Abstract
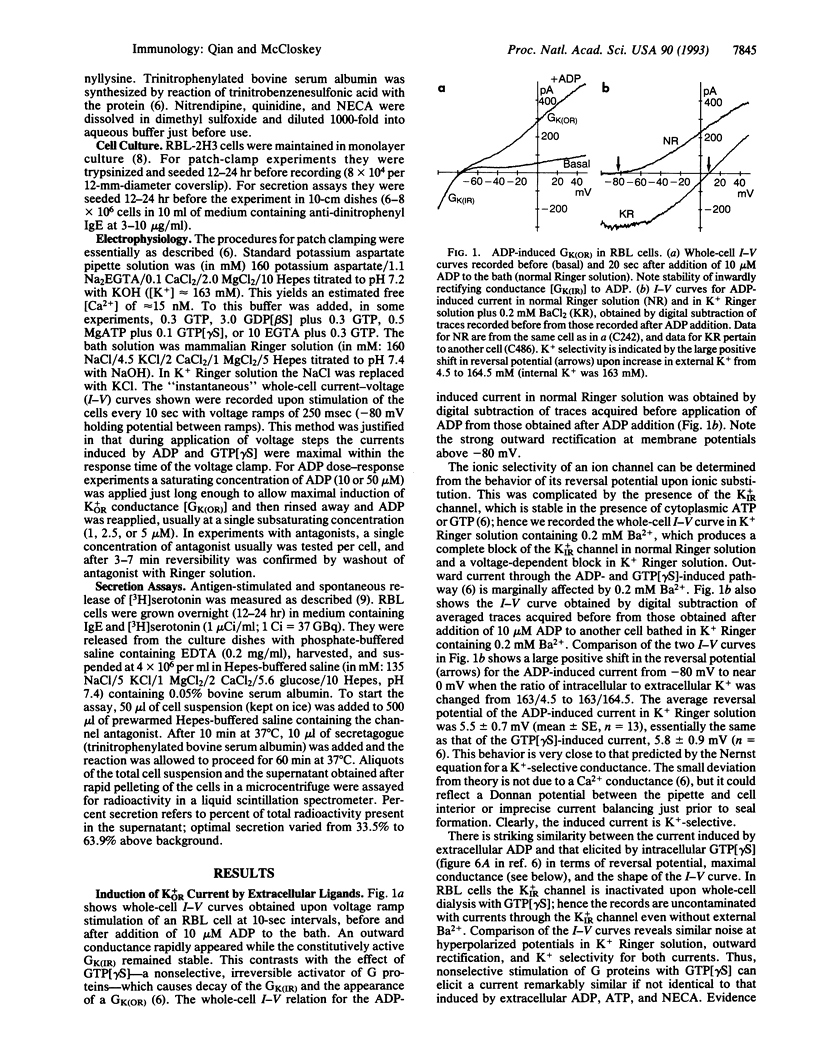
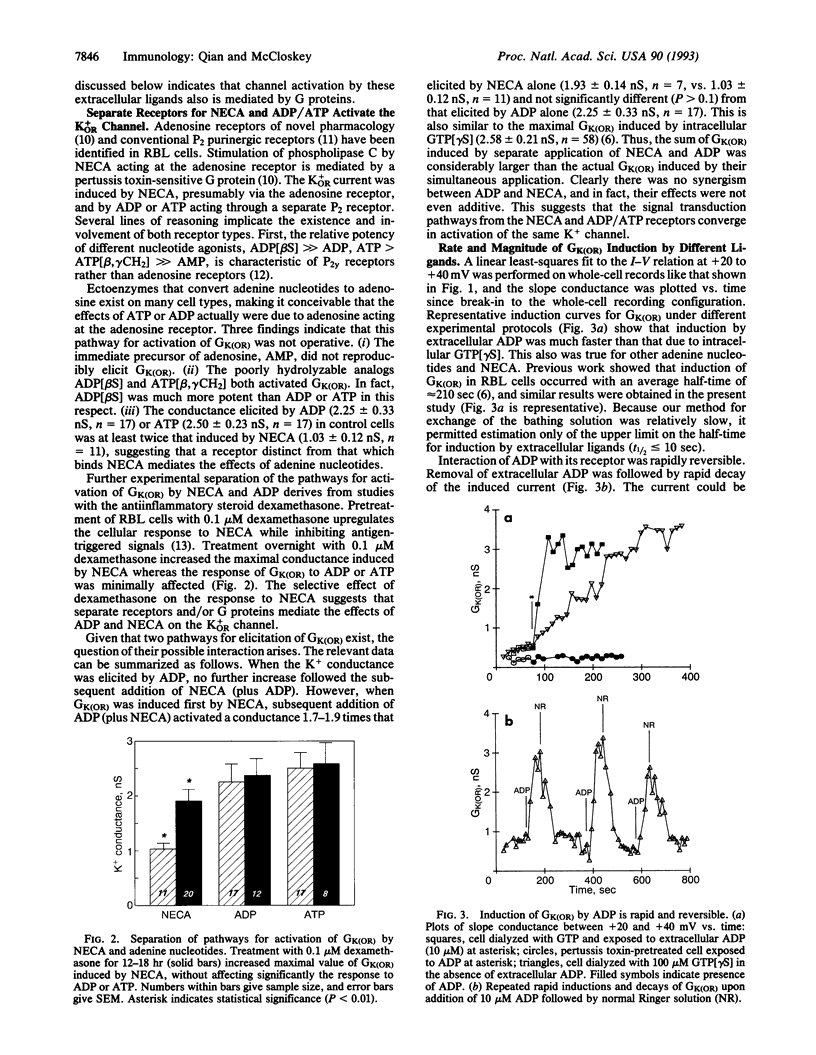
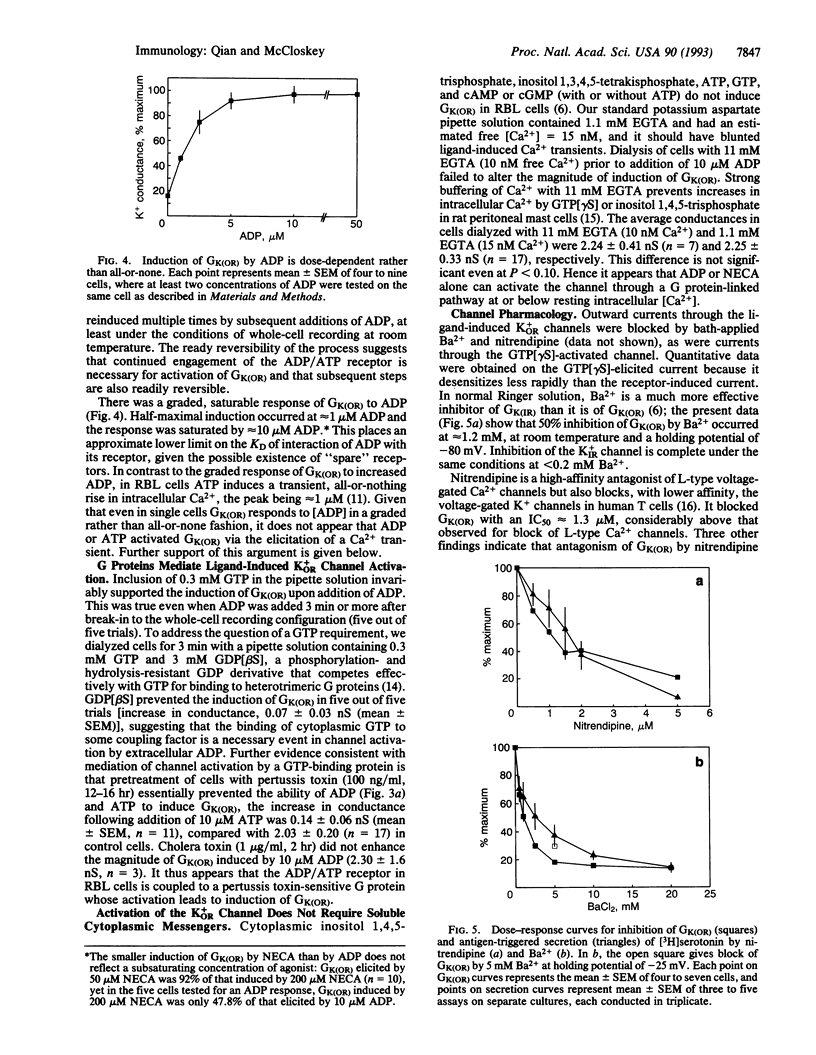
The rat basophilic leukemia (RBL) mast cell line possesses cell surface receptors for adenosine whose ligation markedly potentiates antigen-driven Ca2+ influx and secretion. Here we show that engagement of these receptors and of separate P2 purinergic receptors rapidly activates an outwardly rectifying K+ conductance [GK(OR)] in RBL cells. Activation of GK(OR) by the ligands 5'-(N-ethylcarboxamido)adenosine (NECA), ADP, and ATP was prevented by cytoplasmic guanosine 5'-[beta-thio]diphosphate as well as by pretreatment of the cells with pertussis toxin, implicating mediation by a G protein. Multiple cycles of induction and decay of GK(OR) were produced upon application and removal of ligand. Induction of GK(OR) by either ligand was much faster than the induction caused by guanosine 5'-[gamma-thio]triphosphate (t1/2 < 10 sec vs. 210 sec.). In control cells the maximal whole-cell conductance elicited by ADP (2.25 +/- 0.30 nS) or ATP (2.50 +/- 0.33 nS) was about twice as large as that induced by NECA (1.03 +/- 0.11 nS), and similar to that previously reported for the guanosine 5'-[gamma-thio]triphosphate-elicited GK(OR) in RBL cells (2.58 +/- 1.59 nS). Treatment of RBL cells with dexamethasone upregulated Ca2+ responses to NECA, and it also nearly doubled the maximal conductance elicited by NECA without appreciable effect on responses to ADP or ATP. The failure of water-soluble second messengers to activate GK(OR) and the inability of 11 mM EGTA (< 10 nM Ca2+) to prevent activation by ADP suggest that the relevant pathway is membrane-delimited. Two ion-channel blockers inhibited antigen-stimulated