Abstract
Background: Platelets are involved in multiple links of the process of thrombus formation and development. The aim of this study is to investigate the impact of pretreatment platelet parameters, such as plateletcrit (PCT), platelet mean distribution width (MDW), and mean platelet volume (MPV) in a cohort of patients with locally advanced pancreatic adenocarcinoma treated with a combination of chemotherapy and radiation therapy. Methods: A retrospective analysis was conducted of 163 locally advanced unresectable consecutive pancreatic adenocarcinoma patients who received chemoradiotherapy in Zhejiang cancer hospital from January 2009 to December 2011. The effects of platelet parameters on overall survival (OS) were assessed using Kaplan-Meier analysis. Independent prognostic factors were identified in the multivariate Cox analysis. Results: The median of the PC, PCT, MDW, MPV and CRP were 175×109, 20.0%, 14.0%, 10.8 fl and 7.0 mg/L, respectively. MDW was positively correlated with PC (r=0.156, P=0.047) and CRP (r=0.591, P<0.001). The median survival time of high MDW group was significantly worse than that of low MDW group (14.0 m Vs 11.0 m, P=0.008). Patients with high PCT were found to have shorter overall survival time (15.0 m Vs 11.0 m, P=0.018). Multivariate analysis indicated that MDW and N stage were two independent prognostic factors for overall survival (P<0.05). Patients with higher MDW had a 1.48-fold increased risk of death compared to those with low MDW. Conclusions: MDW is an independent negative prognostic factor for overall survival in pancreatic adenocarcinoma patients.
Keywords: Pancreatic adenocarcinoma, platelet, platelet parameters, prognosis
Introduction
The prognosis of cancer patients is not only determined by tumor characteristics, patient-related factors are also important factors. Cancer-associated thrombosis is a key determinant of disease progression and survival in most cancers [1]. Platelets are the smallest circulating blood cells and their major function is to stop bleeding by clumping and clogging blood vessel injuries. Platelets are involved in multiple links of the process of thrombus formation and development. In fact, increased circulating platelet counts have been reported with unfavorable prognosis in pancreatic cancer [2-5]. Several evidences support a role for platelets in contributing to systematic inflammation and promoting thrombosis [6,7]. The changes in platelet parameters were reported to reflect the platelet activation [8]. It has been indicated that platelet count (PC) and its parameters including plateletcrit (PCT), platelet mean distribution width (MDW), and mean platelet volume (MPV) might be useful together with other acute phase reactants to define inflammation activation. Recently, Ma et al. [9] showed that the combination of thrombocytosis and maximal aggregation rate are independent negative prognostic factors for overall survival in epithelial ovarian cancer patients, but there is no study regarding these markers in pancreatic cancer.
In our previous study [10], the rate of thrombocytosis was 13.1% in pancreatic adenocarcinoma and a significant association can be found between thrombocytosis and the poor survival of patients with pancreatic adenocarcinoma as evidenced by a multivariate analysis. Therefore, this study was undertaken to identify the potential prognostic significance of pretreatment indices including plateletcrit (PCT), platelet mean distribution width (MDW), and mean platelet volume (MPV) in a cohort of patients with locally advanced pancreatic adenocarcinoma treated with a combination of chemotherapy and radiation therapy.
Patients and methods
Patient’s selection
A retrospective analysis was conducted of 168 locally advanced unresectable consecutive pancreatic adenocarcinoma patients who received chemoradiotherapy in Zhejiang cancer hospital from January 2009 to December 2011. All patients were newly confirmed to have pancreatic adenocarcinoma and had not received treatment previously. Patients with other malignancies were excluded from this study. Those patients with concomitant disease or conditions suspected of influencing platelet parameters, such as hematological disease, coronary artery disease, end-stage renal disease, heart failure, cerebrovascular disease, peripheral arterial disease, were excluded. Finally, a total of 163 patients were enrolled in current study. Medical records were reviewed, and each patient’s gender, age, tumor location, clinical TNM stage, treatment response, and overall survival after treatment were obtained. The extent of the disease was determined by TNM staging according to the new IASLC staging system.
Complete blood count (CBC) test results were obtained within 1 week prior to treatment. Our study was approved by the institutional review board of the hospital. All patients provided informed consent before treatment.
