Abstract
Cardiopulmonary bypass (CPB) is associated with a marked systemic inflammatory response. Although dexmedetomidine (Dex) is routinely used in cardiac surgery, the effect in reducing the inflammatory response in coronary artery bypass graft surgery (CABG) with CPB remains unclear. In this study, Dex was administered at a loading dose of 0.5 μg/kg for 10 min, followed by a continuous infusion of 0.5 μg/kg per hour until the completion of CABG with CPB. The levels of inflammatory cytokines in the serum, including tumor necrosis factor-alpha (TNFalpha), interleukin (IL)-6, IL-8 and IL-10, were measured to explore the inflammatory regulating function of Dex. In addition, troponin-I (cTnI) and creatine kinase (CK-MB) levels were studied to explore the myocardial protection provided by Dex. In this study, we showed Dex inhibited the increase in cTnI and CK-MB, attenuated the production of pro-inflammatory cytokines TNF-alpha, IL-6 and IL-8, and promoted anti-inflammatory cytokine IL-10 production. These findings demonstrate that Dex regulates anti-inflammatory as well as myocardial protection potential in CABG with CPB.
Keywords: Dexmedetomidine, myocardial injury, inflammatory response, coronary artery bypass grafting
Introduction
Dexmedetomidine (Dex), a highly selective α2-adrenergic receptor agonist, is widely used for sedation and analgesia in the ICU or as an anaesthetic adjuvant [1]. Some clinical reports have demonstrated the effect in cardiac surgery: Dex can maintain a stable hemodynamic status during coronary artery bypass graft (CABG) surgery [2], and is a safe substitution for propofol [3]. Dex is associated with a decrease in postoperative mortality and incidence of postoperative complications in patients undergoing cardiac surgery [4]. In addition, Dex suppresses the stress responses against intubation and surgery [5,6]. Furthermore, it can decrease the heart rate (HR), and reduce myocardial oxygen consumption due to its sympatholytic property. Therefore, Dex is widely used in CABG surgery as anaesthetic adjuvant.
CABG with cardiopulmonary bypass (CPB) is a traditional surgical approach for treating coronary artery stenosis. During the surgery, a significant systemic inflammatory response can be observed. It can be caused by CPB, surgical trauma, pulmonary and myocardial ischemia-reperfusion injury. This inflammatory responses cause proinflammatory cytokine release, complement activation, neutrophil infiltration and endothelial cell swelling which can cause extra myocardial injury [7,8]. In order to minimise the extra myocardial damage, it is important to limit inflammatory responses during CPB procedures.
Inflammation is the key to pathophysiology of myocardial ischemia-reperfusion injury [9]. Several studies and clinical trials have revealed that inflammation increases during myocardial ischemia and reperfusion [10-12]. Several studies have demonstrated that Dex has strong anti-inflammatory properties [13]. Dex reduces the mortality rate and systemic inflammatory responses during polymicrobial sepsis, mitigate pulmonary inflammatory response caused by ventilator-induced lung injury, and alleviate delirium by suppressing neuroinflammation mediated by microglia [14-16]. In addition, a study with astrocytes demonstrated that Dex has therapeutic effects on neuronal inflammatory reactions mediated by Jun N-terminal kinase (JNK) signaling pathway [17].
In order to improve the clinical outcome, therapeutic strategies to attenuate reperfusion injury and systemic inflammatory response are needed. Several drugs such as propofol, opioids, and inhalational anesthetics have cardioprotective effects against ischemia-reperfusion injury [18-22]. Inflammation and myocardial injury resulting from on-pump CABG was much higher than that from off-pump CABG [20].
However, the relationship between Dex and inflammatory cytokines remains unclear. Hence, we investigated whether Dex has an anti-inflammatory effect on myocardial ischemia-reperfusion injury. In order to evaluate the effect of Dex on myocardial injury and inflammatory response, we recruited patients who underwent CPB surgery. We hypothesized that Dex offers myocardial protection by inhibiting the inflammatory response. In this study, myocardial ischemia markers cardiac troponin I (cTn I) and creatine kinase MB (CK-MB) were chosen to evaluate myocardial protection, and pro-inflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), IL-8, and anti-inflammatory cytokine IL-10 were chosen to evaluate the inflammatory response in CABG.
