Abstract
Background: This relationship between hypertension (HTN) and osteoporosis (OP) is not well documented among the population in China. The study sought to study the relationship between HTN and OP in Chinese postmenopausal women. Methods: This cross-sectional study involved 1878 Chinese postmenopausal women with an average age of 62.38 years. OP was diagnosed by standardized quantitative ultrasound at the calcaneus and HTN was defined by blood pressure data and/or the use of antihypertensive medication. The relationship for OP and HTN were calculated using univariate and multivariate regression analyses. Results: The prevalence of OP was 28.17% in the postmenopausal women, and there was a significant difference in the prevalence of OP between the two groups according to HTN (P value = 0.003). Univariate analysis demonstrates a positive correlation between HTN and OP. After adjustment for relevant potential confounding factors, multivariate logistic regression analyses detected significant associations between HTN and OP (P value = 0.096). In participants with HTN, the OR for OP was 1.209 (95% CI: 0.967-1.513). Conclusion: The prevalence of OP was more frequent in Chinese postmenopausal women with HTN, and HTN was independently and significantly associated with OP.
Keywords: Hypertension, osteoporosis, postmenopausal women
Introduction
Osteoporosis (OP), a risk factor for hip and other fractures, is associated with increased mortality, concomitant morbidity, and reduced quality of life [1]. In postmenopausal women, the rate of bone resorption increases remarkably and remains elevated for up to 40 years after cessation of ovarian function, leading to continuous, progressive bone loss, as the biochemical, cellular, and molecular biology effects of estrogen on bone remodeling and menopause lead to significant bone loss [2]. China is undergoing an increasing OP pandemic with an increasingly aging population [3]. About one third of the women aged 60-69 years and half of those aged 70 years or over have OP. Much lower prevalence of OP was observed in Chinese men in similar age groups [4]. Hypertension (HTN), also known as high blood pressure or arterial hypertension is a chronic medical condition in which the blood pressure in the arteries is elevated [5]. HTN, a public health priority worldwide, is considered a major risk factor for an array of cardiovascular and related diseases involving stroke, myocardial infarction, heart failure, chronic kidney disease, etc [6]. The prevalence of HTN has been increasing in China for decades and reached 18.8% in 2002 [7].
Owing to an aging population and lifestyle changes, OP and HTN are two common diseases in older individuals, and both are important issues in public health and clinical management. Previous epidemiological and biological studies regarding the pathogenesis of OP and HTN supported the hypothesis that the diseases share a similar etiopathology, involving low calcium intake and level, vitamin D and vitamin K deficiency, and low or very high nitric oxide levels [8]. Many studies that evaluated a relationship between HTN and OP have been published in the last few years; however, however, evidence from Chinese populations is limited. The main purpose of this study was to explore the associations between HTN and OP in a large-scale sample of Chinese postmenopausal women.
Methods
Study population
We performed a risk-factor study for OP using a random sample of the Chinese population. Participants were recruited from rural and urban communities in Shanghai. Participants aged 30-90 years were included in this study. More than 2,000 postmenopausal women were invited to a screening visit between 2011 and 2014. Some participants with chronic diseases and conditions that might potentially affect bone mass, structure, or metabolism were excluded. Briefly, the exclusion criteria were as follows: a history of 1) serious residual effects of cerebral vascular disease; 2) serious chronic renal disease (Glomerular filtration rate-GFR <30 mL/min/1.73 m2); 3) serious chronic liver disease or alcoholism; 4) significant chronic lung disease; 5) corticosteroid therapy at pharmacologic levels; 6) evidence of other metabolic or inherited bone disease, such as hyper- or hypoparathyroidism, Paget disease, osteomalacia, or osteogenesis imperfecta; 7) recent (within the past year) major gastrointestinal disease, such as peptic ulcer, malabsorption, chronic ulcerative colitis, regional enteritis, or significant chronic diarrhea; 8) Cushing syndrome; 9) hyperthyroidism; and 10) any neurologic or musculoskeletal condition that would be a non-genetic cause of low bone mass. A total of 1905 individuals were available to the data analysis in this study. Written consent was obtained from all patients before the study, which was performed in accordance with the ethical standards in the Declaration of Helsinki, and approved by the Medicine Ethical Committee of Shanghai Tongji Hospital. The total of 1878 participants with complete records were available for data analysis.
