Abstract
Recent studies have demonstrated that epithelial ovarian cancer (EOC) are factual several different diseases. A two-tier system divides EOC into type I and type II EOC. HE4 has been used as a complementary biomarker for diagnosing EOC. This study aimed to evaluate the different clinicopathologic characteristics and HE4 expression levels in types I and II EOCs. This retrospective study included 127 EOC patients. Data related to patient demographics, cancer stages, grades, histology, operation procedures, residual disease, adjuvant chemotherapy, recurrence, and survival were collected. A total of 134 ovarian carcinoma tissue specimens and 40 matching borderline ovarian tumor specimens were chosen from the pathology department archives. Immunohistochemistry was used to assess HE4 expression in EOC and borderline ovarian tumor tissue specimens. Of the 127 patients, there were 42 type I EOC patients (7 low grade serous carcinomas, 8 mucinous carcinomas, 12 low grade endometrioid carcinomas and 15 clear cell carcinomas) and 85 type II EOC patients (83 high grade serous carcinomas and 2 high grade endometrioid carcinomas). The median followed--up time was 18.3 months. There were significant differences between the two types of EOC in terms of the menopausal state, FIGO stage and pathological differentiation, but there were no differences in the residual tumor and chemotherapy treatment. In type I EOC, the median follow--up time was 31 months and the median progression--free survival was 72 months (95% CI: 40.34-103.66). There were 15 (35.7%) relapsed or progressive patients. In type II EOC, the median follow-up time was 17 months (0-60 m), and the median progression--free survival was 27 months (95% CI: 17.83-36.17). There were 47 (55.3%) relapsed or progressive patients. There was a significant difference between the two types of EOCs in terms of progression--free survival (P<0.001). Among the 44 type I specimens, 25 demonstrated positive expression of HE4 (56.8%). In contrast, 78 (86.7%) type II EOC demonstrated positive expression levels. There was a significant difference between type I and type II EOCs in terms of HE4 expression. Additionally, there was a significant difference between high grade serous carcinoma and borderline serous tumor, but no difference was observed between low grade serous carcinoma and borderline serous tumor or other types of EOC and corresponding borderline tumors. The different clinicopathologic characteristics between type I and type II EOC indicate that the two--tier EOC system reasonable and reliable. HE4 would be a powerful biomarker to distinguish type II EOC from borderline tumors but it is less useful in type I EOC. Type I EOC is generated from the corresponding borderline tumor.
Keywords: Epithelial ovarian cancer, borderline tumor, human epididymis protein 4, immunohistochemistry, the two--tier system of EOC
Introduction
Epithelial ovarian cancer (EOC) is the most lethal gynecologic malignancy in the Western world. The lack of clearly identified precancerous lesions, reliable screening approaches, and non-specific early symptoms results in late diagnosis [1]. Because of the great heterogeneity in molecular and biological status, epithelial ovarian cancer is essentially a group of 5 distinct diseases with discrepant clinical patterns. These distinct diseases may require specific treatments [2]. Traditionally EOC has been thought to arise from epithelial cells that cover the ovary surface. Recent studies have indicated that EOC also arises from the fallopian tube epithelium and from the endometrium through retrograde menstruation [3,4].
Based on morphological and molecular genetics, a novel tumor origination model has been proposed. This model divides EOC into type I and type II tumors. Type I tumors are suggested to behave in an indolent manner and are more often confined to the ovary at presentation, with a stable genome and without TP53 mutations, although somatic mutations are frequently detected. Type II tumors are more aggressive and genetically highly unstable; the majority have TP53 mutations, and approximately half of all cases have mutations, hypermethylation, or dysfunction of BRCA1/2 [3,5]. These aggressive tumors account for 75% of all EOC cases and are responsible for 90% of deaths from the disease.
HE4, which has a stable 4-disulfide core protein associated with the WFDC2 gene was first introduced as a potential biomarker for EOC in 2003 [6]. It has been reported that HE4 displayed excellent performance in diagnosing ovarian cancer, predicting optimal cytoreduction and prognosis, assessing the treatment and differentiating ovarian cancer from benign tumors [7-12].