secretion with IC50 values similar to those at which they blocked GK(OR), suggesting that activity of the outwardly rectifying K+ channel may be important for stimulus-response coupling in these cells. Potentiation of the secretory response by NECA may reflect, in part, the activation of GK(OR), which serves to repolarize the membrane more effectively than does the constitutive mechanism, thereby enhancing antigen-driven Ca2+ influx. This channel and its functionally associated receptors may allow neighboring cells of the host to modulate the response of mast cells to exogenous antigen.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali H., Cunha-Melo J. R., Saul W. F., Beaven M. A. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J Biol Chem. 1990 Jan 15;265(2):745–753. [PubMed] [Google Scholar]
- Barsumian E. L., Isersky C., Petrino M. G., Siraganian R. P. IgE-induced histamine release from rat basophilic leukemia cell lines: isolation of releasing and nonreleasing clones. Eur J Immunol. 1981 Apr;11(4):317–323. doi: 10.1002/eji.1830110410. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Overview. Purinergic mechanisms. Ann N Y Acad Sci. 1990;603:1–18. doi: 10.1111/j.1749-6632.1990.tb37657.x. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. ATP induces nucleotide permeability in rat mast cells. Nature. 1979 Jun 7;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- Collado-Escobar D., Cunha-Melo J. R., Beaven M. A. Treatment with dexamethasone down-regulates IgE-receptor-mediated signals and up-regulates adenosine-receptor-mediated signals in a rat mast cell (RBL-2H3) line. J Immunol. 1990 Jan 1;144(1):244–250. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Hide M., Beaven M. A. Calcium influx in a rat mast cell (RBL-2H3) line. Use of multivalent metal ions to define its characteristics and role in exocytosis. J Biol Chem. 1991 Aug 15;266(23):15221–15229. [PubMed] [Google Scholar]