Data collection
All study subjects underwent complete clinical baseline characteristics evaluation, which included a physical examination and response to a structured, nurse-assisted, self-administrated questionnaire to collect information on age, gender, residential region, visit date, family history, lifestyle, dietary habits, physical activity level during leisure time, use of vitamins and medications, smoking, alcohol consumption, and self-reported medical history. Body weight and height were measured according to a standard protocol. Smoking and alcohol consumption were categorized as never, current (smoking or consuming alcohol regularly in the past 6 months), or ever (cessation of smoking or alcohol consumption for more than 6 months). Regular exercise was defined as any kind of physical activity 3 or more times per week. Education was commonly divided into five stages: illiteracy, primary school, junior high school, senior high school and group. Dietary habits, including consumption of meat, fish and potato food was evaluated by a semi-quantitative food frequency questionnaire (group 1: seldom, group 2: once or twice per week, group 3: once per 2 days, and group 4: always).
Self-reported medical and therapy history was categorized as “no” or “yes.” Diabetes mellitus (DM) was defined by oral glucose tolerance test (OGTT) and either HbAlc ≥6.5% or the use of insulin or hypoglycemic medications. HTN was defined as blood pressure ≥140/90 mmHg, or a history of hypertension medication.
The study outcomes
The bone mineral density (BMD g/cm2) was measured at calcaneus by standardized quantitative ultrasound (QUS, Hologic Inc., Bedford, MA, USA) utilizing T-scores based on WHO criteria [9], which were obtained from the automated equipment. T-score refers to the ratio between patient’s BMD and that of young adult population of same sex and ethnicity. T-score of >-1 was taken as normal, between -1 and -2.5 osteopenic and <-2.5 as osteoporotic. Daily calibration was performed during the entire study period by a trained technician. The coefficients of variation of the accuracy of the QUS measurement were 0.9%. The QUS technology is less expensive, portable and also has the advantage of not using ionizing radiation, so it is safer than dual energy X-ray absorptiometry (DEXA).
Statistical analysis
Continuous variables were analyzed to determine whether they followed normal distributions, using the Kolmogorov-Smirnov Test. Variables that were not normally distributed were log-transformed to approximate a normal distribution for analysis. Results are described as mean ± SD or median, unless stated otherwise. Differences in variables among subjects grouped by HTN were determined by one way analysis of variance. Between groups, differences in properties were detected by χ2 analysis. Univariate regression analysis was performed to determine variables associated with outcomes (T-score or OP), and to estimate confounding factors possibly disturbing the relation of HTN to outcomes (T-score or OP). For the associations analysis, there model have been developed. Tests were two-sided, and a P-value of <0.05 was considered significant. Multivariable regression (MR) was performed to control potential confounding factors and determine the independent contribution of variables to outcomes (T-score or OP). Under MR models, tests were two-sided, and a P-value of <0.1 was considered significant. Results were analyzed using the Statistical Package for Social Sciences for Windows, version 16.0 (SPSS, Chicago, IL, USA). Odds ratios (OR) with 95% confidence intervals (CI) were calculated for the relative risk of HTN with the outcome of OP.
Results
Clinical characteristics of subjects
The clinical baseline characteristics of the 1878 Chinese postmenopausal women are listed in Table 1. In the total sample, the mean age was 62.38 years. There was significant difference in age between groups according to HTN (P<0.001). There were also significant differences in education, CAD, DM and Vitamin C supplement between groups according to HTN (P value <0.05 for all). An average T-score of -1.86 was reported in the total sample. A high prevalence of OP (28.17%) was reported in the postmenopausal women sample. Significant differences in prevalence of OP between the two groups were reported (P value = 0.003).