In this study, we aimed to evaluate the clinicopathologic significance of the two--tier grading system by comparing the different origins of EOC and to investigate the expression performance of HE4 in type I and type II EOC.
Materials and methods
Study population
A total of 133 patients who diagnosed with epithelial ovarian cancer and who underwent primary cytoreduction at Peking University People’s Hospital between January 2003 and March 2009 were retrospectively enrolled in this study. Six patients were excluded because of incomplete clinic data (such as surgery record missing or undefined residual tumor size). Finally, 127 patients with intact clinic features and pathologic records were studied. All enrolled patients underwent at least one cycle of cisplatin based-chemotherapy. The local ethics committee at Peking University People’s Hospital approved this study.
The EOC cases were further divided into type I and type II tumors. Type I included low grade serous, low grade endometrioid, mucinous and clear cell carcinomas. Type II included high grade serous carcinomas, high grade endometrioid, undifferentiated carcinoma and malignant mixed mesodermal tumors (MMMT) [13]. According to the two-tier system, the eligible study population (n=127) was comprised of women with 42 type I and 85 type II EOCs. Patient demographics, cancer stages, grades, histology, surgical procedures, residual disease, adjuvant chemotherapy, recurrence, and survival were collected. Optimal cytoreduction was defined as a residual tumor size of <1 cm. All patients were followed-up from 1 to 5 years, and the median followed-up time was 18.3 months. The endpoint was progression--free survival (PFS).
Then, from the pathology department archives, we obtained 134 ovarian carcinoma tissue specimens from the study population, including 44 type I and 90 type II specimens. Additionally, we obtained 40 matching borderline ovarian tumor specimens. In total, 174 specimens were used to measure the HE4 expression through immunohistochemistry.
Immunohistochemistry
Immunohistochemical studies were performed using 4 μm serial paraffin-embedded sections, with the streptavidin-biotin-peroxidase complex method. Immunohistochemistry was used to evaluate HE4 expression in 174 specimens, according to the manufacturer’s protocol: (1) Tissue slides were deparaffined and hydrated regularly and then incubated for 10 min with deionized water containing 3% H2O2 to constrain endogenous peroxidase; (2) EDTA (pH8.0) restoration solution was applied in a microwave, followed by rinsing with 1×PBS after naturally cooling, and then, 100 μL of rabbit anti-human HE4 polyclonal antibody (Signet Laboratories Inc., USA) in a 1:80 dilution was used for incubation overnight at 4°C. The tissues were rinsed three times in a 1×PBS rinse for 2 min before moving forward to the next procedures. Primary reagent was added for a 20--30 min incubation period at 37°C, and then the same procedure was used for the secondary reagent. Finally, 3,3-diaminobenzidine-hydrogen-peroxide was used as a chromogen for 10 mins. The sections were counterstained with hematoxylin and then fixed and enveloped with ethanol hydrochloride. A 1×PBS rinse was used as a negative control. Human epididymis was used as a positive control, and the human normal ovarian surface tissue as a negative control; the blank control was PBS instead of an HE4 antibody.
Two pathologists, who were blinded to the clinical outcomes of the patients, independently scored the results of the staining. The staining intensity of the tissue specimens was observed through a light microscope, which demonstrated that positive protein expression of HE4 was manifested as brown or reddish brown granules, primarily in the cytoplasm. Eight distinctive fields were randomly selected with a high power lens (400×), and total cells and positive cells were mounted. The score1 was judged by the percentage of positive cells, which ranged from 0--4: 0=0--5% positive cells, 1=5%--25% positive cells, 2=25%--50% positive cells, 3=50%--75% positive cells and 4= above 75% positive cells. Meanwhile, score2 was assessed according to the intensity of staining: 0 (none), 1+ (weak), 2+ (moderate), and 3+ (strong). The immunohistochemical results were based on the multiple of score1 and 2: 0--2 (-), 3--4 (+), 5--8 (2+), 9--12 (3+). For statistical purposes, “+” was considered to be weakly positive, while “2+” and “3+” were referred to as strongly positive.
