS and VB in the three combination therapy groups between patients with or without the YMDD mutation. LdT had a low genetic barrier to resistance and was cross resistant with LAM; ETV had a high genetic barrier to resistance and had cross resistance to LAM; but only ETV showed resistance through the polymerase residue 204 pathway, as well as through replacement of residues 184, 202, or 250. Because of its resistance mechanism, ETV might be more efficacious combined with ADV in patients with an YMDD mutation than were LAM or LdT. However, our results indicated that there were no significant differences among the 3 optimized combination therapies in ALT normalization rates, HBV DNA decline amplitudes, virological response rates, HBeAg/HBeAb conversion rates or virological breakthrough rates at week 96 (Tables 2 and 3). Moreover, LAM therapy often led to mutations in the YMDD motif [27]. Consistent with the report of Berg et al. [24], in our study the presence of a YMDD mutation before treatment did not affect virological response or virological breakthrough rates in any of the optimized therapy groups we tested. This suggested that patients with an YMDD mutation before treatment will also benefit from combination therapy even if they had suboptimal responses to primary ADV monotherapy. We inferred that another nucleotide analogue drug with a compatible cross resistance profile ought to be used as early as possible when a patient’s response to nucleotide analogue monotherapy is suboptimal.
Table 4.
Comparison of YMDD (+) and (–) CHB patients after optimized therapy on complete viral suppression and virological breakthrough rate
| Group | CVS (%) | P-value | VB (%) | P-value | ||
|---|---|---|---|---|---|---|
|
|
|
|||||
| YMDD (+) | YMDD (-) | YMDD (+) | YMDD (-) | |||
| Group B, n = 24 | 6/9 (66.7) | 8/15 (53.3) | 0.678 | 0/9 (0.0) | 2/15 (13.3) | 0.511 |
| Group C, n = 26 | 7/15 (46.6) | 7/11 (63.6) | 0.697 | 2/15 (13.3) | 0/11 (0.0) | 0.492 |
| Group D, n = 27 | 10/17 (58.8) | 6/10 (60.0) | 1.00 | 2/17 (11.8) | 0/10 (0.0) | 0.260 |
| Total, n = 77 | 23/41 (56.1) | 21/36 (58.3) | 0.843 | 4/41 (9.8) | 2/36 (5.6) | 0.516 |
CVS, complete viral suppression; VB, virological breakthrough rate.
Recent reports had indicated that although combinations of two nucleotide analogue drugs strongly inhibited HBV DNA replication and efficiently lowered the risk of resistance to drugs with a low genetic barrier, resistance still cannot be completely avoided [20,22,23]. We also found that ADV combined with LMV, LdT, or ETV can strongly inhibit HBV DNA replication, and resistance incidence was much lower compared to ADV treatment alone. However, 4 patients showed virological breakthrough in each of our 3 groups by week 96 (Table 2), and there were 3, 2 and 2 patients whose HBV DNA decline amplitudes were < 1 log copies/mL in groups B, C and D, respectively, as well as 4 in the control group (data not shown). Determination of the resistance genotypes of these patients is ongoing in our laboratory. Patients that had virological breakthrough after being treated with ADV and either LMV or LdT were changed to ADV combined with ETV; those that had been treated with ADV combined with ETV were changed to ADV combined with ETV (1 mg/day) in 2 cases, or received tenofovir disoproxil fumarate monotherapy in the other two cases. Patients whose HBV DNA decline amplitudes were < 1 log copies/mL were continued on the primary treatment instead of altering it. Additional study is needed to determine whether prolonging combination treatments beyond 96 weeks causes virological breakthrough rates within the optimized therapy groups to rise, or reveals differences among patients receiving optimized therapies or ADV monotherapy.
