Abstract
Background: This study was aimed at evaluating the associations between frequency of meat food intake and osteoporosis (OP) in general Chinese postmenopausal women. Methods: We conducted a large-scale, community-based, cross-sectional study to investigate the associations by using self-report questionnaire to access frequency of meat food intake. The total of 1905 participants was available to data analysis in this study. Multiple regression models controlling for confounding factors to include frequency of meat food intake variable were performed to investigate the relationships for OP. Results: Positive correlations between frequency of meat food intake and T-score were reported (β = 0.12, P value < 0.001). Multiple regression analysis indicated that the frequency of meat food intake was significantly associated with OP (P < 0.1 for model 1 and model 2). The postmenopausal women with high frequency of meat food intake had a lower prevalence of OP. Conclusion: The findings indicated that frequency of meat food intake was independently and significantly associated with OP. The prevalence of OP was less frequent in Chinese postmenopausal women preferring meat food habits.
Keywords: Frequency, meat food intake, osteoporosis, Chinese postmenopausal women, association
Introduction
Osteoporosis (OP) is a progressive systemic bone disease characterized by low bone mass and micro-architecture deterioration of bone tissue. Decreased bone mass and bone damage lead to increased bone fragility and facture [1]. As the global older population increases, the prevalence of OP and the incidence of osteoporosis-related fractures is becoming a major social and medical concern [2]. There is higher prevalence of OP among postmenopausal women and the elderly [3]. In the United States and Europe, more than 30% of postmenopausal women have OP [4]. Ho et al. reported that the prevalence of OP was as high as 60% in Chinese postmenopausal women [5].
Generally, primary prevention is the most cost-effective approach for managing common diseases. Modifiable lifestyle factors such as diet, exercise, smoking, and alcohol consumption are important in preventing or deterring the development of OP and reducing the risk of osteoporosis-related fractures [6,7]. Diet factors play a key role in the maintenance of optimal bone health [8]. Numerous studies have shown meat consumption is associated with cardiovascular diseases [9,10] and certain cancers [11]. Several studies indicated that frequency of meat consumption was associated with bone mass and fracture rates in humans [12-14]. However, other studies reported no significant associations between meat consumption and the outcome [15,16]. The associations between meat consumption and OP are controversial.
Moreover, little is known about the relationship between meat consumption and outcomes in Chinese postmenopausal women. Thus, the main purpose of the present study was to evaluate the extent to which meat consumption was associated with OP among Chinese postmenopausal women by using self-reported questionnaire applied to a large-scale sample.
Methods
Study population
We performed a risk-factor study for OP using a random sample of the Chinese population. Participants were recruited from rural and urban communities in Shanghai. Participants aged 30-90 years were included in this study. More than 2,000 postmenopausal women were invited to a screening visit between 2011 and 2014. Written consent was obtained from all patients before the study, which was performed in accordance with the ethical standards in the Declaration of Helsinki, and approved by the Medicine Ethical Committee of the Changfeng Healthcare Center. Some participants with chronic diseases and conditions that might potentially affect bone mass, structure, or metabolism were excluded. Briefly, the exclusion criteria were as follows: a history of 1) serious residual effects of cerebral vascular disease; 2) serious chronic renal disease (Glomerular filtration rate-GFR < 30 mL/min/1.73 m2); 3) serious chronic liver disease or alcoholism; 4) significant chronic lung disease; 5) corticosteroid therapy at pharmacologic levels; 6) evidence of other metabolic or inherited bone disease, such as hyper- or hypoparathyroidism, Paget disease, osteomalacia, or osteogenesis imperfecta; 7) recent (within the past year) major gastrointestinal disease, such as peptic ulcer, malabsorption, chronic ulcerative colitis, regional enteritis, or significant chronic diarrhea; 8) Cushing syndrome; 9) hyperthyroidism; and 10) any neurologic or musculoskeletal condition that would be a non-genetic cause of low bone mass.