Table 1.
The baseline characteristics of participants
| Variable | Total sample | non-HTN | HTN | P value |
|---|---|---|---|---|
| Demographical information | ||||
| N | 1878 | 1041 | 837 | - |
| Age | 62.38±9 | 60.5±8.84 | 64.71±8.64 | <0.001 |
| Height | 156.31±5.73 | 156.42±5.82 | 156.13±5.67 | 0.826 |
| Weight | 58.92±8.49 | 57.58±9.17 | 61.08±6.88 | 0.063 |
| Lifestyle | ||||
| Education | 250 (13.31%) | 147 (14.12%) | 103 (12.31%) | <0.001 |
| Exercise | 1345 (71.62%) | 745 (71.57%) | 600 (71.68%) | 0.955 |
| Smoking | 15 (0.8%) | 10 (0.96%) | 5 (0.6%) | 0.135 |
| Drink | 37 (1.97%) | 22 (2.11%) | 15 (1.79%) | 0.863 |
| Dietary habit | ||||
| Meat | 837 (44.57%) | 480 (46.11%) | 357 (42.65%) | 0.134 |
| Coffee | 91 (5.08%) | 54 (5.47%) | 37 (4.6%) | 0.405 |
| Potato | 258 (13.74%) | 151 (14.51%) | 107 (12.78%) | 0.281 |
| Oil | 19.09±8.99 | 19.1±8.77 | 19.09±9.25 | 0.982 |
| Medical and therapy history | ||||
| CAD | 179 (9.9%) | 42 (4.14%) | 137 (17.28%) | <0.001 |
| DM | 210 (11.36%) | 67 (6.52%) | 143 (17.42%) | <0.001 |
| RA | 104 (5.73%) | 51 (5.08%) | 53 (6.54%) | 0.183 |
| VC | 242 (12.89%) | 113 (10.85%) | 129 (15.41%) | 0.003 |
| VD | 76 (4.05%) | 44 (4.23%) | 32 (3.82%) | 0.606 |
| Outcome | ||||
| T-score | -1.86±0.74 | -1.84±0.73 | -1.89±0.76 | 0.196 |
| OP | 529 (28.17%) | 264 (25.36%) | 265 (31.66%) | 0.003 |
Note: HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA-Rheumatoid arthritis, OP-osteoporosis.
Association analysis for outcomes
Univariate linear regression analyses were developed to include demographical information, medical history, and lifestyle to estimate the association of various clinical factors and T-score (Table 2). The variables age, Vitamin C supplement, and meat food preference were significantly associated with the T-score (P<0.05 for all). The results of comparison of T-score between groups according to HTN showed that the mean T-score was -1.84 and -1.89 in the two groups, respectively (Figure 1). There were no significantly differences between the two groups (P value = 0.196).
Table 2.
Univariate linear regression analysis for associations among variables and T-score
| Variable | β | SE | P value | 95% CI for β |
|---|---|---|---|---|
| Age | -0.033 | 0.002 | <0.001 | -0.036--0.029 |
| Height | 0.0192 | 0.014 | 0.194 | -0.012-0.048 |
| Weight | -0.002 | 0.011 | 0.835 | -0.021-0.019 |
| Education | 0.088 | 0.015 | <0.001 | 0.060-0.116 |
| Exercise | 0.063 | 0.037 | 0.091 | -0.010-0.138 |
| Smoking | -0.091 | 0.094 | 0.330 | -0.273-0.093 |
| Drink | 0.031 | 0.053 | 0.599 | -0.081-0.145 |
| Meat intake | 0.121 | 0.034 | <0.001 | 0.053-0.188 |
| CAD | -0.116 | 0.052 | 0.048 | -0.231--0.001 |
| DM | 0.039 | 0.054 | 0.467 | -0.067-0.146 |
| RA | -0.133 | 0.075 | 0.075 | -0.279-0.013 |
| Vitamin C | -0.114 | 0.051 | 0.024 | -0.213--0.015 |
| Vitamin D | -0.058 | 0.086 | 0.508 | -0.225-0.112 |
| HTN | -0.045 | 0.035 | 0.196 | -0.112-0.023 |
Note: HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA-Rheumatoid arthritis.