Materials and methods
Patients and exclusion criteria
65 patients who had elective CABG surgery with CPB from March 2013 till August 2014 were randomly allocated into two groups. Patients were administrated with Dexmedetomidine (Dex) (Yichang Human well Pharmaceutical Co. Ltd., Hubei, China) or sterilised saline (Shandong San Lu Pharmaceutical Co. Ltd., Shandong, China).
Exclusion criteria include patients with systemic inflammatory diseases, received immunosuppressive or anti-inflammatory medication within a month before administration, previous cardiac surgery, myocardial infarction within six weeks before administration, and severely impaired left ventricular function (ejection fraction <40%). Patients were also excluded if an emergency operation for unstable angina was performed. The study was approved by the Medical Research Ethics Committee of the Second Affiliated Hospital, Nanchang University. All patients signed informed consent forms before involved in this study.
The patients’ invasive arterial blood pressure and heart rate (HR) were continuously monitored (Datex-Ohmeda) and recorded. The mean arterial pressure (MAP) and HR were determined at the following time points: baseline (before administration of Dex or normal saline) (T1), 15 mins after declamping the aorta (T2), 2 h after declamping the aorta (T3), and 24 h after declamping the aorta (T4).
Anaesthesia
Cardiovascular medications, including beta-adrenergic blockers, calcium-channel blockers, nitrates, etc. were continued until morning of the surgery. In the operation room, a pulse oximeter and five ECG leads were attached to the patient, and leads II were continuously monitored. A 20 G radial artery catheter was inserted to monitor arterial blood pressure. A double cavity central venous catheter was inserted into the right internal jugular vein before induction of anaesthesia Patients started Dex pump infusion at a loading dose of 0.5 µg/kg for 10 mins, followed by a continuous pump infusion 0.5 µg/kg per hour until the completion of the surgery. The control group received same infusion of normal saline for the same period. Propofol was used for post-operative sedation in the ICU.
Anesthesia was induced with 0.5-1.0 µg/kg sufentanil, 0.15 mg/kg cisatracurium and 2 mg/kg propofol. After tracheal intubation, anesthesia was maintained with sevoflurane (1.5-2.5%), continuous infusion of 0.1 mg/kg per hour cisatracurium and 1 mg/kg per hour of propofol. A bolus dose of 0.3 µg/kg sufentanil was given before skin incision. Phenylephrine was infused when MAP or systolic pressure decreased below 50 and 80 mmHg, respectively. Intraoperative anticoagulation was achieved using 3 mg/kg of heparin before CPB. The activated clotting time (ACT) was maintained above 480 seconds during CPB. Anticoagulation was reversed with 1.5 mg protamine per 1 mg of heparin at the end of CPB.
Surgical procedure
Routine surgery was performed through a median sternotomy in all patients by the same surgical team. The left internal mammary artery (with pedicle) and the greater saphenous vein were harvested. CPB was performed using a roller pump and membrane oxygenator. Hemodilution was primed with a solution of 1000 ml each of Ringer’s lactate and hydroxyethyl starch. Systemic hypothermia was maintained at 31-35°C. Cardioplegia with high-dose (100 mEq/L) and low-dose (40 mEq/L) potassium was given in a ratio of 1:4 of total blood volume during bypass. MAP was maintained between 50-80 mmHg, and CPB flow was maintained between 2-2.5 L/m per minute. The left anterior descending coronary arteries were revascularized with the pedicled left internal mammary artery flaps. Saphenous vein grafts were used to revascularize the other coronary artery branches. Pharmacological support was given in accordance with the patients’ hemodynamic needs.
Detection of blood parameters
Central venous blood samples were taken at T1, T2, T3 and T4. Blood samples were centrifuged at 1000 rpm for 10 min at 4°C, and then plasma levels of cTn I and CK-MB were detected using an ADVIA Centaur CP System® (Siemens, USA) by chemical luminous technology. Serum levels of TNF-alpha, IL-6, IL-8 and IL-10 were detected using sandwich enzyme immunoassay sets® (CytoSets, Biosource, USA) by enzyme-linked immunosorbent assay (ELISA). All assays were performed according to the manufacturers’ instructions.