Data collection
All study subjects underwent complete clinical baseline characteristics evaluation, which included a physical examination and response to a structured, nurse-assisted, self-administrated questionnaire to collect information on age, gender, residential region, visit date, family history, lifestyle, dietary habits, physical activity level during leisure time, use of vitamins and medications, smoking, alcohol consumption, and self-reported medical history. Body weight and height were measured according to a standard protocol. Smoking and alcohol consumption were categorized as never, current (smoking or consuming alcohol regularly in the past 6 months), or ever (cessation of smoking or alcohol consumption for more than 6 months). Regular exercise was defined as any kind of physical activity 3 or more times per week. Education was commonly divided into four stages: preschool, primary school, secondary school, and college. Self-reported medical and therapy history was categorized as “no” or “yes.” HTN was defined as blood pressure ≥ 140/90 mmHg, or a history of hypertension medication. Diabetes mellitus (DM) was defined by oral glucose tolerance test (OGTT) and either HbAlc ≥ 6.5% or the use of insulin or hypoglycemic medications.
Dietary habits, including consumption of meat food was evaluated by a semi-quantitative food frequency questionnaire (group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always). To determine frequency of fish food preference, the participants were asked, “How often you eat meat food?” The possible answers were: “seldom,” “once or twice per week,” “once every 2 days,” or “always,” and the answers were taken as a subjective assessment. To answer the question, the participants were required to decide two issues based on their impressions: 1) whether or not the consumed meat were actually fish; and 2) the frequency with which they consumed meat foods.
The study outcomes
The bone mineral density (BMD g/cm2) was measured at calcaneus by standardized quantitative ultrasound (QUS, Hologic Inc., Bedford, MA, USA) utilizing T-scores based on WHO criteria [17], which were obtained from the automated equipment. T-score refers to the ratio between patient’s BMD and that of young adult population of same sex and ethnicity. T-score of > -1 was taken as normal, between -1 and -2.5 osteopenic and < -2.5 as osteoporotic. Daily calibration was performed during the entire study period by a trained technician. The coefficients of variation of the accuracy of the QUS measurement were 0.9%. The QUS technology is less expensive, portable and also has the advantage of not using ionising radiation, so it is safer than dualenergy X-ray absorptiometry (DEXA).
Statistical analysis
Continuous variables were analyzed to determine whether they followed normal distributions, using the Kolmogorov-Smirnov Test. Variables that were not normally distributed were log-transformed to approximate a normal distribution for analysis. Results are described as mean ± SD or median, unless stated otherwise. Differences in variables among subjects grouped by frequency of meat food intake were determined by one way analysis of variance. Among groups, differences in properties were detected by χ2 analysis. Univariate regression analysis was performed to determine variables associated with outcomes (T-score or OP), and to estimate confounding factors possibly disturbing the relation of frequency of fish food intake to outcomes (T-score or OP). For the associations analysis, there model have been developed. In model 1, frequency of meat food intake was categorized by group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always. In model 2: frequency of meat food intake was categorized by low frequency and high frequency groups. Tests were two-sided, and a P-value of < 0.05 was considered significant. Multivariable regression (MR) was performed to control potential confounding factors and determine the independent contribution of variables to outcomes (T-score or OP). Under MR models, tests were two-sided, and a P-value of < 0.1 was considered significant. Results were analyzed using the Statistical Package for Social Sciences for Windows, version 16.0 (SPSS, Chicago, IL, USA). Odds ratios (OR) with 95% confidence intervals (CI) were calculated for the relative risk of frequency of fish food intake with the outcome of OP.
Results
Clinical characteristics of subjects
The clinical baseline characteristics of the 1905 Chinese postmenopausal women are listed in Table 1. In the total sample, the mean age was 62.39 years. The minority proportions of subjects having current smoking and alcohol habits were reported (0.79% and 2.10% for smoking and drink intake, respectively). The prevalence of HTN, coronary artery disease (CAD), DM and Rheumatoid arthritis (RA) were 45.57%, 10.03%, 11.47%, and 5.71%, respectively. An average T-score of -1.86 was reported and the prevalence of OP was 28.29% in our study sample. There were significant differences in age and education among groups according to frequency of fish food intake (P value < 0.05 for all). Significant differences in T-Score and the prevalence of OP among the four groups were reported (P value = 0.003 for T-score and 0.045 for the prevalence of OP).