Figure 1.
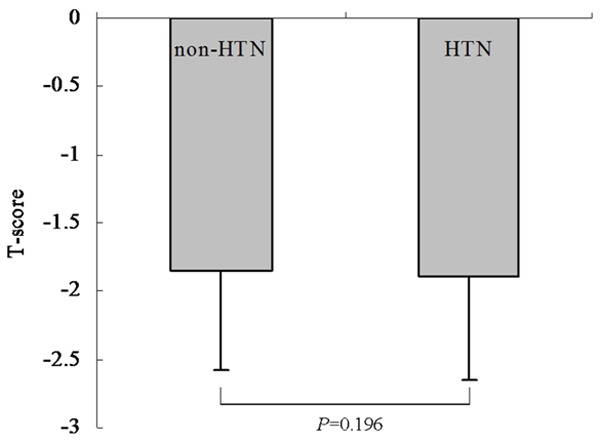
Comparison of T score among groups according to education level in Chinese postmenopausal women. The results of comparison of T-score among groups according to HTN. The mean T-score was -1.84 and -1.89 in the two groups, respectively. There were no significantly differences between the two groups (P value = 0.196).
Univariate logistic analyses were performed to evaluate associations with OP. The results indicate that age, education, HTN, CAD, Vitamin C, Vitamin D, frequency of meat intake and RA were significantly associated with OP (P value <0.05 for all, Table 3). The comparison of prevalence of OP between the two groups according to HTN revealed that the prevalence of osteoporosis was 25.36% and 31.66% in the two groups, respectively (Figure 2). There were significant differences between the two groups (P value = 0.003). Univariate analysis demonstrates a positive correlation between RA and OP. Multivariate logistic regression analyses were employed to evaluate the association between HTN and the OP outcome. After adjustment for relevant potential confounding factors, the multivariate logistic regression analyses detected significant associations between HTN and OP (P value = 0.096, Table 4). In participants with HTN, the OR for OP was 1.209 (95% CI: 0.967-1.513).
Table 3.
Univariate logistic regression analysis for associations among variables and osteoporosis
| Variable | β | S.E. | P value | OR | 95.0% CI |
|---|---|---|---|---|---|
| Age | 0.095 | 0.006 | <0.001 | 1.104 | 1.09 2-1.118 |
| Height | 0.008 | 0.052 | 0.871 | 1.008 | 0.915-1.113 |
| Weight | 0.005 | 0.034 | 0.886 | 1.005 | 0.940-1.075 |
| Education | -0.24 | 0.043 | <0.001 | 0.787 | 0.722-0.858 |
| Exercise | -0.236 | 0.112 | 0.033 | 0.792 | 0.636-0.981 |
| Smoking | 0.032 | 0.275 | 0.913 | 1.032 | 0.601-1.769 |
| Drink | -0.194 | 0.191 | 0.315 | 0.825 | 0.565-1.202 |
| Meat intake | -0.254 | 0.102 | 0.013 | 0.775 | 0.632-0.948 |
| CAD | 0.498 | 0.161 | 0.002 | 1.647 | 1.198-2.26 |
| DM | 0.173 | 0.158 | 0.255 | 1.196 | 0.879-1.625 |
| RA | 0.482 | 0.210 | 0.021 | 1.617 | 1.075-2.429 |
| Vitamin C | 0.313 | 0.146 | 0.030 | 1.369 | 1.037-1.817 |
| Vitamin D | 0.497 | 0.238 | 0.038 | 1.636 | 1.028-2.604 |
| HTN | 0.311 | 0.103 | 0.003 | 1.366 | 1.114-1.668 |
Note: HTN-hypertension, CAD-coronary artery disease, DM-diabetes mellitus, RA-Rheumatoid arthritis.