Statistical analysis
Statistical analyses were performed using SPSS version 19.0 software program (IBM, USA). Individual data points were excluded as outliers if >2 SD from the mean. Data is displayed as mean ± SD. Difference in groups were considered significant if P<0.05. Data was analysed using One-way ANOVA and T test. Statistical significance in differences between the groups was concluded when P<0.05.
Results
Preoperative character of patients showed no difference, nor surgical parameters
To investigate the effect of Dex treatment on the inflammatory responses, we firstly excluded any confounding including age, gender, weights and drugs been taken before operation. Patients experienced elective CABG by CPB was randomly assigned into two groups received either Dex (n=32) or saline (n=32). Patients showed no difference in their age, sex, body mass index or ejection fraction (Table 1). It is also noticed that the medications of patients taken had no difference cross these two groups (Table 1). The cross-clamping time, duration of CPB, number of grafted vessels and inotropic support showed no significance between two groups (Table 2).
Table 1.
Characteristics of patients
| Parameters | Control group (n=32) | Dex group (n=32) | p-value |
|---|---|---|---|
| Age (years) | 62.9 (7.2) | 61.8 (7.8) | 0.66 |
| Gender (male/female) | 24/8 | 20/12 | 0.71 |
| Body mass index (kg m-2) | 28.2 (3.0) | 27.7 (3.2) | 0.62 |
| LV ejection fraction (%) | 54.7 (6.6) | 58.4 (6.1) | 0.11 |
| Preoperative medications | |||
| Calcium channel blockers, n (%) | 8 (25) | 4 (12.5) | 0.37 |
| Nitrates, n (%) | 20 (62.5) | 12 (37.5) | 0.16 |
| β-blockers, n (%) | 18 (56.3) | 28 (87.5) | 0.11 |
| ACEIs, n (%) | 16 (50) | 22 (68.8) | 0.47 |
| Statins, n (%) | 18 (56.3) | 24 (75) | 0.46 |
| Diuretics, n (%) | 16 (50) | 12 (37.5) | 0.72 |
Data are shown as incidence (%) or mean ± (SD). BMI, body mass index; EF, ejection fraction; ACEI, angiotensin-converting enzyme inhibitor.
Table 2.
Comparison of surgical parameters
| Parameters | Control group (n=32) | Dex group (n=32) | p-value |
|---|---|---|---|
| Cross-clamping time (min) | 44 (19) | 46 (20) | 0.68 |
| Duration of CPB (min) | 88 (36) | 91 (27) | 0.37 |
| Number of grafted vessels | 3.3 (0.8) | 3.5 (0.8) | 0.59 |
| Need for inotropic support, n (%) | 14 (43.7) | 12 (37.5) | 0.80 |
Values are provided as incidence (%) or mean ± (SD). CPB, cardiopulmonary bypass.
Dexmedetomidine induces lower arterial pressure and heart rate
15 min (T2) till 2 h (T3) after declamping the aorta, the MAP of patients received Dex is lower (82.4±16.5 at T2 and 85.4±13.9 at T3 respectively), as compared with their baseline (97.6±18.4 in T1) (Table 3). In addition, Dex treated patients also had a lower MAP at T2 when compared control group (82.4±16.5 at T2 in Dex group and 96.9±14.6 in control group) (Table 3). Patients also had a reduced heart rate at T2 after administration of Dex to both control group and their baseline (62.4±7.5 in Dex at T2; 79.3±8.6 in control at T2 and 71.3±9.8 in Dex at T1) (Table 3).
Table 3.
Patients showed reduced MAP and HR during and after surgery
| Variable | Group | T1 | T2 | T3 | T4 |
|---|---|---|---|---|---|
| MAP (mmHg) | Control | 94.4 (12.7) | 96.9 (14.6) | 89.3 (19.1) | 93.5 (17.7) |
| Dex | 97.6 (18.4) | 82.4 (16.5)*,# | 85.4 (13.9)* | 88.5 (20.3) | |
| HR (bpm) | Control | 70.2 (10.3) | 79.3 (8.6)# | 83.4 (8.8) | 81.7 (9.3) |
| Dex | 71.3 (9.8) | 62.4 (7.5)*,# | 80.6 (7.5) | 76.8 (10.6) |
Data are presented as the mean ± (SD).
P<0.05 as compared to T1;
P<0.05 as compared to the control group;
MAP, mean blood pressure; HR, heart rate.