Table 1.
Baseline characteristics of subjects
| Variable | Total sample | Frequency of meat food intake* | P value | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Group 1 | Group 2 | Group 3 | Group 4 | |||
| Demographical information | ||||||
| N | 1905 | 199 | 857 | 527 | 322 | - |
| Age | 62.39±8.99 | 63.96±9.59 | 62.93±8.99 | 61.92±8.96 | 60.76±8.34 | < 0.001 |
| Height | 156.35±5.71 | 155.64±4.96 | 156.94±5.67 | 155.07±6.46 | 156.2±5.94 | 0.714 |
| Weight | 58.85±8.47 | 57.82±7.91 | 58.9±7.07 | 60.5±12.37 | 57.9±9.2 | 0.834 |
| Lifestyle | ||||||
| Education | 254 (13.33%) | 19 (9.55%) | 120 (14%) | 55 (10.44%) | 60 (18.63%) | < 0.001 |
| Exercise | 1364 (71.6%) | 134 (67.34%) | 630 (73.51%) | 375 (71.16%) | 225 (69.88%) | 0.279 |
| Smoking | 15 (0.79%) | 2 (1.01%) | 8 (0.93%) | 4 (0.76%) | 1 (0.31%) | 0.572 |
| Drink | 40 (2.1%) | 5 (2.51%) | 25 (2.92%) | 6 (1.14%) | 4 (1.24%) | 0.210 |
| Oil | 19.14±9.05 | 18.37±8.07 | 19.14±9.11 | 19.04±9.05 | 19.78±9.45 | 0.375 |
| Medical history | ||||||
| HTN | 837 (44.57%) | 89 (45.41%) | 391 (46.27%) | 228 (43.93%) | 129 (40.57%) | 0.362 |
| CAD | 183 (10.03%) | 13 (6.67%) | 89 (10.8%) | 48 (9.6%) | 33 (10.78%) | 0.350 |
| DM | 214 (11.47%) | 25 (12.63%) | 86 (10.29%) | 61 (11.91%) | 42 (13.17%) | 0.491 |
| RA | 105 (5.71%) | 16 (8.12%) | 43 (5.19%) | 30 (5.95%) | 16 (5.18%) | 0.431 |
| Therapy history | ||||||
| Vitamin C | 247 (12.97%) | 30 (15.08%) | 121 (14.12%) | 50 (9.49%) | 46 (14.29%) | 0.045 |
| Vitamin D | 78 (4.09%) | 6 (3.02%) | 35 (4.08%) | 22 (4.17%) | 15 (4.66%) | 0.912 |
| Outcome | ||||||
| T-score | -1.86±0.74 | -1.97±0.74 | -1.9±0.74 | -1.81±0.72 | -1.78±0.78 | 0.003 |
| OP | 539 (28.29%) | 61 (30.65%) | 262 (30.57%) | 127 (24.1%) | 89 (27.64%) | 0.061 |
Frequency of meat food intake were categorized by group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always;
HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA-Rheumatoid arthritis, OP-Osteoporosis.
Univariate analysis for T-score and OP
Univariate linear regression analyses were developed to include demographical information, medical history, and lifestyle to estimate the association of various clinical factors and Tscore (Table 2). The variables age, education, Vitamin C supplement and meat food preference were significantly associated with the T-score (P < 0.05 for all).
Table 2.
Univariate linear regression analysis for associations among variables and T-score
| Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|
| Age | -0.033 | 0.002 | < 0.001 | -0.036-0.029 |
| Height | 0.019 | 0.015 | 0.195 | -0.01-0.048 |
| Weight | -0.002 | 0.01 | 0.836 | -0.022-0.018 |
| Education | 0.088 | 0.014 | < 0.001 | 0.06-0.116 |
| Exercise | 0.063 | 0.038 | 0.096 | -0.011-0.137 |
| Smoking | -0.09 | 0.093 | 0.333 | -0.273-0.093 |
| Drink | 0.031 | 0.059 | 0.592 | -0.084-0.147 |
| HTN | -0.045 | 0.035 | 0.196 | -0.112-0.023 |
| CAD | -0.116 | 0.058 | 0.048 | -0.23--0.001 |
| DM | 0.039 | 0.054 | 0.467 | -0.067-0.146 |
| Gout | -0.235 | 0.249 | 0.346 | -0.723-0.253 |
| RA | -0.133 | 0.075 | 0.075 | -0.279-0.013 |
| Vitamin C | -0.114 | 0.051 | 0.024 | -0.213-0.015 |
| Vitamin D | -0.058 | 0.085 | 0.5 | -0.225-0.11 |
| Frequency of meat intake | 0.12 | 0.034 | < 0.001 | 0.053-0.187 |
Note: HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA-Rheumatoid arthritis.