Treatment schedule
All patients were treated with intensity modulated radiation therapy (IMRT). Patients were immobilized in a supine position, arms overhead, with thermoplastic cast. Patients underwent CT-simulation on CT scanner (GE, Lightspeed, USA), using 5 mm slices with contrast enhancement. Radiation plans for IMRT were generated using Pinnacle Version 8.0. All patients underwent external beam radiation therapy with 6 MV X-rays. The area of solid macroscopic tumors in pancreas, the surrounding tissue infiltrated, and the regional lymph node metastases were defined as the gross tumor volume (GTV). The GTV plus a margin of at least 5 mm, including any areas of microscopic spread and the regional lymph nodes (peripancreatic, celiac, superior mesenteric, portal hepatic, retroperitoneal), was defined as the clinical target volume (CTV). The plan target volume (PTV) was defined as the CTV plus 0.5-1.0 cm to account for the daily setup variation and respiratory movement. A fractional daily dose of 1.8 Gy (Gray) (5 days per week, over 5 weeks) at an isocenter was prescribed. The median delivered dose of IMRT was 50 Gy (range: 44.0-55.8 Gy). The dose to the adjacent normal structures was constrained as follows: The liver dose was limited to V5<75%, V20<50%, V30<30%, and a mean dose less than 28 Gy. The kidney dose was limited to V12<50% and V22.5<30%. Spinal cord maximum dose was held to 45 Gy. The CTV encompassed at least 95% isodose line. The dose volume histogram (DVH) was obtained for CTV, PTV, spinal cord, liver, and kidney. The small bowel contour was confined to the small bowel loops within 3 cm of the PTV and was limited to maximal dose <54 Gy.
Concurrent chemoradiotherapy, with or without adjuvant chemotherapy, was used in 101 patients. The chemotherapy regimens consisted of S1 at 50 mg/m2 twice daily from days 1 to 14 or gemcitabine at 1,000 mg/m2 on day 1 and day 8 of a 21 days cycle. The CR (complete response), PR (partial response), SD (stable disease), and PD (progressive disease) were assessed at an interval of at least 4 weeks to confirm the objective response. All patients received standardized follow-up, occurring at 3 months interval for 2 years, 6 months interval the 3rd year, and yearly thereafter. Evaluations comprised a physical examination, pancreatic CT, complete blood count, liver and kidney function tests, abdominal ultrasound, chest radiography, and pelvic CT.
Statistical analysis
Overall survival was calculated from the initial event (radiotherapy) to the death or censoring. The cutoff values of PCT, MDW, and MPV were determined using receiver operating characteristic (ROC) curve analysis, and the dependent variable was the OS for 2 years. The optimal cutoff levels for PCT, MDW, and MPV were established at 18.00%, 14.15%, and 12.00 fl, respectively, and these cutoff values were used to categorize high and low PCT, MDW, and MPV groups. Chi square test or Fisher exact test was used for comparisons of categorical variables. The correlation between PC, PCT, MDW, MPV and C-reactive protein (CRP) was determined through linear correlation analysis. Survival curves were estimated by the univariate Kaplan-Meier method. The log-rank test was applied to check the significant difference in the curves among groups. Furthermore, we used the Cox proportional hazards model with the backward selection method for multivariate analysis. All statistical calculations were performed with SPSS 13.0 software for Windows (Chicago, IL, USA). Two-sided P-values of <0.05 were considered to be statistically significant.
Results
Characteristics of patients
Preoperative PC, PCT, MDW, and MPV were confirmed in 163 pancreatic adenocarcinoma patients. Patient characteristics are shown in Table 1. The study population had a median age of 61 years (range: 34-83 years). Tumor invasion depths of T1, T2, T3, and T4 were observed in 17 (10.4%), 28 (17.2%), 75 (46.0%), and 43 (26.4%) of the patients, respectively. On the basis of image parameters, tumor was found with regional lymph node metastases in 86 (52.8%) patients. The overall follow-up durations ranged from 4 to 48 months (median, 12.0 months). 137 of 163 (84.0%) patients died of disease progression.
Table 1.