Figure 2.
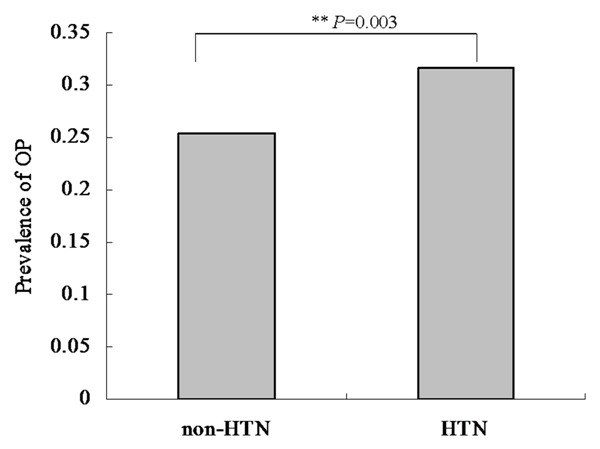
Comparison of prevalence of osteoporosis among groups according to education level in Chinese postmenopausal women. The results of comparison of prevalence of osteoporosis among groups according to HTN. The prevalence of osteoporosis was 25.36% and 31.66% in the two groups, respectively. There were significantly differences between the two groups (P value = 0.003).
Table 4.
Multiple variables logistic regression analysis for associations between hypertension and osteoporosis
| Variable | β | S.E. | P value | OR | 95% CI |
|---|---|---|---|---|---|
| HTN | 0.19 | 0.114 | 0.096 | 1.209 | 0.967-1.513 |
Note: HTN-hypertension, adjusted for age, smoking, alcohol intake, education, exercise, medical and therapy history.
Discussion
We conducted a community-based, cross-sectional study to explore the relationship between OP and HTN in a sample of 1878 Chinese postmenopausal women. The standardized quantitative ultrasound (QUS) used in this study has many advantages over dual X-ray absorptiometry (DXA) to diagnose osteoporosis, including lower cost, no ionizing radiation damage, convenience, speed, and portability. In addition, several studies have shown that the osteoporotic fracture predictions by QUS were equal to and possibly better than DXA [10,11].
Significant associations between OP and HTN among postmenopausal women were observed in univariate analysis as well as in the multivariate analysis adjusted for age, smoking, alcohol intake, education, exercise, and medical and therapy history. In addition, we also verified that the variables age, vitamin C supplement, and meat consumption were significantly associated with the T-score. Our results were consistent with previous findings of significant associations between OP and HTN. In a large British cohort, Cappuccio et al. studied the relation between bone mass and blood pressure in white postmenopausal elderly women [12]. The researchers found that femoral neck BMD was inversely related to systolic and diastolic blood pressure (BP) but only with the highest BP quartile. Popovic et al. also found associations between the two diseases in 1664 Korean postmenopausal women aged 50 years or older [13]. Yazici et al. revealed that HTN is an independent predictor of spinal osteopenia and OP in logistic regression analysis [14]. Similarly, Afghani et al. reported that women with HTN had significantly lower bone mineral content (BMC) compared with normotensive women [15]. Additionally, SBP was significantly and independently related to BMC in a small population of Hispanic premenopausal women. Briefly, our sample was an adequate representation of the Chinese postmenopausal women population, and our study provides compelling evidence that HTN is significantly and independently positive correlated to OP.