Dexmedetomidine is associated with reduced CTnI and CK-MB level
Blood samples were taken from patients in both groups and cTnl levels and CK-MB were determined. Patients received either Dex or normal saline had an overall increase level of cTnl after declamping the aorta (P<0.01) (Figure 1A). However, compared to control group, Dex treated patients clearly showed 2-fold decrease in cTnl at 2 h (T3) (P<0.01) and 1.6-fold decrease at 24 h (T4) (P<0.01) after declamping the aorta (Figure 1A). At the meantime, patients in both group had an overall increase in the level of CK-MB when compared to the baseline (P<0.05 or P<0.01). Dex appeared to reduce the level of CK-MB during and after surgery (P<0.05 or P<0.01) (Figure 1B).
Figure 1.
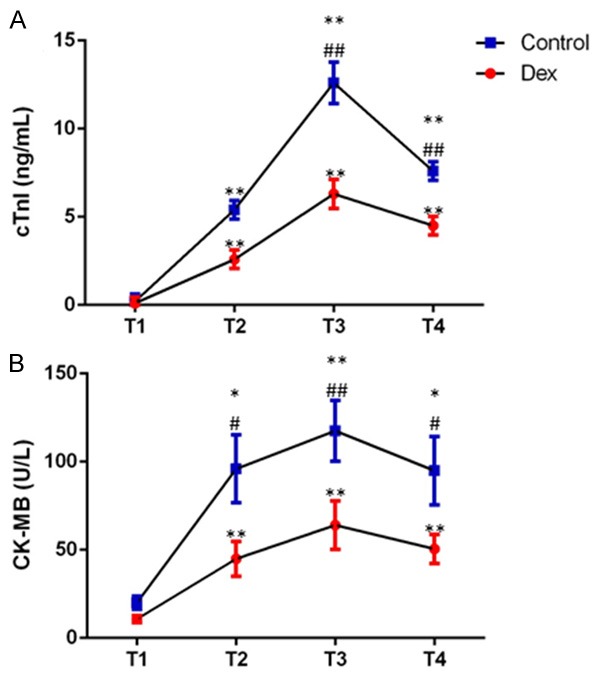
Blood cTnI and CK-MB levels were elevated during and after surgery in both groups. Data were collected at baseline (T1), and 15 mins (T2), 2 h (T3) and 24 h (T4) after declamping the aorta. Data are presented as mean (SD). *P<0.05 as compared to T1; **P<0.01 as compared to T1; #P<0.05 as compared to the control group, ##P<0.01 as compared to the control group.
Reduced plasma proinflammation in patients received dexmedetomidine
Knowing that Dexmedetomidine promotes cardiovascular function, we checked some proinflammatory cytokines as well as anti-proinflammatory cytokine to evaluate immune responses of patients during and after surgery. There was an acute upregulation (P<0.05 or P<0.01) of TNF-α, IL-6 and IL-8 during and after surgery in both groups when compared with before surgery (Figure 2A-C). However, the upregulation of TNF-α, IL-6 and IL-8 is not to the same extend in patients received Dex (P<0.05 or P<0.01) (Figure 2A-C). Dex successfully inhibited the excess production of proinflammatory cytokines. The anti-inflammatory cytokine IL-10 is also elevated throughout T2 till T4, in both groups (Figure 2D), and it is clearly that patients had Dex appeared more IL-10 has been produced. Overall, Dex showed it can limit proinflammatory cytokine production, and promote anti-inflammation.
Figure 2.
