Abstract
Objective: Growth differentiation factor-15 (GDF-15) has been identified as a strong biomarker of cardiovascular diseases; however, no evidence are available concerning the relationship of GDF-15 and atrial fibrosis in patients with atrial fibrillation (AF) and rheumatic heart disease (RHD). Methods: Twenty patients with rheumatic heart disease were divided into two groups, 10 cases with AF and 10 cases with sinus rhythm (SR). Clinical data and blood samples were collected; left atrial appendage was taken by the surgeon in the process of valve replacement. Masson stained sections and mRNA levels of cardiac fibrosis biomarkers were used to determine the level of cardiac fibrosis, the expression level of GDF-15 was evaluated via immunohistochemistry, enzyme-linked immunosorbent assay (ELISA) and real-time polymerase chain reaction (PCR). Results: Compared with SR group, more collagen deposited in the atrial tissue of AF group. The distribution of GDF-15 in the AF group was significantly higher than SR group (P<0.05). In addition, plasma GDF-15 level and mRNA level of GDF-15 in atrial tissue of AF showed the same trend as the result of immunohistochemistry. After linear correlation analysis, the expression level of GDF-15 was found to be positively related to the degree of cardiac fibrosis. Conclusion: GDF-15 might involve in the development and maintenance of atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease, and GDF-15 could be used as a novel biomarker to evaluate myocardial fibrosis in the future.
Keywords: Growth differentiation factor-15, atrial fibrosis, atrial fibrillation, rheumatic heart disease
Introduction
Atrial fibrillation (AF) is the common clinical manifestation of many cardiovascular diseases, such as rheumatic heart disease, coronary heart disease, hypertension, cardiomyopathy, congenital heart disease, pericardial diseases and other cardiovascular disease [1-3]. AF can seriously affect human health, in addition, which could cause serious cardiovascular and cerebrovascular events, has a high morbidity and mortality. Both clinical and basic researches have shown that atrial fibrosis is the most prominent manifestation of atrial structural remodeling in patients with atrial fibrillation, which is due to the heterogeneity of electrical conduction in atrial myocardium, which makes AF easy to occur and maintain [4-6]. Therefore, to block the remodeling of atrial structure maybe a better way to prevent the development and deterioration of atrial fibrillation. Growth differentiation factor-15 (GDF-15) is a member of transforming growth factor (TGF) of beta super family. Recent studies have confirmed that GDF-15 participates in a variety of cardiovascular diseases including acute myocardial ischemia, ischemia reperfusion injury and so on, which can provide prognostic evaluation of ST segment elevation in myocardial infarction patients [7,8]. It is reported that the expression level of GDF-15 is significantly increased in myocardial tissue and peripheral blood [9-11]. The level of peripheral blood GDF-15 was also elevated in patients with chronic heart failure, and was associated with the prognosis of heart failure [12-14]. However, whether GDF-15 could reflect the degree of myocardial fibrosis after AF, whether associated with cardiac fibrosis as a biological indicator is still unclear. Therefore, this study aimed to investigate the relationship of GDF-15 and atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
Materials and methods
Human myocardium samples collection and processing
This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (Wuhan, China) and the samples were obtained according to the regulations of Renmin Hospital of Wuhan University. Twenty patients aged from 30 to 70 with rheumatic heart disease (RHD) who underwent valve replacement served as subjects in the present study. They were divided into two groups: 10 in the sinus rhythm (SR) group and 10 in the atrial fibrillation (AF) group. Exclusion criteria in the study as follows: infective endocarditis, hyperthyroidism, serious liver, kidney, lung dysfunction, malignant tumor, coronary atherosclerotic heart disease and chronic pulmonary heart disease. About 200 mg myocardium samples were collected before extracorporeal circulation established by the surgeon from the right atrium of patients. Each sample was divided into two parts after removed connective tissue and residual blood, one part was quickly put into liquid nitrogen jar and immediately transferred to -80°C refrigerator, another part was immediately washed with saline solution and fixed with 10% neutral buffered formalin for paraffin section.
Materials
GDF-15 Human ELISA Kit (ab155432) was obtained from Abcam. Primary antibody against GDF-15 (ab39999) was purchased from Abcam. TRIzol was purchased from Invitrogen Life Technologies (#15596018). Transcriptor First Strand cDNA Synthesis Kit (#04896866001) was purchased from Roche. Power SYBR® Green PCR Master Mix (#4367659) was purchased from Life Technologies.