Baseline patient characteristics (n=163) and univariate analysis of prognostic factors of OS by the Kaplan-Meier method in pancreatic adenocarcinoma
| Characteristics | Total (n, %) | Median OS time (months) | 2 year OS rate (%) | P |
|---|---|---|---|---|
| Gender | ||||
| Female | 60 (36.8) | 14.0 | 9.6 | 0.887 |
| Male | 103 (63.2) | 12.0 | 14.7 | |
| Age (year) | ||||
| <60 | 74 (45.4) | 14.0 | 15.9 | 0.297 |
| ≥60 | 89 (54.6) | 12.0 | 9.6 | |
| Tumor location | ||||
| Head | 77 (47.2) | 12.0 | 8.5 | 0.397 |
| Body and tail | 86 (52.8) | 13.0 | 15.5 | |
| BMI (kg/m2) | ||||
| <20 | 70 (42.9) | 13.0 | 15.3 | 0.756 |
| ≥20 | 93 (57.1) | 13.0 | 17.3 | |
| Chemotherapy | ||||
| No | 62 (38.) | 10.0 | 5.7 | 0.049 |
| Yes | 101 (62.0) | 14.0 | 16.4 | |
| T stage | ||||
| T1-2 | 45 (27.6) | 13.0 | 12.1 | 0.882 |
| T3-4 | 118 (72.4) | 12.0 | 16.1 | |
| N stage | ||||
| N0 | 77 (47.2) | 14.0 | 13.7 | 0.045 |
| N1 | 86 (52.8) | 11.0 | 11.6 | |
| CRP (mg/L) | ||||
| <3 | 39 (23.9) | 14.0 | 26.3 | 0.161 |
| ≥3 | 124 (76.1) | 12.0 | 13.5 | |
| Platelet count | ||||
| <300×109 | 142 (87.1) | 14.0 | 13.7 | 0.015 |
| ≥300×109 | 21 (12.9) | 9.0 | 5.7 | |
| MPV (fl) | ||||
| <12.00 | 128 (78.5) | 13.0 | 14.3 | 0.381 |
| ≥12.00 | 35 (21.5) | 11.0 | 7.3 | |
| PCT (%) | ||||
| <18.00 | 100 (61.3) | 15.0 | 18.5 | 0.018 |
| ≥18.00 | 63 (38.7) | 11.0 | 13.5 | |
| MDW (%) | ||||
| <14.15 | 85 (52.1) | 14.0 | 20.5 | 0.008 |
| ≥14.15 | 78 (47.9) | 11.0 | 5.4 |
Abbreviations: OS, overall survival; HR, hazard ratio; BMI, body mass index; CRP, C-reactive protein; MPV, mean platelet volume; PCT, plateletcrit; PDW, platelet distribution width; MDW, platelet distribution width.
Relationship between PC, PCT, MDW, MPV and CRP
The median of the PC, PCT, MDW, MPV and CRP were 175×109, 20.0%, 14.0%, 10.8 fl and 7.0 mg/L, respectively. MDW was positively correlated with PC (r=0.156, P=0.047, Figure 1A) and CRP (r=0.591, P<0.001, Figure 1B). However, no significant correlation was observed between PCT, PC, MPV and CRP (P>0.05).
Figure 1.
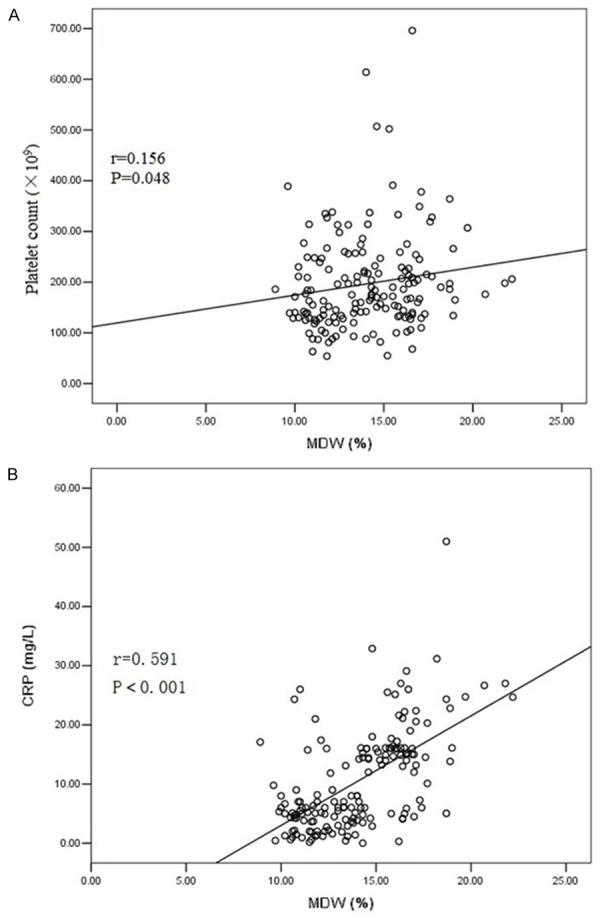
A. Spearman’s correlation revealed that MDW was positively correlated with PC (r=0.156, P=0.047). B. Spearman’s correlation revealed that MDW was positively correlated with CRP (r=0.591, P<0.001).