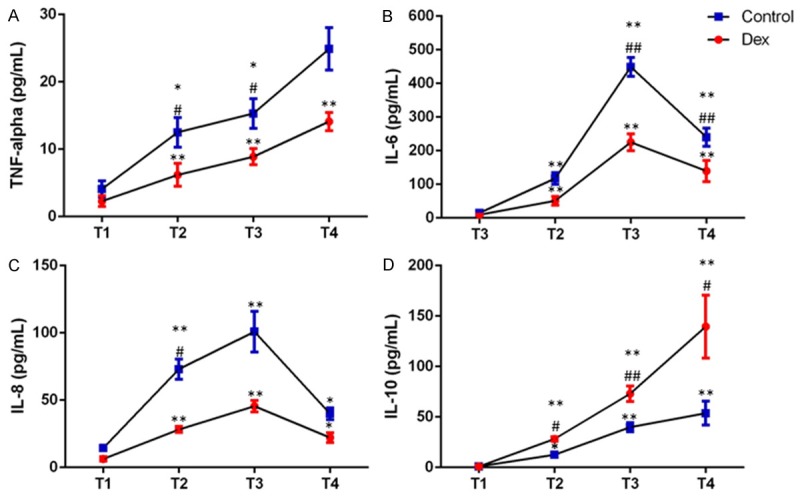
Concentrations of pro-inflammatory cytokines (TNF-alpha-panel A, IL-6-panel B, IL-8-panel C) and anti-inflammatory cytokine (IL-10-panel D) at baseline (T1), and 15 mins (T2), 2 h (T3) and 24 h (T4) after declamping the aorta. Data are presented as mean (SD). *P<0.05 as compared to T1; **P<0.01 as compared to T1; #P<0.05 as compared to the control group, ##P<0.01 as compared to the control group.
Discussion
This study demonstrated that CABG surgery results in the release of cardiac biochemical markers cTnI and CK-MB, and increases the levels of inflammatory cytokines TNF-alpha, IL-6, IL-8 and IL-10. However, in patients received Dex, decrease cTnI and CK-MB levels were observed, as well as reduced levels of TNF-alpha, IL-6 and IL-8, and upregulation of IL-10. Dex-induced downregulation of pro-inflammatory cytokines and upregulation of anti-inflammatory cytokine may be associated with attenuation of systemic inflammatory response, which could reduce the incidences of myocardial injury.
CTnI and CK-MB are unique biomarkers for myocardial necrosis. After declamping the aorta, cardiac resuscitation involves reperfusion recovery of the ischemic heart. We detected an obvious increase in cTnI and CK-MB after declamping the aorta in both groups, which indicates, to some extent, there is myocardial injury. In addition, we observed a significant decrease in the levels of cTnI and CK-MB in the Dex group than those in the control group, suggesting that Dex reduces myocardial injury, and provides organ protection in the myocardium. Dex was previously reported to attenuate ischemia-reperfusion injury in intestinal, renal and brain tissues [20,23].
Inflammatory response results from tissue reperfusion injury, and is also the primary cause of ischemia-reperfusion injury [24]. Tissue injuries activate the innate immune system as well as cytokine cascade which is critical to inflammatory response. The excessive inflammatory response could be more harmful than the original insult [25]. In this study, we detected an increase in the pro-inflammatory cytokines TNF-alpha, IL-6, IL-8 and anti-inflammatory cytokine IL-10 on the first day in both groups, which is similar to the report by Ueki et al. [26] that detected high-mobility group box 1 after CPB. Our data suggested that the expression of inflammatory cytokines increased after myocardial reperfusion, i.e. inflammation occurs after myocardial ischemia-reperfusion injury, which is in accordance with previous studies [27]. CPB, reperfusion injury of the myocardium and lungs, and surgical trauma can cause an inflammatory response. Furthermore, TNF-alpha, IL-6 and IL-8 levels were significantly lower, while IL-10 was obviously higher in the Dex group than in the control group, which demonstrates that Dex decreases systemic inflammatory response. Myocardial protection by Dex may be linked to the inhibition of cytokines. Anti-inflammatory mechanism of Dex could involve excitation of the vagus nerve, and activation of the cholinergic anti-inflammatory pathway [28]. The finding that Dex has an immunomodulatory function suggests that it could be used as a novel adjunct therapeutic agent to regulate the immune response in ischemia-reperfusion injury patients.
Notably, major hemodynamic fluctuations were not found in this study, which is in contrast to a previous report [29], and could be related to the differences in Dex administration methods. According to the recommended administration method [30], Dex is started at a loading dose of 1 μg/kg for more than 10 min, followed by an additional 0.2-0.7 μg/kg per hour for maintenance. High blood pressure and bradycardia often occur at this dose. In order to avoid these conditions, we reduced the Dex loading dose to 0.5 μg/kg for 10 min, followed by a continuous infusion of 0.5 μg/kg per hour, and showed that limiting the loading dose helps in hemodynamic stability.