The comparison of T-scores among groups according to Model 1 revealed that the mean T-score was -1.96, -1.90, -1.81 and -1.78 in the four groups, respectively (Figure 1A). There were significant differences among the four groups (P value = 0.003). Additionally, there were significant differences among groups according to model 2 (Figure 1B, P value < 0.001), univariate analysis demonstrated a positive correlation between frequency of meat food intake and T-score. Univariate logistic analyses were performed to evaluate associations with OP. The results indicate that age, education, HTN, CAD, RA, Vitamin C, Vitamin D and frequency of meat intake were significantly associated with OP (P value < 0.05 for all, Table 3). The comparison of prevalence of OP among groups according to model 1 reported that the prevalence of OP was 31.16%, 30.57%, 23.91% and 27.64% in the four groups, respectively (Figure 2A). There were significant differences among the four groups (P value = 0.045). Significant differences among groups according to model 2 were also reported (Figure 2B, P value = 0.013 for model 2). Univariate analysis demonstrates a negative correlation between frequency of meat food intake and OP.
Figure 1.
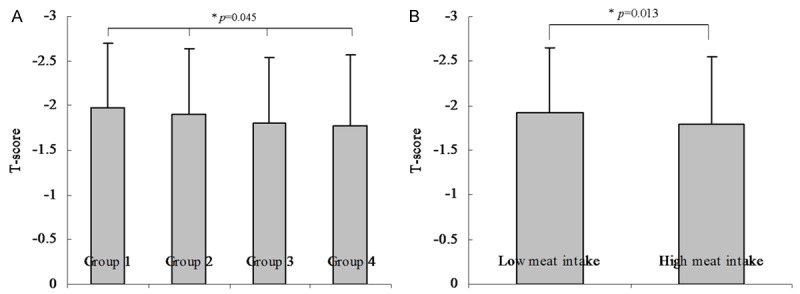
Comparison of T score among groups according to frequency of meat food intake. A: The results of comparison of T-score among groups according to Model 1 (Model 1: frequency of meat food intake were categorized by group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always). The mean T-score was -1.96, -1.90, -1.81 and -1.78 in the four groups, respectively. There were significantly differences among the four groups (P value = 0.003). B: The results of comparison of T-score between groups according to Model 2 (Model 2: frequency of meat food intake was categorized by low frequency and high frequency groups). The mean T-score was -1.92 and -1.80 in the two groups, respectively. There were no significantly differences between the two groups (P value < 0.001).
Table 3.
Univariate logistic regression analysis for associations among variables and osteoporosis
| Variable | β | S.E. | P value | OR | 95.0% CI |
|---|---|---|---|---|---|
| Age | 0.099 | 0.007 | 0 | 1.104 | 1.09-1.119 |
| Height | 0.008 | 0.05 | 0.871 | 1.008 | 0.914-1.112 |
| Weight | 0.005 | 0.033 | 0.886 | 1.005 | 0.941-1.073 |
| BMI | 0 | 0.085 | 1 | 1 | 0.847-1.18 |
| Education | -0.24 | 0.044 | 0 | 0.787 | 0.722-0.858 |
| Exercise | -0.236 | 0.111 | 0.033 | 0.79 | 0.636-0.981 |
| Smoking | 0.03 | 0.276 | 0.913 | 1.031 | 0.601-1.769 |
| Drink | -0.193 | 0.192 | 0.315 | 0.824 | 0.565-1.202 |
| HTN | 0.31 | 0.103 | 0.003 | 1.364 | 1.114-1.668 |
| CAD | 0.498 | 0.162 | 0.002 | 1.646 | 1.198-2.26 |
| DM | 0.178 | 0.157 | 0.255 | 1.195 | 0.879-1.625 |
| Gout | 0.705 | 0.673 | 0.295 | 2.023 | 0.541-7.562 |
| RA | 0.48 | 0.208 | 0.021 | 1.616 | 1.075-2.429 |
| Vitamin C | 0.314 | 0.145 | 0.03 | 1.369 | 1.03-1.817 |
| Vitamin D | 0.492 | 0.237 | 0.038 | 1.636 | 1.028-2.603 |
| Frequency of meat intake | -0.256 | 0.103 | 0.013 | 0.774 | 0.632-0.948 |
Note: HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RARheumatoid arthritis.