Survival analysis
The 1 year, 2 year and 3 year cumulative overall survival rate were 50.2%, 16.3%, 4.7%. We performed univariate analysis for platelets parameters and other clinicopathological variables (including gender, age, tumor location, BMI, T stage, lymph node metastasis, and chemotherapy) to find useful prognostic factors for pancreatic adenocarcinoma (Table 1). The results were shown in Table 1. Chemotherapy, N stage, PC, PCT and MDW were five significant prognostic factors for overall survival (Chemotherapy: P=0.049; N stage: P=0.045; PC: P=0.015; PCT: P=0.018; MDW: P=0.008). The pretreatment PCT was correlated with overall survival, with high PCT patients with shorter overall survival time (Figure 2A). The pretreatment MDW was significantly correlated with overall survival, with low MDW patients with longer overall survival time (Figure 2B).
Figure 2.
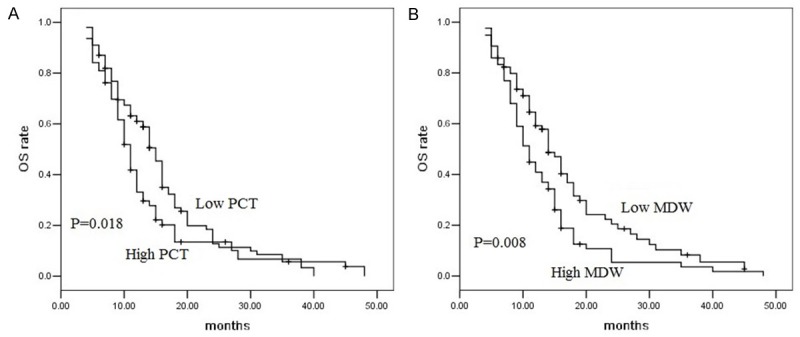
A. Kaplan-Meier overall survival curves of the patients with high pretreatment PCT (n=63) and low PCT (n=100). All patients underwent radiotherapy (P=0.018, log-rank test). B. Kaplan-Meier overall survival curves of the patients with high pretreatment MDW (n=78) and low PCT (n=85). All patients underwent radiotherapy (P=0.008, log-rank test).
We then performed multivariate analysis for these factors whose presence significantly affected prognosis. The Cox proportional hazards regression indicated that MDW and N stage were two independent prognostic factors for overall survival (P<0.05, Table 2). Patients with high MDW had a 1.48-fold increased risk of death compared to those with low MDW. The 95% confidence interval (CI) was 1.05-2.09. However, PC and PCT were not independent prognostic factors adjusted for other prognostic factors (P>0.05).
Table 2.
Multivariable analysis on overall survival
| Variables | Hazard ratio | 95% CI | P |
|---|---|---|---|
| Chemotherapy | |||
| Yes Vs No | 0.73 | 0.51-1.05 | 0.091 |
| N stage | |||
| N0 Vs N1 | 0.70 | 0.49-0.99 | 0.043 |
| T stage | |||
| T3-4 Vs T1-2 | 0.92 | 0.63-1.35 | 0.666 |
| Platelet count | |||
| ≥300×109 Vs <300×109 | 1.48 | 0.90-2.44 | 0.124 |
| PCT (%) | |||
| ≥18.00 Vs <18.00 | 1.32 | 0.92-1.92 | 0.131 |
| MDW (%) | |||
| ≥14.15 Vs <14.15 | 1.48 | 1.05-2.09 | 0.027 |
Abbreviations: CI, confidence interval; PCT, plateletcrit; MDW, platelet distribution width.