In this study, a significant decline in the HR and blood pressure of the patients was observed in the Dex group, which is in accordance with a previous study [31]. Dex reduces myocardial oxygen consumption and cardiac afterload, and provides myocardial protection, especially in coronary artery stenosis patients. The decrease in HR and MAP is due to the inhibition of the central sympathetic neurotransmitters, reduction of the peripheral sympathetic nervous tension, and simultaneous excitation of the vagus nerve [32].
In conclusion, this study demonstrated that Dex could alleviate myocardial injury and attenuate the systemic inflammatory response. The limitation of this study was its small sample size. Hence, future studies should include a larger sample size to confirm these findings.
Acknowledgements
This work was supported by a grant from the reviewers for their helpful comments on this paper.
Disclosure of conflict of interest
None.
References
- 1.Afonso J, Reis F. Dexmedetomidine: current role in anesthesia and intensive care. Rev Bras Anestesiol. 2012;62:118–133. doi: 10.1016/S0034-7094(12)70110-1. [DOI] [PubMed] [Google Scholar]
- 2.Kabukcu HK, Sahin N, Temel Y, Titiz TA. Hemodynamics in coronary artery bypass surgery: effects of intraoperative dexmedetomidine administration. Anaesthesist. 2011;60:427–431. doi: 10.1007/s00101-010-1842-3. [DOI] [PubMed] [Google Scholar]
- 3.Reichert MG, Jones WA, Royster RL, Slaughter TF, Kon ND, Kincaid EH. Effect of a dexmedetomidine substitution during a nationwide propofol shortage in patients undergoing coronary artery bypass graft surgery. Pharmacotherapy. 2011;31:673–677. doi: 10.1592/phco.31.7.673. [DOI] [PubMed] [Google Scholar]
- 4.Ji F, Li Z, Nguyen H, Young N, Shi P, Fleming N, Liu H. Perioperative dexmedetomidine improves outcomes of cardiac surgery. Circulation. 2013;127:1576–1584. doi: 10.1161/CIRCULATIONAHA.112.000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menda F, Koner O, Sayin M, Ture H, Imer P, Aykac B. Dexmedetomidine as an adjunct to anesthetic induction to attenuate hemodynamic response to endotracheal intubation in patients undergoing fast-track CABG. Ann Card Anaesth. 2010;13:16–21. doi: 10.4103/0971-9784.58829. [DOI] [PubMed] [Google Scholar]
- 6.Sulaiman S, Karthekeyan RB, Vakamudi M, Sundar AS, Ravullapalli H, Gandham R. The effects of dexmedetomidine on attenuation of stress response to endotracheal intubation in patients undergoing elective off-pump coronary artery bypass grafting. Ann Card Anaesth. 2012;15:39–43. doi: 10.4103/0971-9784.91480. [DOI] [PubMed] [Google Scholar]
- 7.Franke A, Lante W, Fackeldey V, Becker HP, Kurig E, Zoller LG, Weinhold C, Markewitz A. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: is what we see what we know? Eur J Cardiothorac Surg. 2005;28:569–575. doi: 10.1016/j.ejcts.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 8.Palatianos GM, Balentine G, Papadakis EG, Triantafillou CD, Vassili MI, Lidoriki A, Dinopoulos A, Astras GM. Neutrophil depletion reduces myocardial reperfusion morbidity. Ann Thorac Surg. 2004;77:956–961. doi: 10.1016/j.athoracsur.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594–604. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 10.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin L, Knowlton AA. Innate immunity and cardiomyocytes in ischemic heart disease. Life Sci. 2014;100:1–8. doi: 10.1016/j.lfs.2014.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincent A, Lattuca B, Merlet N, Sportouch-Dukhan C, Barrere-Lemaire S. New insights in research about acute ischemic myocardial injury and inflammation. Antiinflamm Antiallergy Agents Med Chem. 2013;12:47–54. doi: 10.2174/1871523011312010007. [DOI] [PubMed] [Google Scholar]
- 13.Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. Eur J Anaesthesiol. 2011;28:3–6. doi: 10.1097/EJA.0b013e32833e266d. [DOI] [PubMed] [Google Scholar]