Statistics analysis
Statistical analyses were performed using SPSS for Windows, version 16.0 (SPSS Inc., Chicago, IL, USA). The patients were classified according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria [14] as complete responders, partial responders (PR), as having stable disease or progressive disease. To estimate continuous variables, Student’s t tests and the Wilcoxon rank-sum test were used. For categorical variables, x2 and Fisher’s exact tests were used. Progression--free survival (PFS) was defined up to the date of the first progression or death or, for living patients, without progression until the date of last contact. Overall survival (OS) was measured up to the date of death due to any cause or, for living patients, the date of last contact. PFS and OS were estimated using the Kaplan-Meier method, and the differences in survival were compared using the log-rank test. All of the P values obtained were two-sided, and P<0.05 was considered to be statistically significant.
Results
The characteristics
Among the 127 patients, there were 42 with type I EOC, including 7 with low grade serous carcinomas, 8 mucinous carcinomas, 12 low grade endometrioid carcinomas and 15 clear cell carcinomas. There were 85 type II EOC, including 83 high grade serous carcinomas and 2 high grade endometrioid carcinomas. The patients ranged in age from 21 to 69 years, and the median age was 48.6 years.
There were 76 postmenopausal patients and 51 premenopausal patients. Among the type I patients, 20 (47.4%) were postmenopausal, and 22 (52.6%) were premenopausal. Among the type II patients, 65.7% were postmenopausal patients. In terms of the menopausal states of the patients, there was a significant difference between the two types of EOC.
According to FIGO stage criteria, 43 patients were in FIGO stage I-II, and 84 patients were in FIGO stage III-IV. Among the type I patients, there were 31 (73.7%) patients in stage I-II and 11 (26.3%) patients in stage III-IV, while among the type II patients, there were 13 (15.5%) patients in stage I-II and 72 (84.5%) patients in stage III-IV. In terms of the FIGO stage, there was a statistically difference between the two EOC types.
The definition of “optimal” is that the residual tumor (RT) was <1 cm of the diameter of the largest nodule. A total of 95 patients had primary optimal cytoreduction, although 32 patients were considered to have unsatisfied primary cytoreduction. In type I patients, there were 8 patients with residual tumor >1 cm, while in type II patients, there were 24 patients with residual tumor >1 cm. In terms of residual tumor, there was no statistically significant differences between the two EOC types.
There were 29 type I patients with grade 1 tumors. However, among the type II patients, only 2 patients had grade 1 tumors. In terms of the pathological differentiation, there was a statistically significant difference between the two EOC types.
Among the type I patients, 37 were completely or partly relieved, while 67 type II patients were completely or partly relieved. There was no statistically significant difference between the two types of EOC patients in terms of the chemotherapy relief (Table 1).
Table 1.
Clinic pathological characteristics in type I and type II EOC
| Characteristics | Type I | Type II | |||
|---|---|---|---|---|---|
|
|
|||||
| Patients number | Percent (%) | Patients number | Percent (%) | P value | |
| Menopausal state | |||||
| Post-menopausal | 20 | 47.4 | 56 | 65.7 | 0.048 |
| Pre-menopausal | 22 | 52.6 | 29 | 34.3 | |
| Differentiation | |||||
| G1 | 28 | 67.5 | 2 | 2 | <0.0001 |
| G2-G3 | 14 | 32.5 | 83 | 98 | |
| FIGO stage | |||||
| Stage I-II | 31 | 73.7 | 13 | 15.5 | <0.0001 |
| Stage III-IV | 11 | 26.3 | 72 | 84.5 | |
| Residual tumor | |||||
| ≥1 cm | 8 | 18.4 | 24 | 28.6 | 0.26 |
| <1 cm | 34 | 81.6 | 61 | 71.4 | |
| Chemotherapy response | |||||
| No relieved | 5 | 12.1 | 18 | 20.7 | 0.20 |
| Complete/partial responder | 37 | 87.9 | 67 | 79.3 | |
Survival analysis
In type I EOC, the median follow-up time was 31months (4-72 m) and the median progression-free survival was 72 months (95% CI: 40.34-103.66). There were 15 (35.7%) relapsed or progressive patients. In type II EOC, the median follow-up time was 17 months (0-60 m), and the median progression-free survival was 27 months (95% CI: 17.83-36.17). There were 47 (55.3%) relapsed or progressive patients. In terms of the progression--free survival time, there was a significant difference between the two types of EOC (P<0.001). In type I EOC, the 1--year, 3--year and 5--year survival rates were 90.47% (38/42), 71.43% (30/42), and 64.29% (27/42), respectively, whereas in type II EOC, the 1--year, 3--year and 5--year survival rates were 77.65% (66/85), 49.41% (42/85), 41.18% (35/85), respectively. In terms of the 1--year survival rate, there was no statistically significant difference between the two types of EOC, but in terms of the 3--year and 5--year survival rates, there were statistically significant differences between the two types of EOC (Table 2).