The mechanisms of the increased risk of osteoporotic fractures in HTN may be increased loss of calcium in the urine, evidence of secondary increase in parathyroid gland activity, increase in urinary cyclic AMP, tendency to low serum ionized calcium, raised vitamin D levels, and increased intestinal calcium absorption, leading to a negative calcium balance [16]. This increased urinary calcium loss as a function of blood pressure has been observed in animals with HTN [17,18]. This calcium leakage could lead to increased bone turnover and thus increase the risk of OP. Additionally, some pathological conditions such as abnormal lipid metabolism, altered levels of low density lipoprotein (LDL), high density lipoprotein (HDL), plasma homocysteine, and nitric oxide (NO) are reported to be involved in HTN and OP [19-21]. However, the mechanism responsible for HTN-related OP remains unclear. Although strategies including selective estrogen receptor modulators, hormone replacement therapy, calcitonin, and bisphosphonates are now used to manage OP, they have no effect on HTN-related OP [22]. Pharmacological studies with anti-HTN drugs including thiazide, beta-adrenoceptor antagonists, calcium channel, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor antagonists have greatly enhanced our understanding of the relationship between HTN and OP. Their effects are mediated through direct actions on their respective receptors expressed on the osteoblast progenitor cells or have an indirect impact on osteoporosis by ameliorating the detrimental effects of hypertension.
This study has several limitations. First, our analysis was restricted to postmenopausal women with an average age of 62.38 years; thus, the findings are not generalizable to younger women. Additionally, our study, based on a community-based data from eastern China collected between 2011 and 2014, requires more geographic representations in a larger sample. Finally, the causality of the relationship of OP and HTN cannot be evaluated directly since we used a cross-sectional design.
Our findings suggested that HTN is independently and significantly associated with OP. The prevalence of OP was less frequent in Chinese postmenopausal women without HTN. This study suggests that a study of the pathophysiological mechanism underlying HTN might be beneficial in the prevention of OP in Chinese postmenopausal women. Controlling high blood pressure may, as an added benefit, provide protection against fractures.
Acknowledgements
We thank the grant from Shanghai Tongji Hospital to support the study. Grants from the Clinical Medicine Foundation of Shanghai Tongji Hospital, ClinicalTrials.gov Identifier: NCT02451397.
Disclosure of conflict of interest
None.
Abbreviations
- BMD
Bone mineral density
- BM-MNC
Bone marrow-derived mononuclear cell
- BMI
Bodymineral index
- CAD
Coronary artery disease
- CI
Confidence intervals
- DM
Diabetes
- DXA
Dual-energy X-ray
- HTN
Hypertension
- GFR
Glomerular filtration rate
- OR
Odds ratios
- OP
Osteoporosis
- QUS
Quantitative ultrasound
- RA
Rheumatoid arthritis
References
- 1.Gass M, Dawson-Hughes B. Preventing osteoporosis-related fractures: an overview. Am J Med. 2006;119:S3–S11. doi: 10.1016/j.amjmed.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 2.North American Menopause Society. The role of calcium in peri- and postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause. 2006;13:862–877. doi: 10.1097/01.gme.0000243566.25205.0b. quiz 878-880. [DOI] [PubMed] [Google Scholar]
- 3.Ilic K, Obradovic N, Vujasinovic-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif Tissue Int. 2013;92:217–227. doi: 10.1007/s00223-012-9671-9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang ZQ, Ho SC, Chen ZQ, Zhang CX, Chen YM. Reference values of bone mineral density and prevalence of osteoporosis in Chinese adults. Osteoporos Int. 2014;25:497–507. doi: 10.1007/s00198-013-2418-2. [DOI] [PubMed] [Google Scholar]