Figure 2.
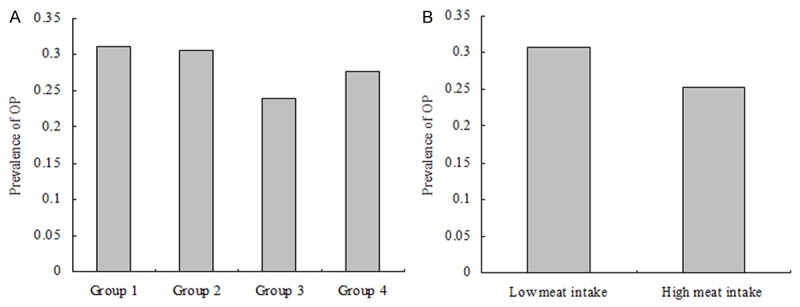
Comparison of prevalence of osteoporosis among groups according to frequency of meat food intake. A: The results of comparison of prevalence of osteoporosis among groups according to Model 1 (Model 1: frequency of meat food intake were categorized by group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always). The prevalence of osteoporosis was 31.16%, 30.57%, 23.91% and 27.64% in the four groups, respectively. There were significantly differences among the four groups (P value = 0.045). B: The results of comparison of prevalence of osteoporosis between groups according to Model 2 (Model 2: frequency of meat food intake was categorized by low frequency and high frequency groups). The prevalence of osteoporosis was 30.68% and 25.32% between the two groups, respectively. There were significantly differences between the two groups (P value = 0.013).
Multiple variable analyses for T-score and OP
Multivariate linear regression analyses were developed to include frequency of meat food intake and the outcome of T-score. After adjustment for relevant potential confounding factors, the multivariate linear regression analyses detected significant associations (β = 0.032, P-value = 0.090, 95% CI: -0.005-0.069 for model 1; and β = 0.060, P-value = 0.068, 95% CI: 0.004-0.124 for model 2, Table 4). Multivariate logistic regression analyses were employed to evaluate the association between frequency of meat food intake and the OP outcome. After adjustment for relevant potential confounding factors, the multivariate logistic regression analyses detected significant associations (P-value = 0.087 for model 1; and P value = 0.081 for model 2, Table 5). In participants with high frequency of meat food intake, the OR for OP was 0.827 in model 1 (95% CI: 0.669-1.024).
Table 4.
Multiple variables linear regression analysis for the associations between frequency of meat food intake and T score
| Model | Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|---|
| Model 1 | Frequency of meat food intake | 0.032 | 0.019 | 0.090 | -0.005-0.069 |
| Model 2 | Frequency of meat food intake | 0.060 | 0.033 | 0.068 | 0.004-0.124 |
Note: Model 1: frequency of meat food intake were categorized by group 1: seldom, group 2: once or twice per week, group 3: once every 2 days, and group 4: always; Model 2: frequency of meat food intake were categorized by low frequency and high frequency groups; and all models adjusted for age, smoking, alcohol intake, education, exercise and medical history.
Table 5.