Table 2.
1--year, 3--year and 5--year survival rates in type I and type II EOC
| Type | 1--year(%) | 3--year(%) | 5--year(%) |
|---|---|---|---|
| Type I | 90.47 | 71.43 | 64.29 |
| Type II | 77.65 | 49.41 | 41.18 |
| P value | 0.91 | 0.023 | 0.023 |
Ovarian serous carcinoma classically represents the theory of a two--tier EOC system. Therefore, in this study, the menopausal state, FIGO stage, chemotherapy resistance and survival were compared between the low grade and high grade serous carcinomas. In terms of the FIGO stage and 5--year survival rate, there were statistically significant differences (P<0.05, data not shown).
Expression of HE4 in type I and type II EOC
In this study, 134 pathologic specimens were obtained from the pathology department, including 44 type I and 90 type II. Moreover, 40 borderline ovarian tumors were chosen from the same department to compare the expression of HE4.
Among the 44 type I specimens, 25 demonstrated positive expression of HE4 (56.8%); in contrast, 78 (86.7%) type II specimens demonstrated positive HE4 expression. There was a significant difference between the type I and type II EOC samples in terms of the expression of HE4 (P=0.0001, Table 3; Figure 1).
Table 3.
HE4 expression in type I and type II EOC
| Total | Positive | Percent (%) | |
|---|---|---|---|
| Type I | 44 | 25 | 56.8 |
| Low grade serous carcinoma | 7 | 2 | 28.6 |
| Mucinous tumor | 9 | 5 | 55.6 |
| Low grade endometrioid carcinoma | 13 | 12 | 92.3 |
| Clear cell carcinoma | 15 | 6 | 40.0 |
| Type II | 90 | 78 | 86.7 |
| High grade serous carcinoma | 88 | 76 | 86.4 |
| High grade endometrioid carcinoma | 2 | 2 | 100 |
Figure 1.
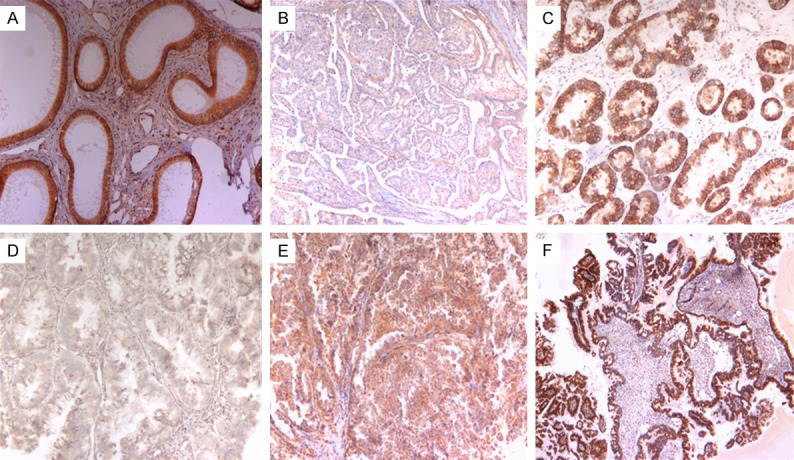
HE4 expression in EOC. A. Positive control: normal epididymis tissue; B. Negative staining of low grade serous carcinoma; C. Positive staining of clear cell carcinoma; D. Negative staining of mucinous carcinoma; E. Weak positive staining of clear cell carcinoma, F. Positive staining of low grade serous carcinoma.