Masson staining
Evidence of interstitial collagen deposition was visualized using Masson staining, and collagen volume (%) was measured using an image quantitative digital analysis system (Image-Pro plus 6.0). Briefly, myocardium tissues were fixed in 10% neutral buffered formalin for at least 24 hours, and embed in paraffin wax according to embedding machine manufactures instructions. Prepare 8 µm sections on the microtome and place on clean, positively-charged microscope slides. Heat in tissue-drying oven for 1 hour at 60°C. After deparaffinization and rehydration through 100% alcohol, 95% alcohol and 70% alcohol, the slides were washed in distilled water, and then re-fixed in Bouin’s solution for 1 hour at 56°C to improve staining quality although this step is not absolutely necessary. Rinse the slides in running tap water for 10 minutes to remove the yellow colour. Firstly, stain in Weigert’s iron hematoxylin working solution for 10 minutes; rinse them in running warm tap water for 10 minutes and wash in distilled water. And then stain in Biebrich scarlet-acid fuchsin solution for 10 minutes and wash in distilled water. Differentiate in phosphomolybdic-phosphotungstic acid solution for 10 minutes until collagen is not red. Transfer sections directly to aniline blue solution and stain for 5 minutes; rinse them briefly in distilled water and differentiate in 1% acetic acid solution for 2 minutes, wash in distilled water. Dehydrate very quickly through 95% ethyl alcohol, absolute ethyl alcohol and clear in xylene, lastly mount with resinous mounting medium. Collagen was stained for blue, but muscle and cytoplasm were stained for red.
Immunohistochemistry
Deposition of GDF-15 in myocardium tissues was measured by the method of immunohistochemistry. As mentioned above, after fixed, embed, sectioned, deparaffinization and rehydration, we chose the most common method of antigen retrieval just as heat-mediated retrieval in citrate buffer. And add 100 µl blocking solution for per slide; incubate 30 minutes at room temperature. Drain the blocking solution from slides; apply 100 µl per slide of diluted primary antibody against GDF-15 at recommended concentration of 1:100; incubate overnight at 4°C. After washed with TBST, apply 100 µl per slide of diluted conjugated secondary antibody and incubate for 30 minutes at room temperature. Apply color development by the method of enzyme substrate for 30 minutes, wash slides in TBST and distilled water, and then dehydrate and mount slides. Brown-yellow granules in myocardium tissues meant a positive result. Computer image analysis was used to determine density of the positively stained area and relative quantitative analysis.
Enzyme-linked immunosorbent assay (ELISA)
Plasma GDF-15 in this study was detected by the method of enzyme-linked immunosorbent assay (ELISA). Briefly, add 100 μL of each standard and sample into appropriate wells. Cover well and incubate for 2.5 hours at room temperature with gentle shaking. Discard the solution and wash with Wash Solution by using a multi-channel Pipette. After the last wash, remove any remaining Wash Buffer by aspirating. Invert the plate and blot it against clean paper towels. Add 100 μL prepared biotinylated antibody to each well; incubate for 1 hour at room temperature with gentle shaking. Discard the solution and repeat the wash as before. Add 100 μL of prepared Streptavidin solution to each well and incubate for 45 minutes at room temperature with gentle shaking. Discard the solution and repeat the wash as before. Add 100 μL of TMB One-Step Substrate Reagent to each well; incubate for 30 minutes at room temperature in the dark with gentle shaking. Lastly, Add 50 μL of Stop Solution to each well and read at 450 nm immediately. For data calculation, calculate the mean absorbance for each set of duplicate standards, controls and samples, and subtract the average zero standard optical density. Plot the standard curve on log-log graph paper, with standard concentration on the x-axis and absorbance on the y-axis. Draw the best-fit straight line through standard points.