- Kanner B. I., Metzger H. Crosslinking of the receptors for immunoglobulin E depolarizes the plasma membrane of rat basophilic leukemia cells. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5744–5748. doi: 10.1073/pnas.80.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque G. F., Holowka D., Baird B. Antigen-triggered membrane potential changes in IgE-sensitized rat basophilic leukemia cells: evidence for a repolarizing response that is important in the stimulation of cellular degranulation. J Immunol. 1989 Jan 1;142(1):236–243. [PubMed] [Google Scholar]
- Labrecque G. F., Holowka D., Baird B. Characterization of increased K+ permeability associated with the stimulation of receptors for immunoglobulin E on rat basophilic leukemia cells. J Biol Chem. 1991 Aug 15;266(23):14912–14917. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- MacDonald A. J., Haig D. M., Bazin H., McGuigan A. C., Moqbel R., Miller H. R. IgE-mediated release of rat mast cell protease II, beta-hexosaminidase and leukotriene C4 from cultured bone marrow-derived rat mast cells. Immunology. 1989 Jul;67(3):414–418. [PMC free article] [PubMed] [Google Scholar]
- Mazingue C., Dessaint J. P., Capron A. [3H]serotonin release: an improved method to measure mast cell degranulation. J Immunol Methods. 1978;21(1-2):65–77. doi: 10.1016/0022-1759(78)90224-7. [DOI] [PubMed] [Google Scholar]