In conclusion, our research revealed that patients with suboptimal response to ADV can improve or normalize their biochemical markers such as ALT, TBIL and ALB when combining ADV with LAM, LdT, or ETV. These therapies had been defined as safe (reference) and patient compliance is satisfactory. Compared to the continued ADV monotherapy, all three optimized therapies significantly inhibited HBV DNA replication, and virological breakthrough rates were greatly reduced. Efficacy with respect to biochemistry, virology, serology and resistance incidence among the three optimized combination therapies was similar. Thus optimized combination therapies of ADV combined with LMV, LdT, or ETV may be reasonable choices for hepatitis B patients with a suboptimal response to ADV monotherapy.
Acknowledgements
This project was supported by the 12th fiveyear National Science and Technology Major Project (No. 2012ZX10004-902).
Disclosure of conflict of interest
None.
References
- 1.Liaw YF. Hepatitis B virus replication and liver disease progression: The impact of antiviral therapy. Antivir Ther. 2006;11:669–679. [PubMed] [Google Scholar]
- 2.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, Chi YC, Zhang H, Hindes R, Iloeje U, Beebe S, Kreter B. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 3.Kramata P, Votruba I, Otova B, Holý A. Different inhibitory potencies of acyclic phosphonomethoxyalkyl nucleotide analogs toward DNA polymerases alpha, delta and epsilon. Mol Pharmacol. 1996;49:1005–1011. [PubMed] [Google Scholar]
- 4.Delaney WE. Progress in the treatment of chronic hepatitis B: long-term experience with adefovir dipivoxil. J Antimicrob Chemother. 2007;59:827–832. doi: 10.1093/jac/dkl551. [DOI] [PubMed] [Google Scholar]
- 5.Lim S, Marcellin P, Tassopoulos N, Hadziyannis S, Chang TT, Tong M, Sievert W, Hu P, Arterburn S, Brosgart C. Clinical trial: effects of adefovir dipivoxil therapy in Asian and Caucasian patients with chronic hepatitis B. Aliment Pharmacol Ther. 2007;26:1419–1428. doi: 10.1111/j.1365-2036.2007.03506.x. [DOI] [PubMed] [Google Scholar]
- 6.European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227–242. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 8.Zoulim F. Mechanism of viral persistence and resistance to nucleoside and nucleotide analogs in chronic hepatitis B virus infection. Antiviral Res. 2004;64:1–15. doi: 10.1016/j.antiviral.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, Chen Y, Heathcote EJ, Rasenack J, Bzowej N, Naoumov NV, Di Bisceglie AM, Zeuzem S, Moon YM, Goodman Z, Chao G, Constance BF, Brown NA, Group GS. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 10.Keeffe EB, Dieterich DT, Han SH, Jacobson IM, Martin P, Schiff ER, Tobias H. A treatment algorithm for the management of chronic hepatitis B virus infection in the United States: 2008 update. Clin Gastroenterol Hepatol. 2008;6:1315–1341. doi: 10.1016/j.cgh.2008.08.021. quiz 1286. [DOI] [PubMed] [Google Scholar]
- 11.Seifer M, Hamatake RK, Colonno RJ, Standring DN. In vitro inhibition of hepadnavirus polymerases by the triphosphates of BMS-200475 and lobucavir. Antimicrob Agents Chemother. 1998;42:3200–3208. doi: 10.1128/aac.42.12.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durantel S, Werle B, Durantel D, Pichoud C, Currie G, Xiong S, et al., editors. Hepatology. 111 River ST, Hoboken, NJ 07030 USA: John Wiley & Sons Inc; 2004. Different profiles of response to Adefovir Dipivoxil and factors that may influence response in patients with chronic Hepatitis B. [Google Scholar]