Expression of HE4 in malignant tumor and borderline tumor
According to the two-tier system, type I EOC has been thought to evolve in a stepwise fashion from ovarian epithelial inclusions, benign cystadenomas and borderline tumors. Therefore, we proposed to compare the same pathological specimen types to evaluate this assumption. In this study, 3 types of EOCs were compared, malignant serous carcinoma and borderline serous tumor, malignant mucinous carcinoma and borderline mucinous tumors, malignant endometrioid carcinoma and borderline endometrioid tumors. The results are shown below (Table 4). From the results, it was easy to determine that there was no statistically significant difference between type I EOC and its corresponding borderline tumors, but there was a significant difference between high grade serous carcinoma and borderline serous tumor, which indicated type I EOC and its borderline tumor would have the same pathological origin, but this was not the case for type II EOC and borderline tumors. There was no clear cell bordering tumor in this study, and thus, for this type, the difference cannot be compared.
Table 4.
HE4 expression in malignant and borderline tumor
| Malignant carcinoma | Borderline tumor | P value | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| N | Positive cases | Percent (%) | N | Positive Cases | Percent (%) | ||
| HGS | 88 | 76 | 86.4 | 16 | 7 | 43.8 | <0.0001 |
| LGS | 7 | 2 | 28.6 | 0.66 | |||
| MT | 9 | 5 | 55.6 | 22 | 8 | 36.4 | 0.42 |
| LGEm | 13 | 12 | 92.3 | 2 | 2 | 100 | 0.87 |
HGS = High grade serous carcinoma, LGS = Low grade serous carcinoma, MT = Mucinous tumor, LGEm = low grade endometrioid carcinoma.
Discussion
In terms of molecular genetic alterations, EOC has been assigned into slow--growing type I and aggressive type II tumors. These types are essentially distinct diseases, as indicated by the differences in epidemiological and genetic risk factors, precursor lesions, patterns of spread, and molecular events during oncogenesis, chemotherapy response, and prognosis. In this study, we aimed to evaluate the clinic pathological characteristics and patient prognosis for both type I and type II EOCs. The results demonstrated that there were statistically significant differences between these two types of EOC in terms of the FIGO stage, tumor differentiation, menopausal state and 5--year progression-free survival rate and that these differences reflected the discrepant clinicopathological patterns in these two types of EOC. Steven et al have compared the descriptive epidemiologies of low grade and high grade lesions, and the authors concluded that the epidemiology of low grade tumors appears to be sufficiently different from that of high grade lesions to support the concept that low grade ovarian serous cancers constitute a distinct clinical, and perhaps biological, entity [15].
Note that in this study, we finally obtained 90 ovarian serous carcinomas, of which only 7 low grade serous carcinomas were enrolled. In 2008, Steven et al conducted a large epidemiologic study, which showed that among Asians/Pacific Islanders, the morbidity of low grade serous carcinoma was 0.11/100,000, while the incidence of high grade serous carcinoma was 2.41/100,000 [15]. The incidence ratio was 21.65 (95% CI: 14.38-33.25). However, these results were also observed among Caucasians and Blacks. Although the incidence varied slightly, the trend that high grade lesions occur significantly more frequently than low grade lesions, regardless of race or ethnicity, was obvious. In addition, the following-up results revealed that the median survival time for low grade serous carcinoma was 99 months (95% CI: 92-104), whereas in the high grade serous carcinoma group, the median survival time was only 57 months (95% CI: 56-58). The statistical significant difference was evident. In this study, the survival rates were also compared. The difference between 1--year and 3--year survival rate was not obvious, but there was a difference between the 5--year survival rate. Unfortunately, there were only 7 low grade serous carcinoma patients, and the results may not be objective, which indicates that further study is necessary to confirm this result.