Multiple variables logistic regression analysis for associations between frequency of meat food intake and osteoporosis
| Model | Variable | β | S.E. | P value | OR | 95% CI |
|---|---|---|---|---|---|---|
| Model 1 | Frequency of meat food intake | -0.103 | 0.060 | 0.087 | 0.902 | 0.802-1.015 |
| Model 2 | Frequency of meat food intake | -0.189 | 0.109 | 0.081 | 0.827 | 0.669-1.024 |
Note: Model 1: frequency of meat food intake were categorized by group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always; Model 2: frequency of fish meat intake were categorized by low frequency and high frequency groups; and all models adjusted for age, smoking, alcohol intake, education, exercise, medical and therapy history.
Discussion
This is a large-scale, community-based, cross-sectional study to investigate associations between meat consumption and OP in Chinese postmenopausal women. We examined more than 2000 Chinese postmenopausal women with varying meat consumption to investigate whether frequency of meat consumption affects OP. The results showed a moderate prevalence of OP inadequacy across all age groups among women with high meat consumption. Some studies have shown that more than 60% of Chinese postmenopausal women have OP leading to low bone mass and bone fragility and facture, which increased mortality and concomitant morbidity and reduced quality of life [5].
In this study, we found that meat consumption was strongly, independently, and significantly associated with OP in Chinese postmenopausal women. Results of univariate and multiple variable analysis provided evidence to support this finding (P-value < 0.01 for all analyses). Our findings were consistent with other studies that reported meat was associated with OP. Zalloua et al. investigated environmental determinants of BMD by using multiple regression models in a rural Chinese population and revealed the role of dietary intake in general [14]. They showed that specific foods, such as fruits and seafood, can positively impact BMD. A 3-year, randomized, placebo-controlled trial was conducted among 342 healthy men and women to determine whether supplemental calcium citrate malate and vitamin D influence associations between protein intake and change in BMD [18]. The findings indicated that increasing protein intake may have a favorable effect on change in BMD in older subjects who were supplemented with calcium citrate malate and vitamin D. Promislow et al. investigated the associations of total animal and vegetable protein with BMD and variations in these associations with calcium intake in a community-dwelling cohort [19]. The data suggested a protective role for dietary animal protein in the skeletal health of elderly women. Devine and his colleagues conducted a cross-sectional and longitudinal study of a population-based sample of 1077 elderly women to study the relation between protein consumption and bone mass in elderly women with allowance for other lifestyle factors that affect bone metabolism [20]. The findings suggested that protein intake for elderly women above the current recommendations may be necessary to optimize bone mass.
In contrast, other studies have shown different results. Sellmeyer et al. conducted a prospective cohort study with a mean of 7.0 years of follow-up to explore whether a high dietary ratio of animal to vegetable foods increases bone loss and the risk of fracture among 1035 community-dwelling white women [16]. This study revealed that an increase in vegetable protein intake and a decrease in animal protein intake may decrease bone loss and the risk of hip fracture. Hannan et al. examined the relation between baseline dietary protein and a subsequent 4-year change in BMD for 391 women and 224 men from the population-based Framingham Osteoporosis Study [15]. This study suggested that protein intake was important in maintaining bone or minimizing bone loss in older individuals; however, higher animal protein intake does not appear to affect the skeleton adversely in this older population. This possibility should be confirmed in other prospective studies and tested in a randomized trial. Although there was debate about whether meat consumption has correlations with OP, our study offered evidence that meat consumption was strongly, independently, and significantly associated with OP in Chinese postmenopausal women. Our findings can help physicians understand more about the importance of diet for the prevention of OP, especially the consumption of meat. In addition, our findings suggest that moderate increased meat consumption may be an efficient method for preventing OP.
Meat foods include not only protein but are also rich in phosphorus and magnesium. Prospective studies showed that individuals with the highest protein intake have the slowest rate of bone loss [15,19]. An increase in dietary protein is also known to increase circulating levels of insulin-like growth factor 1 (IGF-1), and conversely, a low-protein diet decreases IGF-1 [21]. IGF-1 is a key mediator of bone growth [22]. Magnesium (Mg) influences mineral metabolism indirectly through a role in ATP metabolism and as a cofactor for more than 300 proteins, the calciotropic hormones and 1,25(OH)2D. Mg also influences bone health with direct effects on bone quality, such as decreasing hydroxyapatite crystal size and thus preventing larger, more perfect mineral crystals that can lead to brittle bone [23]. Higher meat consumption was associated with improvements in BMD and skeletal metabolism in older individuals [24].