Real-time polymerase chain reaction (PCR) analysis
Expression levels of fibrosis biomarkers including CTGF, Collagen I and III were measured by real-time polymerase chain reaction (PCR) analysis. Total RNA was extracted from the myocardium tissues using TRIzol reagent according to the manufacturer’s instructions and the cDNA was synthesized using oligo (dT) primers with the transcriptor first-strand cDNA synthesis kit. Selected gene differences were confirmed by real-time PCR using SYBR-Green and the results were normalized against GAPDH gene expression. The sequences of all primers used in this study are presented as follows: GAPDH: 5’-GAGTCAACGGATTTGGTCGT-3’ and 5’-TTGATTTTGGAGGGATCTCG-3’; CTGF: 5’-TACCAATGACAACGCCTCCT-3’ and 5’-CCGTCGGTACATACTCCACA-3’; Collagen I: 5’-CCCCAGCCACAAAGAGTCTA-3’ and 5’-TACCTGAGGCCGTTCTGTAC-3’; Collagen III: 5’-GAAGGGCAGGGAACAACTTG-3’ and 5’-TTTGGCATGGTTCTGGCTTC-3’.
Statistical analysis
The data are expressed as the means ± standard deviation (SD). Differences between two groups were performed by an unpaired Student’s t-test using SPSS 19.0 statistical software. A value of P<0.05 was considered to indicate statistically significant difference.
Results
Expansive left atrial diameter showed in AF patients with RHD
All subjects were received preoperative routine testing of urine, stool, blood coagulation, blood biochemistry, X-ray, electrocardiograph, ultrasonic cardiogram and so on. General clinical characteristics included age, gender, past history, AF duration etc. There was no statistically difference in terms of gender, age, classification of cardiac function and LVEF between SR and AF groups (P>0.05). However, when compared with the SR group, it was shown that left atrial diameter (LAD) was remarkably expansive in AF patients (P<0.05) (Table 1).
Table 1.
Expansive left atrial diameter showed in AF patients with RHD
| Clinical characteristics | SR (n = 10) | AF (n = 10) |
|---|---|---|
| Gender (m/w) | 5/5 | 5/5 |
| Age (y) | 49.60±7.69 | 48.80±7.38 |
| AF time (m) | - | 15.70±4.50 |
| NYHA (II/III) | 7/3 | 6/4 |
| LAD (mm) | 34.30±3.95 | 51.60±5.36* |
| RAD (mm) | 37.20±2.74 | 37.60±2.99 |
| LVEF (%) | 57.70±3.68 | 55.20±3.61 |
Note: Gender and grade of heart function were compared by using Fisher exact probability method; m/w = male/female; y = year; m = month; NYHA = New York heart function classification; LAD = left atrial diameter; RAD = right atrial diameter; LVEF = left ventricular ejection fraction;
P<0.05.
Accumulated collagen deposited in the myocardial interstitial of AF patients with RHD
To investigate the role of atrial fibrillation in morphology of patients with rheumatic heart disease, atrial fibrosis was measured by visualizing the total amount of collagen present in the interstitial spaces and the collagen volume. As shown in the present study, interstitial fibrosis was observed in the AF group, and AF group showed significant increases in total collagen volume compared with the SR group (P<0.05). Masson staining indicated that atrial fibrillation had an adverse effect on cardiac fibrosis. To further detect the severity of atrial fibrosis, we found that when compared with SR group, the mRNA levels of cardiac fibrosis biomarkers CTGF, Collagen I and III were significantly increased in atrial tissues of AF group (P<0.05) (Figure 1). These above results indicated that there was more collagen deposited in the myocardial interstitial of AF patients.
Figure 1.
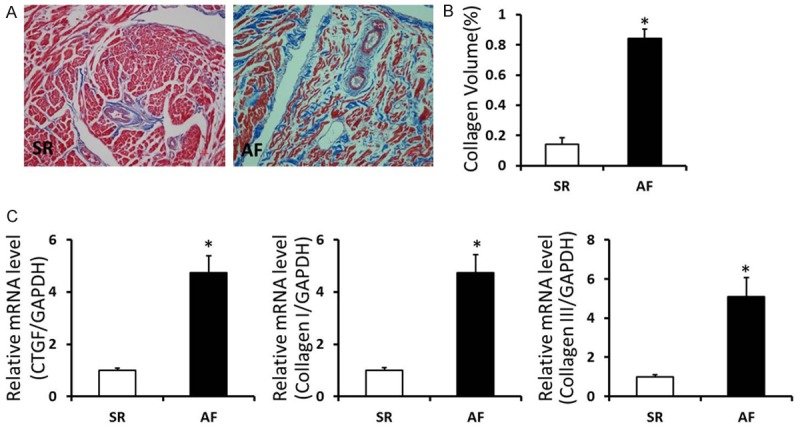
Accumulated collagen deposited in the myocardial interstitial of AF patients with RHD. A. Masson staining in right atrium of SR and AF patients with rheumatic heart disease (Representative image, collagen was stained for blue). B. Quantification of the total collagen volume in SR and AF patients with rheumatic heart disease. C. The mRNA levels of CTGF, Collagen I and III in the atrial tissues of SR and AF patients with rheumatic heart disease. *P<0.05 vs. SR group.
