Abstract
The goal of the present study was to evaluate the clinical and diagnostic value of both serum p53-antibodies (Abs) and preoperative fine needle aspiration cytology (FNAC) for BRAF mutation in patients with papillary thyroid carcinoma (PTC). A total of 312 patients, including thyroid adenoma (85) and PTC (227) were enrolled in this study. Two types of enzyme-linked immunosorbent assays (ELISA), phage-ELISA and p53-ELISA, were used to measure serum p53-Ab levels. Sanger sequencing was used to determine BRAF gene mutation in FNA samples. Phage-ELISA was more efficient than conventional p53-ELISA in measuring serum p53-Abs in PTC patients. BRAF mutation analysis with FNAC significantly improved PTC diagnostic sensitivity from 80.18% to 93.83% (P=0.001) and accuracy from 82.31% to 92.37% (P=0.005). Bothp53-Abs and BRAF mutation were positively associated with lymphatic metastasis and advanced TNM stages. Particularly, serum p53-Abs positively associated with multifocality (P=0.02), while BRAF mutation associated with extrathyoidal extension (P=0.01). Furthermore, PTC patients with both elevated serum p53-Abs and BRAF mutation had a higher prevalence of extrathyoidal extension (P=0.003), lymphnode metastasis (P=0.00), multifocality (P=0.04), and advanced TNM stages (P=0.004). Our results indicate that serum p53-Abs alone might not be a reliable biomarker for PTC diagnosis, but the combined analysis of serum p53-Abs and BRAF mutation in FNAC may be useful for optimizing surgical treatment and prognostic prediction of unfavorable clinicopathologic outcomes.
Keywords: BRAF mutation, p53 antibodies, papillary thyroid carcinoma, fine needle aspiration cytology
Introduction
Thyroid cancer is the most common endocrine malignancy with a rapid rising incidence worldwide in recent years. Papillary thyroid cancer (PTC) is one of the major histological types of thyroid cancer and accounts for 80 to 90% of all thyroid malignancies [1-5]. Therefore, the development of novel strategies for diagnosis and treatment of thyroid cancer is largely dedicated to PTC [6-8].
Extensive efforts have been devoted to search for novel PTC biomarkers, among which serum p53 antibodies (p53-Abs) is a promising target. Since accumulation of mutated, inactive p53 protein is more stable than wild-type p53 protein, p53-Abs has been detected in some cancer patients. Moreover, the positive correlation between p53 mutations, p53 protein accumulation and p53-Abs has also been revealed. The significance and use of p53-Abs as a biomarker of cancer, including PTC, is currently under investigation [9]. However, there is discrepancy between studies on the function of p53-Abs. A previous report suggested that serum p53-Abs may facilitate the early diagnosis of cancer in a subset of smokers with chronic obstructive pulmonary disease, but another study showed prognostic value of serum p53-Abs in lung cancer [10,11]. Thus, whether the presence of p53-Abs correlates with survival of cancer patients is still not clear. Roderick et al. suggested that many anaplastic thyroid carcinoma (ATCs) with papillary components are derived from BRAF-mutated PTC, because of the addition of p53 mutation [12]. Our previous study found thatimmune response is associated with accumulated p53-Abs in PTC, and p53-Abs may be useful as a potential prognostic factor for PTC [13]. On the other hand, the high prevalence of BRAF mutations in the transition from well-differentiated to poorly differentiated and ATCs make it a potentially important marker for tumor diagnosis and prognosis [5,14]. Meanwhile, little is known about the value of the combination detection of BRAF mutation and p53-Abs to make decision for surgery guidelines in PTC.
To this end, we investigated the clinical prognostic value of the combined detection of serum p53-Abs and the BRAFT1799A (V600E) (BRAFV600E) mutation in PTC patients. The aim of our study was to determine whether BRAF mutation and serum p53-Abs could be used to optimize PTC diagnosis and provide surgery guidelines, especially regarding the extent of thyroidectomy and neck dissection. In this study we analyzed the BRAF mutation using fine-needle aspiration cytology (FNAC) and detected serum p53-Abs with phage display technology. In addition, we investigated the correlation of these two biomarkers and clinical parameters in PTC patients.
Materials and methods
Patients, FNA specimens and clinicopathologic data
A total of 312 patients were enrolled in this study, including 85 cases with thyroid adenoma or nodular goiters (25 men, 60 women; median age 50.9, range from 26 to 67) and 227 PTC patients (53 men, 174 women; median age 42.0, range from 18 to 63). All patients were recruited from China-Japan Union Hospital, Changchun, Jilin from December 2013 to April 2014. The records of patient name, age, clinical stage, and lymph node status were collected for this study. Serum samples were obtained before PTC patients received any treatment and those samples were stored at -40°C until used. Clinical staging was defined according to the international TNM classification of Malignant Tumors proposed by American Joint Committee on Cancer (AJCC).