This study has several limitations. First, our analysis was restricted to postmenopausal women; thus, the findings are not generalizable to premenopausal women. Additionally, our study, based on a community-based data from eastern China between 2011 and 2014, requires more geographic representations in a larger sample. Finally, the study’s sample was composed entirely of Chinese postmenopausal women, which thus limits the generalizability of our results.
In conclusion, our findings suggested that frequency of meat consumption was independently and significantly associated with OP. The prevalence of OP was less frequent in Chinese postmenopausal women who preferred meat food. This study suggests that a change in meat consumption might be beneficial in the prevention of OP in Chinese postmenopausal women.
Acknowledgements
We thank the grant from Shanghai Tongji Hospital to support the study. Grants from the Clinical Medicine Foundation of Shanghai Tongji Hospital Clinical Trials. Gov Identifier: NCT02451397.
Disclosure of conflict of interest
None.
Abbreviations
- BMD
Bone mineral density
- BM-MNC
Bone marrow-derived mononuclear cell
- BMI
Body mass index
- CAD
Coronary artery disease
- CI
Confidence intervals
- DM
Diabetes
- DXA
Dual-energy X-ray
- HTN
Hypertension
- GFR
Glomerular filtration rate
- OR
Odds ratios
- OP
Osteoporosis
- QUS
Quantitative ultrasound
- RA
Rheumatoid arthritis
References
- 1.Watts NB, Lewiecki EM, Miller PD, Baim S. National Osteoporosis Foundation 2008 Clinician’s Guide to Prevention and Treatment of Osteoporosis and the World Health Organization Fracture Risk Assessment Tool (FRAX): what they mean to the bone densitometrist and bone technologist. J Clin Densitom. 2008;11:473–477. doi: 10.1016/j.jocd.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17:1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- 3.Rapuri PB, Gallagher JC, Haynatzka V. Protein intake: effects on bone mineral density and the rate of bone loss in elderly women. Am J Clin Nutrit. 2003;77:1517–1525. doi: 10.1093/ajcn/77.6.1517. [DOI] [PubMed] [Google Scholar]
- 4.Bock O, Felsenberg D. Bisphosphonates in the management of postmenopausal osteoporosis--optimizing efficacy in clinical practice. Clin Intervent Aging. 2008;3:279–297. doi: 10.2147/cia.s2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho SC, Chen YM, Woo JL. Educational level and osteoporosis risk in postmenopausal Chinese women. Am J Epidemiol. 2005;161:680–690. doi: 10.1093/aje/kwi047. [DOI] [PubMed] [Google Scholar]
- 6.Bohaty K, Rocole H, Wehling K, Waltman N. Testing the effectiveness of an educational intervention to increase dietary intake of calcium and vitamin D in young adult women. J Am Acad Nurse Pract. 2008;20:93–99. doi: 10.1111/j.1745-7599.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 7.Kukuljan S, Nowson CA, Sanders KM, Nicholson GC, Seibel MJ, Salmon J, Daly RM. Independent and combined effects of calciumvitamin D3 and exercise on bone structure and strength in older men: an 18-month factorial design randomized controlled trial. J Clin Endocrinol Metab. 2011;96:955–963. doi: 10.1210/jc.2010-2284. [DOI] [PubMed] [Google Scholar]
- 8.Rizzoli R. Nutritional aspects of bone health. Best practice & research. Clin Endocrinol Metab. 2014;28:795–808. doi: 10.1016/j.beem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 9.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Goldbourt U, Greenland P. Fish consumption and incidence of stroke: a metaanalysis of cohort studies. Stroke. 2004;35:1538–1542. doi: 10.1161/01.STR.0000130856.31468.47. [DOI] [PubMed] [Google Scholar]