Discussion
To the best of our knowledge, our study is the first study to analyze the relationship between platelet parameters (MPV, PCT and MDW) and prognosis in pancreatic adenocarcinoma. In the present study, we found a significant association between thrombocytosis, high PCT or high MDW and poor overall survival in locally advanced pancreatic adenocarcinoma treated with radiation therapy or chemoradiotherapy. Furthermore, according to the multivariate analysis, MDW is one of the independent prognostic factors.
Platelets activation plays a significant role in the process of thrombus formation and development. The alteration in platelet parameters could reflect the platelet activation. MPV represents the average volume of a single platelet and is an important biological variable and that larger platelets have higher thrombotic potential. MPV is a simple marker showing platelet function and activation that can be influenced by inflammation. Increased MPV levels were reported in myocardial infarction, acute ischemic stroke and Crohn’s disease [11]. Furthermore, MPV is a useful inflammatory marker to differentiate cancer patients from healthy controls [12,13]. The increase in MPV levels is considered to be related with early diagnosis of gastric cancer but not with TNM stage [12]. However, no significant correlation was observed between MPV and TNM stage. There is only one study investigate the association between MPV and pancreatic cancer. In 2011, Karaman et al. [14] evaluate the MPV both in pancreatic adenocarcinomas and pancreatic neuroendocrine tumors. There was not a significant difference among the preoperative MPV between resectable pancreatic adenocarcinomas and unresectable pancreatic adenocarcinomas. In our current study, the preoperative MPV did not related with prognosis in pancreatic adenocarcinoma. This is consistent with previous findings [12,14].
MDW is another platelet parameter which can indicate variation in platelet size and differentially diagnoses thrombocytosis. Elevated MDW is an indication for the anisocytosis of platelets. MDW is an often forgotten platelet indice and clinicians pay less attention than PC and MPV. Recent studies show that MDW is a more specific marker of platelet activation and is associated with vascular damage than PC and MPV [15]. Jindal et al. [16] also reported that higher value of MDW is a special characteristic of diabetics with microvascular complications. In our present study, MDW and PC are both related with overall survival. One possible reason is that tumor cell have been not only shown to have the ability of aggregating platelets, but also it can change the platelet shape and function to more aggressive tumor biology. Chronic inflammation has been proposed as an independent risk factor for the development of pancreatic cancer in a number of important studies [17,18]. CRP is one of the major proteins helpful in determination of activity of chronic inflammation [19]. It is also a diagnostic factor and independent prognostic factor in pancreatic adenocarcinoma [20]. In this study, PC and MDW were positively related with CRP. Moreover, MDW was significantly higher in patients with thrombocytosis than those without thrombocytosis. Spearman’s correlation also revealed that MDW was positively related with PC. Higher level of PC and MDW reflected enhancement activity of bone marrow hematopoietic function. Based on these findings, we speculated that during the process of development of cancer, hematopoietic function of bone marrow could be enhanced by inflammation. Consistent with our study findings, Alexandrakis MG et al. [21] reported that proinflammatory cytokines, such as interleukin-1 beta (IL-1 beta) and interleukin-6 (IL-6), play a significant role in the reactive thrombocytosis. Obesity is another factor which will play important role during pancreatic cancer development. In a multicenter retrospective study, Kasenda B et al. [22] found that increasing BMI was significantly associated with worse survival prognosis (HR 1.22, 95% CI 1.04-1.41, P=0.012). Accumulative evidence shows that obesity is associated with oxidative stress and chronic inflammation [23]. However, it seems there was no relationship between MDW and BMI. Furthermore, BMI was not associated with overall survival. One possible reason is our sample is relatively small. Therefore, further prospective study is needed to confirm the relationship between MDW and BMI.