- 14.Chen C, Zhang Z, Chen K, Zhang F, Peng M, Wang Y. Dexmedetomidine regulates inflammatory molecules contributing to ventilator-induced lung injury in dogs. J Surg Res. 2014;187:211–218. doi: 10.1016/j.jss.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Peng M, Wang YL, Wang CY, Chen C. Dexmedetomidine attenuates lipopolysaccharide-induced proinflammatory response in primary microglia. J Surg Res. 2013;179:e219–225. doi: 10.1016/j.jss.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 16.Xu L, Bao H, Si Y, Wang X. Effects of dexmedetomidine on early and late cytokines during polymicrobial sepsis in mice. Inflamm Res. 2013;62:507–514. doi: 10.1007/s00011-013-0604-5. [DOI] [PubMed] [Google Scholar]
- 17.Zhang X, Wang J, Qian W, Zhao J, Sun L, Qian Y, Xiao H. Dexmedetomidine inhibits tumor necrosis factor-alpha and interleukin 6 in lipopolysaccharide-stimulated astrocytes by suppression of c-Jun N-terminal kinases. Inflammation. 2014;37:942–949. doi: 10.1007/s10753-014-9814-4. [DOI] [PubMed] [Google Scholar]
- 18.Agarwal B, Stowe DF, Dash RK, Bosnjak ZJ, Camara AK. Mitochondrial targets for volatile anesthetics against cardiac ischemia-reperfusion injury. Front Physiol. 2014;5:341. doi: 10.3389/fphys.2014.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erturk E. Ischemia-reperfusion injury and volatile anesthetics. Biomed Res Int. 2014;2014:526301. doi: 10.1155/2014/526301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamada N, Kanaya N, Hirata N, Kimura S, Namiki A. Cardioprotective effects of propofol in isolated ischemia-reperfused guinea pig hearts: role of KATP channels and GSK-3beta. Can J Anaesth. 2008;55:595–605. doi: 10.1007/BF03021433. [DOI] [PubMed] [Google Scholar]
- 21.Minguet G, Brichant JF, Joris J. Opioids and protection against ischemia-reperfusion injury: from experimental data to potential clinical applications. Acta Anaesthesiol Belg. 2012;63:23–34. [PubMed] [Google Scholar]
- 22.Suleiman MS, Zacharowski K, Angelini GD. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol. 2008;153:21–33. doi: 10.1038/sj.bjp.0707526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilic K, Hanci V, Selek S, Sozmen M, Kilic N, Citil M, Yurtlu DA, Yurtlu BS. The effects of dexmedetomidine on mesenteric arterial occlusion-associated gut ischemia and reperfusion-induced gut and kidney injury in rabbits. J Surg Res. 2012;178:223–232. doi: 10.1016/j.jss.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 24.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 26.Ueki M, Kawasaki T, Habe K, Hamada K, Kawasaki C, Sata T. The effects of dexmedetomidine on inflammatory mediators after cardiopulmonary bypass. Anaesthesia. 2014;69:693–700. doi: 10.1111/anae.12636. [DOI] [PubMed] [Google Scholar]
- 27.Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003;75:S715–720. doi: 10.1016/s0003-4975(02)04701-x. [DOI] [PubMed] [Google Scholar]
- 28.Xiang H, Hu B, Li Z, Li J. Dexmedetomidine controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Inflammation. 2014;37:1763–1770. doi: 10.1007/s10753-014-9906-1. [DOI] [PubMed] [Google Scholar]
- 29.Gerlach AT, Murphy CV, Dasta JF. An updated focused review of dexmedetomidine in adults. Ann Pharmacother. 2009;43:2064–2074. doi: 10.1345/aph.1M310. [DOI] [PubMed] [Google Scholar]
- 30.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–466. doi: 10.1097/00000539-200208000-00042. table of contents. [DOI] [PubMed] [Google Scholar]
- 31.Turan G, Ozgultekin A, Turan C, Dincer E, Yuksel G. Advantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgery. Eur J Anaesthesiol. 2008;25:816–820. doi: 10.1017/S0265021508004201. [DOI] [PubMed] [Google Scholar]
- 32.Penttila J, Helminen A, Anttila M, Hinkka S, Scheinin H. Cardiovascular and parasympathetic effects of dexmedetomidine in healthy subjects. Can J Physiol Pharmacol. 2004;82:359–362. doi: 10.1139/y04-028. [DOI] [PubMed] [Google Scholar]