- 10.He K, Song Y, Daviglus ML, Liu K, Van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 11.Geelen A, Schouten JM, Kamphuis C, Stam BE, Burema J, Renkema JM, Bakker EJ, van’t Veer P, Kampman E. Fish consumption, n-3 fatty acids, and colorectal cancer: a meta-analysis of prospective cohort studies. Am J Epidemiol. 2007;166:1116–1125. doi: 10.1093/aje/kwm197. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki T, Yoshida H, Hashimoto T, Yoshimura N, Fujiwara S, Fukunaga M, Nakamura T, Yoh K, Inoue T, Hosoi T, Orimo H. Case-control study of risk factors for hip fractures in the Japanese elderly by a Mediterranean Osteoporosis Study (MEDOS) questionnaire. Bone. 1997;21:461–467. doi: 10.1016/s8756-3282(97)00179-8. [DOI] [PubMed] [Google Scholar]
- 13.Feskanich D, Willett WC, Colditz GA. Calcium, vitamin D, milk consumption, and hip fractures: a prospective study among postmenopausal women. Am J Clin Nutrit. 2003;77:504–511. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 14.Zalloua PA, Hsu YH, Terwedow H, Zang T, Wu D, Tang G, Li Z, Hong X, Azar ST, Wang B, Bouxsein ML, Brain J, Cummings SR, Rosen CJ, Xu X. Impact of seafood and fruit consumption on bone mineral density. Maturitas. 2007;56:1–11. doi: 10.1016/j.maturitas.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- 16.Sellmeyer DE, Stone KL, Sebastian A, Cummings SR. A high ratio of dietary animal to vegetable protein increases the rate of bone loss and the risk of fracture in postmenopausal women. Study of Osteoporotic Fractures Research Group. Am J Clin Nutrit. 2001;73:118–122. doi: 10.1093/ajcn/73.1.118. [DOI] [PubMed] [Google Scholar]
- 17.Kanis J, Johnell O, Gullberg B, Allander E, Elffors L, Ranstam J, Dequeker J, Dilsen G, Gennari C, Vaz AL, Lyritis G, Mazzuoli G, Miravet L, Passeri M, Perez Cano R, Rapado A, Ribot C. Risk factors for hip fracture in men from southern Europe: the MEDOS study. Mediterranean Osteoporosis Study. Osteoporos Int. 1999;9:45–54. doi: 10.1007/s001980050115. [DOI] [PubMed] [Google Scholar]
- 18.Dawson-Hughes B, Harris SS. Calcium intake influences the association of protein intake with rates of bone loss in elderly men and women. Am J Clin Nutrit. 2002;75:773–779. doi: 10.1093/ajcn/75.4.773. [DOI] [PubMed] [Google Scholar]
- 19.Promislow JH, Goodman-Gruen D, Slymen DJ, Barrett-Connor E. Protein consumption and bone mineral density in the elderly: the Rancho Bernardo Study. Am J Epidemiol. 2002;155:636–644. doi: 10.1093/aje/155.7.636. [DOI] [PubMed] [Google Scholar]
- 20.Devine A, Dick IM, Islam AF, Dhaliwal SS, Prince RL. Protein consumption is an important predictor of lower limb bone mass in elderly women. Am J Clin Nutrit. 2005;81:1423–1428. doi: 10.1093/ajcn/81.6.1423. [DOI] [PubMed] [Google Scholar]
- 21.Bonjour JP, Schurch MA, Chevalley T, Ammann P, Rizzoli R. Protein intake, IGF-1 and osteoporosis. Osteoporos Int. 1997;7(Suppl 3):S36–42. doi: 10.1007/BF03194340. [DOI] [PubMed] [Google Scholar]
- 22.Bikle DD, Sakata T, Leary C, Elalieh H, Ginzinger D, Rosen CJ, Beamer W, Majumdar S, Halloran BP. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. doi: 10.1359/jbmr.2002.17.9.1570. [DOI] [PubMed] [Google Scholar]
- 23.Bonjour JP, Gueguen L, Palacios C, Shearer MJ, Weaver CM. Minerals and vitamins in bone health: the potential value of dietary enhancement. Br J Nutrit. 2009;101:1581–1596. doi: 10.1017/S0007114509311721. [DOI] [PubMed] [Google Scholar]
- 24.Christakis G, Christakis P. Drug interactions--nutrients, vitamins, foods (continuing education credit) J Pract Nursing. 1986;36:51–57. [PubMed] [Google Scholar]