PCT is a marker of total platelet mass, which also related with platelet activation. PCT and MPV usually changed in the same direction. PCT could be used as a marker in the discrimination of autoimmune gastritis and functional dyspepsia patients [24]. In our study, increased PCT is related with worse prognosis in univariate analysis. The CR (complete response), PR (partial response), SD (stable disease) and PD (progressive disease) were assessed at an interval of at least 4 weeks after treatment to confirm the objective response. CR and PR were more common in the low PCT group (PCT <18.00%). This might explain the lower mortality in this group compared with the high PCT group (PCT ≥18.00%).
In conclusion, the present study suggests that high MDW and PCT are associated with worse prognosis in pancreatic adenocarcinoma. MDW is a new novel independent prognostic biomarker for overall survival in pancreatic adenocarcinoma.
Acknowledgements
We are grateful to the patients who are included in this study. This study was supported by grants from the Nature Science Foundation of Zhejiang province (NO. LY14H160012).
Disclosure of conflict of interest
None.
References
- 1.Connolly GC, Phipps RP, Francis CW. Platelets and cancer-associated thrombosis. Semin Oncol. 2014;41:302–10. doi: 10.1053/j.seminoncol.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Chadha AS, Kocak-Uzel E, Das P, Minsky BD, Delclos ME, Mahmood U, Guha S, Ahmad M, Varadhachary GR, Javle M, Katz MH, Fleming JB, Wolff RA, Crane CH, Krishnan S. Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta Oncol. 2015;54:971–8. doi: 10.3109/0284186X.2014.1000466. [DOI] [PubMed] [Google Scholar]
- 3.Wang H, Gao J, Bai M, Liu R, Li H, Deng T, Zhou L, Han R, Ge S, Huang D, Ba Y. The pretreatment platelet and plasma fibrinogen level correlate with tumor progression and metastasis in patients with pancreatic cancer. Platelets. 2014;25:382–7. doi: 10.3109/09537104.2013.827782. [DOI] [PubMed] [Google Scholar]
- 4.Smith RA, Bosonnet L, Raraty M, Sutton R, Neoptolemos JP, Campbell F, Ghaneh P. Preoperative platelet-lymphocyte ratio is an independent significant prognostic marker in resected pancreatic ductal adenocarcinoma. Am J Surg. 2009;197:466–72. doi: 10.1016/j.amjsurg.2007.12.057. [DOI] [PubMed] [Google Scholar]
- 5.Dominguez I, Crippa S, Thayer SP, Hung YP, Ferrone CR, Warshaw AL, Fernandez-Del Castillo C. Preoperative platelet count and survival prognosis in resected pancreatic ductal adenocarcinoma. World J Surg. 2008;32:1051–6. doi: 10.1007/s00268-007-9423-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130:2747–60. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 7.Cognasse F, Nguyen KA, Damien P, McNicol A, Pozzetto B, Hamzeh-Cognasse H, Garraud O. The Inflammatory Role of Platelets via Their TLRs and Siglec Receptors. Front Immunol. 2015;6:83. doi: 10.3389/fimmu.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yazici M, Kaya A, Kaya Y, Albayrak S, Cinemre H, Ozhan H. Lifestyle modification decreases the mean platelet volume in prehypertensive patients. Platelets. 2009;20:58–63. doi: 10.1080/09537100802613449. [DOI] [PubMed] [Google Scholar]
- 9.Ma X, Wang Y, Sheng H, Tian W, Qi Z, Teng F, Xue F. Prognostic significance of thrombocytosis, platelet parameters and aggregation rates in epithelial ovarian cancer. J Obst Gynaecol Res. 2014;40:178–83. doi: 10.1111/jog.12151. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Zhu Y, Liu L. Elevated pretreatment plasma D-dimer levels and platelet counts predict poor prognosis in pancreatic adenocarcinoma. Onco Targets Ther. 2015;8:1335–40. doi: 10.2147/OTT.S82329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greisenegger S, Endler G, Hsieh K, Tentschert S, Mannhalter C, Lalouschek W. Is elevated mean platelet volume associated with a worse outcome in patients with acute ischemic cerebrovascular events? Stroke. 2004;35:1688–91. doi: 10.1161/01.STR.0000130512.81212.a2. [DOI] [PubMed] [Google Scholar]