- 13.Fung SK, Chae HB, Fontana RJ, Conjeevaram H, Marrero J, Oberhelman K, Hussain M, Lok AS. Virologic response and resistance to adefovir in patients with chronic hepatitis B. J Hepatol. 2006;44:283–290. doi: 10.1016/j.jhep.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang TT, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Ma J, Brosgart CL, Borroto-Esoda K, Arterburn S, Chuck SL Group ADS. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology. 2006;131:1743–1751. doi: 10.1053/j.gastro.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Reijnders JG, De Man RA, Pas SD, Schutten M, Janssen HL, editors. Hepatology. 111 River ST, Hoboken, NJ 07030 USA: John Wiley & Sons Inc; 2007. Entecavir: A rescue therapy for chronic hepatitis B patients with a limited virological response to adefovir? [Google Scholar]
- 16.van Boemmel F, Trojan J, Feucht HH, Moeller B, Hueppe D, Wiedenmann B, et al., editors. Hepatology. 111 River ST, Hoboken, NJ 07030 USA: John Wiley & Sons Inc; 2007. Tenofovir shows limited efficacy in treatment of HBV infections resistant against adefovir. [Google Scholar]
- 17.Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385–1391. doi: 10.1002/hep.21189. [DOI] [PubMed] [Google Scholar]
- 18.Manolakopoulos S, Bethanis S, Koutsounas S, Goulis J, Vlachogiannakos J, Christias E, Saveriadis A, Pavlidis C, Triantos C, Christidou A. Long-term therapy with adefovir dipivoxil in hepatitis B e antigen-negative patients developing resistance to lamivudine. Aliment Pharmacol Ther. 2008;27:266–273. doi: 10.1111/j.1365-2036.2007.03567.x. [DOI] [PubMed] [Google Scholar]
- 19.Lampertico P, Marzano A, Levrero M, Santantonio T, Di Marco V, Brunetto M, Andreone P, Sagnelli E, Fagiuoli S, Mazzella G. Adefovir and lamivudine combination therapy is superior to ADV monotherapy for lamivudine-resistant patients with HBeAG-negative chronic hepatitis B. Digestive and Liver Disease. 2007;39:A7. [Google Scholar]
- 20.Lampertico P, Vigano M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133:1445–1451. doi: 10.1053/j.gastro.2007.08.079. [DOI] [PubMed] [Google Scholar]
- 21.Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661–662. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 22.Hui CK, Zhang HY, Bowden S, Locarnini S, Luk JM, Leung KW, Yueng YH, Wong A, Rousseau F, Yuen KY. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol. 2008;48:714–720. doi: 10.1016/j.jhep.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Sung JJ, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, Brosgart CL, Woessner MA, Scott SA, Gray DF. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008;48:728–735. doi: 10.1016/j.jhep.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Berg T, Marcellin P, Moeller B, Trinh HN, Chan S, Suarez E, Snow-Lampart A, Peschell KJ, Borroto-Esoda K, Hirsch KR. Tenofovir disoproxil fumarate (TDF) versus emtricitabine plus TDF (FTC/TDF) for treatment of chronic hepatitis B (CHB) in patients with persistent viral replication receiving adefovir dipivoxil: Final week 168 results. Hepatology. 2010;52:136A. [Google Scholar]
- 25.Kim HJ, Park JH, Park DI, Cho YK, Sohn CI, Jeon WK, Kim BI. Rescue therapy for lamivudine-resistant chronic hepatitis B: Comparison between entecavir 1.0 mg monotherapy, adefovir monotherapy and adefovir add-on lamivudine combination therapy. J Gastroenterol Hepatol. 2010;25:1374–1380. doi: 10.1111/j.1440-1746.2010.06381.x. [DOI] [PubMed] [Google Scholar]
- 26.Petersen J, Ratziu V, Buti M, Janssen HL, Brown A, Lampertico P, Schollmeyer J, Zoulim F, Wedemeyer H, Sterneck M. Entecavir plus tenofovir combination as rescue therapy in pretreated chronic hepatitis B patients: an international multicenter cohort study. J Hepatol. 2012;56:520–526. doi: 10.1016/j.jhep.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 27.DH MBS. Natural History of Chronic Hepatitis B Infection. http://depts.washington.edu/hepstudy/hepB/mgmt/treatment/discussion.html. 2013.