All nodules were collected using ultrasound (US)-guided (US-FNAC) and the same thyroidologist evaluated all samples. Informed consent for FNAC, including the evaluation of BRAF mutation, was obtained from all patients prior to biopsy. The procedure of freehand US-FNAC was performed with a 22G-gauge needle. Each lesion underwent FNA at least twice. Samples were expressed onto glass slides and immediately fixed in 95% alcohol for both Papanicolaou staining and May- Grunwald-Giemsa staining. The remaining specimens were stored at -80°C until used. A pathologist evaluated all slides. All the cases were categorized into 5 groups using The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC): (I) nondiagnostic, (II) benign, (III) atypia/follicular lesion of undetermined significance (AUS/FLUS), (IV) follicular neoplasm/suspicious of follicular neoplasm, (V) suspicious of malignancy, and (VI) malignant [15].
All patients recruited in this study underwent thyroidectomy. Cervical lymph adenectomy was typically performed for treatment of PTC patients with abnormal lymph nodes that were found by intraoperative examination. Meanwhile, tumor tissues taken from the nodule for pathological examination matched those from preoperative FNA. Resected specimens were fixed in 10% formalin, paraffin-embedded, and conserved for BRAF mutation analysis. Data were collected at the China-Japan Union Hospital as described previously. The ethics committee of China-Japan Union Hospital approved our study, and written informed consent was obtained from all patients.
Detection of serum p53-Abs using enzyme-linked immunosorbent assay (ELISA)
Two types of ELISA methods were used in this study. Phage-ELISA: A peptide with a sequence of SDLWKLLP, termed SP, is a 20-27 amino acid domain located at the N-terminal in the p53 protein. This peptide was displayed on the surface of phage following our protocol, which has been proven to bindserum p53-Abs. This phage was prepared and purified according to our previous methods [13]. Phage-ELISA was performed in apolystyrene 96-well microtiter plate (Nunc, Roskilde, Denmark) coated with 50 μl phage (60 μg/ml in 0.05 M carbonate buffer, pH 9.6). Plates were subsequently washed three times with phosphate buffer saline with Tween-20 (PBST) and then twice with PBS. Excess binding sites were blocked by adding 200 μl blocking buffer (5% powdered nonfat milk dissolved in PBS). After washing wells, 50 μl of diluted serum sample (1/200 in blocking buffer) was added and incubated for 1 h at 37°C. Plates were washed and incubated with 50 μl of peroxidase-conjugated goat anti-human IgG Abs (1/5,000 dilution) for 45 min at 37°C. After wash, 100 μl of Tetra-Methyl-Benzidine solution (TMB, AMRESCO, American) was added into the wells. The reaction was stopped by adding 50 μl 2 M H2SO4 into each well. Finally, the absorbance of each well at 450 nm was recorded by a microtiter plate reader (Multiskan Ascent, Labsystems, Finland). All samples were measured twice at different times. Sera from 150 normal volunteers were examined under the optimal conditions of the ELISA to determine the cut-off values [13]. The cut-off value considered as positive in ELISA was conventionally defined as an absorbance value greater than the mean +2 standard deviations (SDs) of the normal cohort. Because hundreds of samples were analyzed at different time periods, each run of ELISA always included 2 control sera. These two sera represented a range above and below the mean of 150 normal people. The average reading value of these two samples was used to normalize all absorbance values measured in different ELISA runs. p53-ELISA. The procedure of p53-ELISA was similar to phage-ELISA, except a different concentration of phage (5 μg/ml) was used for coating [16,17].
BRAF mutation analysis
Samples of FNA and frozen tumor tissue from PTC patients were micro dissected for DNA isolation. Two primers (forward, 50-AATGCTTGCTCTGATAGGAAAA-30; reverse, 50-AG CATCTCAGGGCCAAAAAT-30) were used to amplify a 230 bp fragment of the exon 15 of BRAF that covers the possible mutation site, BRAFV600E. BRAFV600E mutationin PTC patients’ DNA samples obtained by FNA were confirmed through DNA sequencing (Sanger method).
Statistical analysis
The difference of serum p53-Abs in benign and PTC groups between two ELISA methods was statistically analyzed using Chi square test. Correlations of both serum p53-Abs and BRAF mutation with clinicopathologic parameters were evaluated by Chi square test or Fisher’s exact test as appropriate. Statistical analysis was performed using SPSS version 13.0 for Windows (SPSS Inc., Chicago, IL). P<0.05 was considered statistically significant.