- 12.Kilincalp S, Ekiz F, Basar O, Ayte MR, Coban S, Yilmaz B, Altinbas A, Basar N, Aktas B, Tuna Y, Erbis H, Ucar E, Erarslan E, Yüksel O. Mean platelet volume could be possible biomarker in early diagnosis and monitoring of gastric cancer. Platelets. 2014;25:592–4. doi: 10.3109/09537104.2013.783689. [DOI] [PubMed] [Google Scholar]
- 13.Baldane S, Ipekci SH, Sozen M, Kebapcilar L. Mean platelet volume could be a possible biomarker for papillary thyroid carcinomas. Asian Pac J Cancer Prev. 2015;16:2671–4. doi: 10.7314/apjcp.2015.16.7.2671. [DOI] [PubMed] [Google Scholar]
- 14.Karaman K, Bostanci EB, Aksoy E, Kurt M, Celep B, Ulas M, Dalgic T, Surmelioglu A, Hayran M, Akoglu M. The predictive value of mean platelet volume in differential diagnosis of non-functional pancreatic neuroendocrine tumors from pancreatic adenocarcinomas. Eur J Int Med. 2011;22:E95–8. doi: 10.1016/j.ejim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Amin MA, Amin AP, Kulkarni HR. Platelet distribution width (PDW) is increased in vaso-occlusive crisis in sickle cell disease. Ann Hematol. 2004;83:331–5. doi: 10.1007/s00277-003-0833-8. [DOI] [PubMed] [Google Scholar]
- 16.Jindal S, Gupta S, Gupta R, Kakkar A, Singh HV, Gupta K, Singh S. Platelet indices in diabetes mellitus: indicators of diabetic microvascular complications. Hematology. 2011;16:86–9. doi: 10.1179/102453311X12902908412110. [DOI] [PubMed] [Google Scholar]
- 17.Ekbom A, McLaughlin JK, Karlsson BM, Nyren O, Gridley G, Adami HO, Fraumeni JF Jr. Pancreatitis and pancreatic cancer: a populationbased study. J Natl Cancer Inst. 1994;86:625–7. doi: 10.1093/jnci/86.8.625. [DOI] [PubMed] [Google Scholar]
- 18.Zechner D, Radecke T, Amme J, Burtin F, Albert AC, Partecke LI, Vollmar B. Impact of diabetes type II and chronic inflammation on pancreatic cancer. BMC Cancer. 2015;15:51. doi: 10.1186/s12885-015-1047-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–54. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 20.Zhang P, Zou M, Wen X, Gu F, Li J, Liu G, Dong J, Deng X, Gao J, Li X, Jia X, Dong Z, Chen L, Wang Y, Tian Y. Development of serum parameters panels for the early detection of pancreatic cancer. Int J Cancer. 2014;134:2646–55. doi: 10.1002/ijc.28584. [DOI] [PubMed] [Google Scholar]
- 21.Alexandrakis MG, Passam FH, Perisinakis K, Ganotakis E, Margantinis G, Kyriakou DS, Bouros D. Serum proinflammatory cytokines and its relationship to clinical parameters in lung cancer patients with reactive thrombocytosis. Respir Med. 2002;96:553–8. doi: 10.1053/rmed.2002.1328. [DOI] [PubMed] [Google Scholar]
- 22.Kasenda B, Bass A, Koeberle D, Pestalozzi B, Borner M, Herrmann R, Jost L, Lohri A, Hess V. Survival in overweight patients with advanced pancreatic carcinoma: a multicentre cohort study. BMC Cancer. 2014;14:728. doi: 10.1186/1471-2407-14-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah D, Romero F, Duong M, Wang N, Paudyal B, Suratt BT, Kallen CB, Sun J, Zhu Y, Walsh K, Summer R. Obesity-induced adipokine imbalance impairs mouse pulmonary vascular endothelial function and primes the lung for injury. Sci Rep. 2015;5:11362. doi: 10.1038/srep11362. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Tuzun A, Keskin O, Yakut M, Kalkan C, Soykan I. The predictive value of mean platelet volume, plateletcrit and red cell distribution width in the differentiation of autoimmune gastritis patients with and without type I gastric carcinoid tumors. Platelets. 2014;25:363–6. doi: 10.3109/09537104.2013.821607. [DOI] [PubMed] [Google Scholar]