In the following study, HE4 was used to verify the discrepancy between the two types of EOC. Recently, Kristjansdottir B and colleagues have studied the diagnostic performance of the biomarkers HE4 and CA125 in type I and type II EOCs, and they found that the combination of HE4 and CA125 resulted in the best diagnostic power when comparing benign tumors to type II EOC and that the diagnostic safety for the dual markers HE4 and CA125 is not acceptable in the early stage of type I EOC [1]. In the present study, the positive expression of HE4 was more obvious in type II EOC, compared to the expression level in type I EOC. Similarly, the expression levels of HE4 in low grade and high grade serous carcinomas were also compared, and the result was as expected. In low grade serous carcinomas, the positive rate of HE4 expression was only 28.6% (2/7), while the positive rate of HE4 expression reached 86.4% (76/88). Drapkin et al have demonstrated a 93% expression rate for HE4 in high grade serous carcinoma, 100% in high grade endometrioid tumors, and 50% in clear cell tumors, but there was no mucinous staining when studying 92 late stage EOC patients [16]. On the one hand, HE4 might be a promising marker for type II EOC, but on the other hand, the different expression levels of HE4 in the two types of EOC displayed the reliability of the two-tier system.
According to the two-tier system, type I EOC was suggested to behave in a successive and gradual manner and was considered to have its precursor lesion, the borderline tumor. In this study, to verify the stepwise development, the borderline tumor and malignant tumor in the same pathologic origin were compared. The results were encouraging. The differences between borderline tumors and type I EOC was negligible, but the difference between borderline tumor and type II EOC, actually between borderline serous tumor and high grade serous carcinomas was remarkable. HE4 could be a representative biomarker for identifying type II EOC and borderline tumors, but it is not suitable for distinguishing type I EOC from borderline tumors. Some reports have mainly studied the diagnostic performance of HE4 and CA125 in all EOC groups compared to benign cohorts, and they have found less diagnostic power [17-19]. We believe that this result is due to the poor performance of the HE4 biomarker in type I EOC, which was found in this study. We suggest that further research is needed, with a focus on new histology--specific markers to correctly diagnose each subgroup. These findings suggested that different types of ovarian carcinomas develop along different molecular pathways. Type I tumors are clinically indolent and are usually present at low stages. They exhibit a shared lineage between benign cystic neoplasms and the corresponding carcinomas often through an intermediate (borderline tumor) step, supporting the morphological continuum of tumor progression in these neoplasms [3,13]. In contrast, type II tumors are highly aggressive neoplasms, and afflicted patients usually present at an advanced stage. The current preponderance of available evidence supports the premise that a significant subset and possibly all high grade serous carcinomas, the prototypic of type II tumors, originate from the fallopian tube [20-22].
The ultimate goal to increase survival rates for EOC patients is to find early stage lesions, regardless of type. In this study, early stage EOC patients comprised 34.6% of the malignant cohort (24.4% type I and 10.2% type II). The HE4 biomarker tested in this study was inferior in type I EOC diagnostics, but it seemed to be an asset in the diagnosis of type II EOC. The dualistic model highlights the heterogeneity of ovarian carcinoma and indicates that one screening test will not be effective in detecting all types of EOC [13]. Finding early markers that are specific for all histology subgroups is a future challenge. Achieving a better understanding of the pathogenesis, molecular biology, and behavior of EOC is crucial to advance the process of improving early diagnosis and survival for patients with EOC.
Acknowledgements
This study was supported by National Natural Science Foundation of China (NO. 81172454).
Disclosure of conflict of interest
None.