Elevated GDF-15 levels in plasma and myocardiac tissues of AF patients with RHD
To detect the level of GDF-15, we performed the experiments of ELISA, Immunohistochemistry staining and real-time PCR assay. Compared with SR group, the plasma GDF-15 level was remarkably increased in AF patients (P<0.05). Immunohistochemistry staining was performed in tissue sections for accessing the expression level of GDF-15, the results showed that distribution of small brown-yellow GDF-15 granules in atrial tissues of AF group was markedly higher than that in SR group. Similar to the above data, the mRNA level of GDF-15 in atrial tissues was also significantly increased in AF group (Figure 2). These data indicated that the GDF-15 level was elevated in plasma and myocardiac tissues of AF patients, which might involve in the development and maintenance of atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
Figure 2.
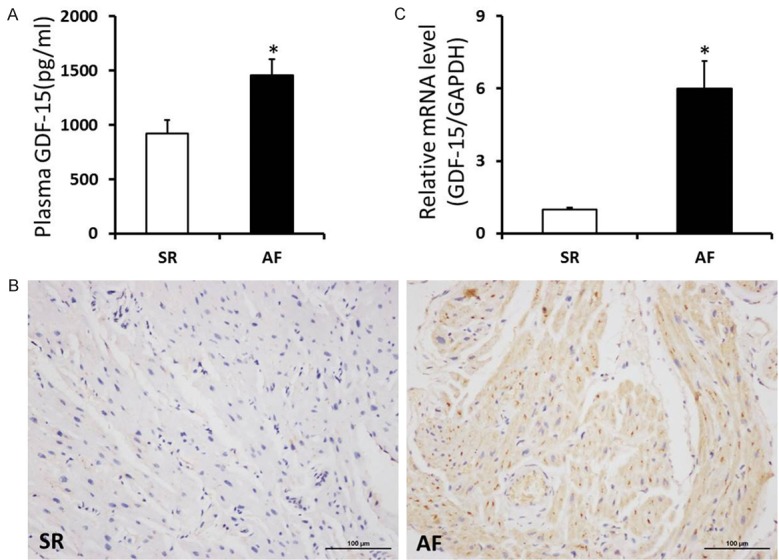
Elevated GDF-15 levels in plasma and myocardiac tissues of AF patients with RHD. A. Level of GDF-15 in plasma was significantly increased in AF patients with rheumatic heart disease. B. Representative immunohistochemistry staining images of GDF-15 in SR and AF patients with rheumatic heart disease. C. The mRNA levels of GDF-15 in the atrial tissues of SR and AF patients with rheumatic heart disease. *P<0.05 vs. SR group.
GDF-15 levels positively related to atrial fibrosis in AF patients with RHD
To explore the relationship of GDF-15 and atrial fibrosis in AF group, we investigated the linear correlation analysis between them. We found that the plasma and mRNA level of GDF-15 in atrial tissues were all positively related to the degree of atrial fibrosis in the AF group (Table 2). Therefore, these results indicated that GDF-15 could be used as a novel biomarker to evaluate myocardial fibrosis in AF patients with rheumatic heart disease in the future.
Table 2.