- 5.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, Smith SC Jr, Svetkey LP, Taler SJ, Townsend RR, Wright JT Jr, Narva AS, Ortiz E. 2014 evidencebased guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311:507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 6.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 7.Tao S, Zhou B. Epidemiology of hypertension in China. Chin Med J. 1999;112:878–882. [PubMed] [Google Scholar]
- 8.Yang S, Nguyen ND, Center JR, Eisman JA, Nguyen TV. Association between hypertension and fragility fracture: A longitudinal study. Osteoporos Int. 2014;25:97–103. doi: 10.1007/s00198-013-2457-8. [DOI] [PubMed] [Google Scholar]
- 9.Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int. 1994;4:368–381. doi: 10.1007/BF01622200. [DOI] [PubMed] [Google Scholar]
- 10.Moayyeri A, Adams JE, Adler RA, Krieg MA, Hans D, Compston J, Lewiecki EM. Quantitative ultrasound of the heel and fracture risk assessment: an updated meta-analysis. Osteoporos Int. 2012;23:143–153. doi: 10.1007/s00198-011-1817-5. [DOI] [PubMed] [Google Scholar]
- 11.Babatunde OO, Forsyth JJ. Quantitative Ultrasound and bone’s response to exercise: a meta analysis. Bone. 2013;53:311–318. doi: 10.1016/j.bone.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 12.Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Lancet. 1999;354:971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 13.Park JS, Choi SB, Rhee Y, Chung JW, Choi EY, Kim DW. Parathyroid hormone, calcium, and sodium bridging between osteoporosis and hypertension in postmenopausal korean women. Calcif Tissue Int. 2015;96:417–429. doi: 10.1007/s00223-015-9972-x. [DOI] [PubMed] [Google Scholar]
- 14.Yazici S, Yazici M, Korkmaz U, Engin Erkan M, Erdem Baki A, Erden I, Ozhan H, Ataoğlu S. Relationship between blood pressure levels and bone mineral density in postmenopausal Turkish women. Arch Med Sci. 2011;7:264–270. doi: 10.5114/aoms.2011.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Afghani A, Johnson CA. Resting blood pressure and bone mineral content are inversely related in overweight and obese Hispanic women. Am J Hypertens. 2006;19:286–292. doi: 10.1016/j.amjhyper.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 16.Brickman AS, Nyby MD, Vonhungen K, Eggena P, Tuck ML. Calcitropic Hormones, Platelet Calcium, And Blood-Pressure In Essential-Hypertension. Hypertension. 1990;16:515–522. doi: 10.1161/01.hyp.16.5.515. [DOI] [PubMed] [Google Scholar]
- 17.Cirillo M, Galletti F, Strazzullo P, Torielli L, Melloni MC. On the Pathogenetic Mechanism Of Hypercalciuria In Genetically Hypertensive Rats Of the Milan Strain. Am J Hypertens. 1989;2:741–746. doi: 10.1093/ajh/2.10.741. [DOI] [PubMed] [Google Scholar]
- 18.Umemura S, Smyth DD, Nicar M, Rapp JP, Pettinger WA. Altered Calcium Homeostasis In Dahl Hypertensive Rats-Physiological And Biochemical-Studies. J Hypertens. 1986;4:19–26. doi: 10.1097/00004872-198602000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Parhami F, Basseri B, Hwang J, Tintut Y, Demer LL. High-density lipoprotein regulates calcification of vascular cells. Circ Res. 2002;91:570–576. doi: 10.1161/01.res.0000036607.05037.da. [DOI] [PubMed] [Google Scholar]
- 20.Resnick LM, Laragh JH, Sealey JE, Alderman MH. Divalent-Cations in Essential-Hypertension-Relations Between Serum Ionized Calcium, Magnesium, And Plasma-Renin Activity. N Engl J Med. 1983;309:888–891. doi: 10.1056/NEJM198310133091504. [DOI] [PubMed] [Google Scholar]
- 21.Yesil Y, Ulger Z, Halil M, Halacli B, Yavuz BB, Yeşil NK, Kuyumcu ME, Cankurtaran M, Ariogul S. Coexistence of osteoporosis (OP) and coronary artery disease (CAD) in the elderly: It is not just a by chance event. Arch Gerontol Geriatr. 2012;54:473–476. doi: 10.1016/j.archger.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 22.Body JJ. How To Manage Postmenopausal Osteoporosis? Acta Clin Belg. 2011;66:443–447. doi: 10.2143/ACB.66.6.2062612. [DOI] [PubMed] [Google Scholar]