References
- 1.Kristjansdottir B, Levan K, Partheen K, Sundfeldt K. Diagnostic performance of the biomarkers HE4 and CA125 in type I and type II epithelial ovarian cancer. Gynecol Oncol. 2013;131:52–58. doi: 10.1016/j.ygyno.2013.07.094. [DOI] [PubMed] [Google Scholar]
- 2.Prat J. New insights into ovarian cancer pathology. Ann Oncol. 2012;23(Suppl 10):x111–117. doi: 10.1093/annonc/mds300. [DOI] [PubMed] [Google Scholar]
- 3.Kurman RJ, Shih IeM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–443. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seidman JD, Yemelyanova A, Zaino RJ, Kurman RJ. The fallopian tube-peritoneal junction: a potential site of carcinogenesis. Int J Gynecol Pathol. 2011;30:4–11. doi: 10.1097/PGP.0b013e3181f29d2a. [DOI] [PubMed] [Google Scholar]
- 5.Senturk E, Cohen S, Dottino PR, Martignetti JA. A critical re-appraisal of BRCA1 methylation studies in ovarian cancer. Gynecol Oncol. 2010;119:376–383. doi: 10.1016/j.ygyno.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 6.Hellstrom I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellstrom KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 7.Hamed EO, Ahmed H, Sedeek OB, Mohammed AM, Abd-Alla AA, Abdel Ghaffar HM. Significance of HE4 estimation in comparison with CA125 in diagnosis of ovarian cancer and assessment of treatment response. Diagn Pathol. 2013;8:11. doi: 10.1186/1746-1596-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angioli R, Plotti F, Capriglione S, Aloisi A, Montera R, Luvero D, Miranda A, Cafa EV, Damiani P, Benedetti-Panici P. Can the preoperative HE4 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? Gynecol Oncol. 2013;128:579–583. doi: 10.1016/j.ygyno.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 9.Braicu EI, Fotopoulou C, Van Gorp T, Richter R, Chekerov R, Hall C, Butz H, Castillo-Tong DC, Mahner S, Zeillinger R, Concin N, Vergote I, Sehouli J. Preoperative HE4 expression in plasma predicts surgical outcome in primary ovarian cancer patients: results from the OVCAD study. Gynecol Oncol. 2013;128:245–251. doi: 10.1016/j.ygyno.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Chan KK, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, Sabaratnam S, Hebbar S, Sickan J, Schodin BA, Sumpaico WW. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol. 2013;128:239–244. doi: 10.1016/j.ygyno.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 11.Kondalsamy-Chennakesavan S, Hackethal A, Bowtell D, Obermair A. Differentiating stage 1 epithelial ovarian cancer from benign ovarian tumours using a combination of tumour markers HE4, CA125, and CEA and patient’s age. Gynecol Oncol. 2013;129:467–471. doi: 10.1016/j.ygyno.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Brennan DJ, Hackethal A, Metcalf AM, Coward J, Ferguson K, Oehler M, Quinn MA, Janda M, Leung Y, Freemantle M, Webb PM, Spurdle AB, Obermair A. Serum HE4 as a Prognostic Marker in Endometrial Cancer-a Population Based Study. Gynecol Oncol. 2014;132:159–65. doi: 10.1016/j.ygyno.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 13.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 15.Plaxe SC. Epidemiology of low-grade serous ovarian cancer. Am J Obstet Gynecol. 2008;198:459.e451–458. doi: 10.1016/j.ajog.2008.01.035. discussion 459.e458-459. [DOI] [PubMed] [Google Scholar]
- 16.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 17.Partheen K, Kristjansdottir B, Sundfeldt K. Evaluation of ovarian cancer biomarkers HE4 and CA-125 in women presenting with a suspicious cystic ovarian mass. J Gynecol Oncol. 2011;22:244–252. doi: 10.3802/jgo.2011.22.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devan SM, Pailoor J, Sthaneshwar P, Narayanan V. Pattern of Tissue Expression of CA-125 and HE4 in Primary Epithelial Ovarian Tumours and Correlation with Serum CA-125 Levels. Asian Pac J Cancer Prev. 2013;14:4545–4548. doi: 10.7314/apjcp.2013.14.8.4545. [DOI] [PubMed] [Google Scholar]
- 19.Moszynski R, Szubert S, Szpurek D, Michalak S, Krygowska J, Sajdak S. Usefulness of the HE4 biomarker as a second-line test in the assessment of suspicious ovarian tumors. Arch Gynecol Obstet. 2013;288:1377–1383. doi: 10.1007/s00404-013-2901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crum CP, Drapkin R, Kindelberger D, Medeiros F, Miron A, Lee Y. Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res. 2007;5:35–44. doi: 10.3121/cmr.2007.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carlson JW, Miron A, Jarboe EA, Parast MM, Hirsch MS, Lee Y, Muto MG, Kindelberger D, Crum CP. Serous tubal intraepithelial carcinoma: its potential role in primary peritoneal serous carcinoma and serous cancer prevention. J. Clin. Oncol. 2008;26:4160–4165. doi: 10.1200/JCO.2008.16.4814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Fadare O, Xiang L, Kong B, Zheng W. Ovarian serous carcinoma: recent concepts on its origin and carcinogenesis. J Hematol Oncol. 2012;5:8. doi: 10.1186/1756-8722-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