GDF-15 levels positively related to atrial fibrosis in AF patients with RHD (r, correlation coefficient)
| Plasma GDF-15 | mRNA of GDF-15 | |
|---|---|---|
| Collagen Volume | 0.622 | 0.752 |
| CTGF | 0.822 | 0.783 |
| Collagen I | 0.837 | 0.678 |
| Collagen III | 0.862 | 0.817 |
Discussion
Atrial fibrillation is the most common persistent arrhythmia in clinic, which increases the risk of stroke and death [15,16]. Epidemiological data show that there are about 800-1000 million of atrial fibrillation patients per year in China, which has become serious public health problem that seriously endanger the people’s health. The current view is that occurrence and maintenance of atrial fibrillation are closely related to atrial remodeling which includes electrical remodeling and structural remodeling, and atrial fibrosis is the most important part of the structural remodeling, collagen accumulation in interstitial cells would not only affect the entire mechanics of atrial function, but would also cause local electrical heterogeneity in conduction resulting in arrhythmias especially atrial fibrillation [17,18]. In the present study, we observed that level of atrial fibrosis markedly increased via Masson staining and biomarkers respectively in atrial fibrillation patients with rheumatic heart disease; however, there were only fewer collagen fibers in the sinus rhythm group of patients, which consistent with previous views that atrial structural remodeling were closely related to the generation and maintenance of atrial fibrillation.
GDF-15 is a member of the transforming growth factor of beta super family, in order to reduce the inflammatory response which can inhibit cardiac hypertrophy, cardiac remodeling, cell apoptosis and activation of macrophages [19-21]. Under physiological conditions, GDF-15 is weakly expressed in cardiac tissues, but in the condition of inflammation or pressure overload, ischemia/reperfusion injury, heart failure, atherosclerosis and other stress conditions, the expression of GDF-15 can be activated in cardiomyocytes [22-24]. Recent studies indicate that GDF-15 is an independent predictor of adverse outcomes in patients with heart failure, acute coronary syndrome and acute pulmonary embolism [25,26]. In addition, recent studies have found that inflammation may play an important role in the occurrence and maintenance of atrial fibrillation [27,28]. Therefore, GDF-15 as an inflammatory marker, it is speculated that it may be involved in the occurrence and development of atrial fibrillation. In this study, our result firstly proved that compared with SR group, more GDF-15 diffusely distributed in the myocardium of atrial fibrillation patients, and the plasma level of GDF-15 was also significantly elevated in atrial fibrillation group. Most importantly, we found that the plasma and mRNA level of GDF-15 in atrial tissues were all positively related to the degree of atrial fibrosis in the atrial fibrillation, which would be expected to become one of the important biomarkers for predicting atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
In conclusion, we show that GDF-15 might involve in the development and maintenance of atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease, and GDF-15 could be used as a novel biomarker to evaluate myocardial fibrosis in patients with atrial fibrillation and rheumatic heart disease in the future. However, there were still some limits in the present study as follows: 1) further expand the sample size to increase the intensity of the results; 2) explore the mechanism of elevated GDF-15, and to find a new target for the intervention of myocardial fibrosis in patients with atrial fibrillation and rheumatic heart disease.
Acknowledgements
We thank Prof. Jun Xia who helped us collect the samples and thank all of the members of Cardiovascular Research Institute of Wuhan University for their expert technical assistance and highly supportive advice. The present study was supported by grants from the Natural Science Foundation of Hubei Province (No. 2013CKB004).
Disclosure of conflict of interest
None.
References