- McCloskey M. A., Cahalan M. D. G protein control of potassium channel activity in a mast cell line. J Gen Physiol. 1990 Feb;95(2):205–227. doi: 10.1085/jgp.95.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. Depolarization of rat basophilic leukemia cells inhibits calcium uptake and exocytosis. J Cell Biol. 1987 Mar;104(3):783–792. doi: 10.1083/jcb.104.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. IgE receptor-mediated depolarization of rat basophilic leukemia cells measured with the fluorescent probe bis-oxonol. J Immunol. 1987 Mar 1;138(5):1564–1570. [PubMed] [Google Scholar]
- Mohr F. C., Fewtrell C. The relative contributions of extracellular and intracellular calcium to secretion from tumor mast cells. Multiple effects of the proton ionophore carbonyl cyanide m-chlorophenylhydrazone. J Biol Chem. 1987 Aug 5;262(22):10638–10643. [PubMed] [Google Scholar]
- Neher E. The influence of intracellular calcium concentration on degranulation of dialysed mast cells from rat peritoneum. J Physiol. 1988 Jan;395:193–214. doi: 10.1113/jphysiol.1988.sp016914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk Y., Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992 Sep 17;359(6392):241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- Penner R., Matthews G., Neher E. Regulation of calcium influx by second messengers in rat mast cells. Nature. 1988 Aug 11;334(6182):499–504. doi: 10.1038/334499a0. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Bismuth G., Debré P., Trautmann A. Nitrendipine-induced inhibition of calcium influx in a human T-cell clone: role of cell depolarization. Cell Calcium. 1991 Apr;12(4):313–323. doi: 10.1016/0143-4160(91)90005-y. [DOI] [PubMed] [Google Scholar]
- Sagi-Eisenberg R., Pecht I. Membrane potential changes during IgE-mediated histamine release from rat basophilic leukemia cells. J Membr Biol. 1983;75(2):97–104. doi: 10.1007/BF01995629. [DOI] [PubMed] [Google Scholar]