- 1.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol. 2009;53:401–408. doi: 10.1016/j.jacc.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 2.Hirsh BJ, Copeland-Halperin RS, Halperin JL. Fibrotic atrial cardiomyopathy, atrial fibrillation, and thromboembolism: mechanistic links and clinical inferences. J Am Coll Cardiol. 2015;65:2239–2251. doi: 10.1016/j.jacc.2015.03.557. [DOI] [PubMed] [Google Scholar]
- 3.Andrade J, Khairy P, Dobrev D, Nattel S. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res. 2014;114:1453–1468. doi: 10.1161/CIRCRESAHA.114.303211. [DOI] [PubMed] [Google Scholar]
- 4.Adam O, Zimmer C, Hanke N, Hartmann RW, Klemmer B, Bohm M, Laufs U. Inhibition of aldosterone synthase (CYP11B2) by torasemideprevents atrial fibrosis and atrial fibrillation in mice. J Mol Cell Cardiol. 2015;85:140–150. doi: 10.1016/j.yjmcc.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Huang B, Scherlag BJ, Ritchey JW, Embi AA, Hu J, Hou Y, Po SS. Structural changes in the progression of atrial fibrillation: Potential role of glycogen and fibrosis as perpetuating factors. Int J Clin Exp Pathol. 2015;8:1712–1718. [PMC free article] [PubMed] [Google Scholar]
- 6.Lu WH, Bayike M, Liu JW, Wang S, Xie X, Yang YC, Liu F, Li N, Liu ZQ, He PY, Muhuyati Association between aldosterone synthase (CYP11B2) -344C/T polymorphism and atrial fibrillation among Han and Kazak residents of the Xinjiang region. Int J Clin Exp Med. 2015;8:5513–5519. [PMC free article] [PubMed] [Google Scholar]
- 7.Kahli A, Guenancia C, Zeller M, Grosjean S, Stamboul K, Rochette L, Girard C, Vergely C. Growth differentiation factor-15 (GDF-15) levels are associated with cardiac and renal injury in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass. PLoS One. 2014;9:e105759. doi: 10.1371/journal.pone.0105759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan LY, Jin ZG, Zong M, Wang QZ, Ju Y, Sun LS, Gu YY, Yu P, Zhang DF, Liu ZM. Growth differentiation factor 15, ischemia modified albumin and pregnancy-associated plasma protein A in patients with coronary artery disease. Clin Lab. 2014;60:973–982. doi: 10.7754/clin.lab.2013.130448. [DOI] [PubMed] [Google Scholar]
- 9.Fuernau G, Poenisch C, Eitel I, de Waha S, Desch S, Schuler G, Adams V, Werdan K, Zeymer U, Thiele H. Growth-differentiation factor 15 and osteoprotegerin in acute myocardial infarction complicated by cardiogenic shock: a biomarker substudy of the IABPSHOCK II-trial. Eur J Heart Fail. 2014;16:880–887. doi: 10.1002/ejhf.117. [DOI] [PubMed] [Google Scholar]
- 10.Dominguez-Rodriguez A, Abreu-Gonzalez P, Hernandez-Baldomero IF, Avanzas P, Bosa-Ojeda F. Change in growth differentiation factor 15, but not C-reactive protein, independently predicts major cardiac events in patients with non-ST elevation acute coronary syndrome. Mediators Inflamm. 2014;2014:929536. doi: 10.1155/2014/929536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaggin HK, Szymonifka J, Bhardwaj A, Belcher A, De Berardinis B, Motiwala S, Wang TJ, Januzzi JL Jr. Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2014;2:65–72. doi: 10.1016/j.jchf.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Izumiya Y, Hanatani S, Kimura Y, Takashio S, Yamamoto E, Kusaka H, Tokitsu T, Rokutanda T, Araki S, Tsujita K, Tanaka T, Yamamuro M, Kojima S, Tayama S, Kaikita K, Hokimoto S, Ogawa H. Growth differentiation factor-15 is a useful prognostic marker in patients with heart failure with preserved ejection fraction. Can J Cardiol. 2014;30:338–344. doi: 10.1016/j.cjca.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 13.Lok DJ, Klip IT, Lok SI, Bruggink-Andre de la Porte PW, Badings E, van Wijngaarden J, Voors AA, de Boer RA, van Veldhuisen DJ, van der Meer P. Incremental prognostic power of novel biomarkers (growth-differentiation factor-15, high-sensitivity C-reactive protein, galectin-3, and high-sensitivity troponin-T) in patients with advanced chronic heart failure. Am J Cardiol. 2013;112:831–837. doi: 10.1016/j.amjcard.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 14.Wollert KC, Kempf T. GDF-15 in heart failure: providing insight into end-organ dysfunction and its recovery? Eur J Heart Fail. 2012;14:1191–1193. doi: 10.1093/eurjhf/hfs158. [DOI] [PubMed] [Google Scholar]
- 15.Chyou JY, Hunter TD, Mollenkopf SA, Turakhia MP, Reynolds MR. Individual and Combined Risk Factors for Incident Atrial Fibrillation and Incident Stroke: An Analysis of 3 Million At-Risk US Patients. J Am Heart Assoc. 2015:4. doi: 10.1161/JAHA.114.001723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi XM, Chen FK, Liang Z, Li J, Lin K, Guo JP, Shan ZL. Is dabigatran efficacy enough to prevent stroke in atrial fibrillation patient with high CHADS2 score during peri-procedural catheter radiofrequency ablation? A case report with literature review. Int J Clin Exp Med. 2015;8:6592–6600. [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Yan H, Wugeti N, Guo Y, Zhang L, Ma M, Guo X, Jiao C, Xu W, Li T. Microelectrode array measurement of potassium ion channel remodeling on the field action potential duration in rapid atrial pacing rabbits model. Int J Clin Exp Med. 2015;8:249–256. [PMC free article] [PubMed] [Google Scholar]
- 18.Li M, Yi X, Ma L, Zhou Y. Hepatocyte growth factor and basic fibroblast growth factor regulate atrial fibrosis in patients with atrial fibrillation and rheumatic heart disease via the mitogen-activated protein kinase signaling pathway. Exp Ther Med. 2013;6:1121–1126. doi: 10.3892/etm.2013.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu XY, Nie Y, Wang FF, Bai Y, Lv ZZ, Zhang YY, Li ZJ, Gao W. Growth differentiation factor (GDF)-15 blocks norepinephrine-induced myocardial hypertrophy via a novel pathway involving inhibition of epidermal growth factor receptor transactivation. J Biol Chem. 2014;289:10084–10094. doi: 10.1074/jbc.M113.516278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heger J, Schiegnitz E, von Waldthausen D, Anwar MM, Piper HM, Euler G. Growth differentiation factor 15 acts anti-apoptotic and prohypertrophic in adult cardiomyocytes. J Cell Physiol. 2010;224:120–126. doi: 10.1002/jcp.22102. [DOI] [PubMed] [Google Scholar]
- 21.Song H, Yin D, Liu Z. GDF-15 promotes angiogenesis through modulating p53/HIF-1alpha signaling pathway in hypoxic human umbilical vein endothelial cells. Mol Biol Rep. 2012;39:4017–4022. doi: 10.1007/s11033-011-1182-7. [DOI] [PubMed] [Google Scholar]
- 22.Tantawy AA, Adly AA, Ismail EA, Youssef OI, Ali ME. Growth differentiation factor-15 in children and adolescents with thalassemia intermedia: Relation to subclinical atherosclerosis and pulmonary vasculopathy. Blood Cells Mol Dis. 2015;55:144–150. doi: 10.1016/j.bcmd.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Lambert JR, Whitson RJ, Iczkowski KA, La Rosa FG, Smith ML, Wilson RS, Smith EE, Torkko KC, Gari HH, Lucia MS. Reduced expression of GDF-15 is associated with atrophic inflammatory lesions of the prostate. Prostate. 2015;75:255–265. doi: 10.1002/pros.22911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breit SN, Johnen H, Cook AD, Tsai VW, Mohammad MG, Kuffner T, Zhang HP, Marquis CP, Jiang L, Lockwood G, Lee-Ng M, Husaini Y, Wu L, Hamilton JA, Brown DA. The TGF-beta superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors. 2011;29:187–195. doi: 10.3109/08977194.2011.607137. [DOI] [PubMed] [Google Scholar]
- 25.Duran L, Kayhan S, Guzel A, Ince M, Kati C, Akdemir HU, Yavuz Y, Zengin H, Okuyucu A, Murat N. The prognostic values of GDF-15 in comparison with NT-proBNP in patients with normotensive acute pulmonary embolism. Clin Lab. 2014;60:1365–1371. doi: 10.7754/clin.lab.2013.130827. [DOI] [PubMed] [Google Scholar]
- 26.Lankeit M, Kempf T, Dellas C, Cuny M, Tapken H, Peter T, Olschewski M, Konstantinides S, Wollert KC. Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med. 2008;177:1018–1025. doi: 10.1164/rccm.200712-1786OC. [DOI] [PubMed] [Google Scholar]
- 27.Geng HH, Li R, Su YM, Pan HY, Pan M, Ji XP. A functional single-nucleotide polymorphism in interleukin-6 promoter is associated with p wave dispersion in hypertensive subjects with atrial fibrillation. Int J Clin Exp Med. 2014;7:4434–4440. [PMC free article] [PubMed] [Google Scholar]
- 28.Letsas KP, Weber R, Burkle G, Mihas CC, Minners J, Kalusche D, Arentz T. Pre-ablative predictors of atrial fibrillation recurrence following pulmonary vein isolation: the potential role of inflammation. Europace. 2009;11:158–163. doi: 10.1093/europace/eun309. [DOI] [PubMed] [Google Scholar]