To evaluate the diagnostic value of cytology, BRAFV600E mutation and serum p53-Abs in PTC, all results were compared with a “gold standard” (i.e. the postoperative definitive pathologic diagnosis). The true-positive (TP), true-negative (TN), false-positive (FP) and false-negative (FN) results were identified. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy for each detection method were calculated.
Sensitivity=TP/(TP+FN)×100
Specificity=TN/(TN+FP)×100
Positive predictive value (PPV)=TP/(TP+FP)×100
Negative predictive value (NPV)=TN/(TN+FN)×100
Accuracy=(TP+TN)/(TP+TN+FP+FN)×100
Results
Detection of serum p53-Abs in benign and PTC groups by two ELISA methods
Sera from 85 thyroid adenoma patients (benign control group) and 227 PTC patients (PTC group) were examined by two ELISA methods. Both methods demonstrated that the positive rates of p53-Abs in the PTC group were higher than the benign control group (Table 1). There was a statistically significant difference in the p53-Abs detection rate between benign and PTC groups by phage-ELISA (P=0.048), but not with p53-ELISA (P=0.143). The results of the chi-square test showed that in 227 patients, 41 (18.6%) cases were positive with serum p53-Abs detected by either p53-phage-ELISA or p53-ELISA, suggesting that the combination of two ELISA methods might be more effective for identifying positive cases. Also, the number of positive cases in the PTC group was more than that in the benign control group (P=0.032).
Table 1.
The detection rates of serum p53-Abs in benign and PTC groups using two ELISA methods
| Population (rate) with positive p53 Abs in sera | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Group | phage-ELISA | p53-ELISA | Phage-p53-ELISA | ||||
|
|
|||||||
| N | N (%) | P * | N (%) | P * | N (%) | P * | |
| Benign control group | 85 | 4 (4.7) | 0.048 | 5 (5.9) | 0.143 | 7 (8.2) | 0.032 |
| PTC group | 227 | 28 (12.3) | 26 (11.5) | 41 (18.6) | |||
χ2-test. P<0.05 was considered statistically significant.
Diagnostic value of FNAC, BRAFV600E mutation and serum p53-Abs in PTC patients
86 specimens of FNA from PTC patients were used to detect the BRAF mutation. The correlation of BRAF mutation and pathological diagnosis for specimens of FNA or the corresponding primary PTC tumors were investigated in our study. We found that BRAF status in 97.67% (84/86) patients was consistent with their pathological diagnosis, but only two cases were inconsistent with the preoperative FNA cytological diagnoses of ‘a typia lesion of undetermined significance’ and ‘benign’.
The quality of FNAC in 312 thyroid nodules was assessed by parameters including sensitivity (80.18%), specificity (88.24%), PPV (94.79%), NPV (62.50%) and accuracy (82.37%). Supplement of BRAF mutation data in FNAC results significantly improved sensitivity from 80.18% to 90.75% (P=0.001), NPV from 62.5% to 78.13% (P=0.013) and accuracy from 82.37% to 90.06% (P=0.005). The collective evaluation of all three examinations revealed that sensitivity and accuracy were further increased to 93.83% and 92.31%, respectively (Table 2).
Table 2.
Diagnostic values of FNAC, BRAFV600E mutation, and p53-Abs in thyroid nodules
| (pathologic diagnosis/test) | Sensitivity | Specificity | PPV | NPV | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| TP | FN | FP | TN | ||||||
| (+/+) | (+/-) | (-/+) | (-/-) | ||||||
| FNC | 182 | 45 | 10 | 75 | 80.18% | 88.24% | 94.79% | 62.50% | 82.37% |
| P53-Abs | 41 | 186 | 7 | 78 | 18.06% | 91.76% | 85.42% | 29.55% | 38.14% |
| BRAF | 136 | 91* | 0 | 85 | 59.91% | 100.00% | 100.00% | 48.30% | 70.83% |
| FNC+BRAF | 206 | 21 | 10 | 75 | 90.75% | 88.24% | 95.37% | 78.13% | 90.06% |
| FNC+BRAF+p53-Abs | 213 | 14 | 10 | 75 | 93.83% | 88.24% | 95.52% | 84.27% | 92.31% |
There were two cases witha BRAF mutation in the surgical specimens, but no mutation in the matched FNAC specimens.
Role of the BRAFV6000E mutation analysis for cancer diagnosis in surgically proven thyroid nodules
Among 227 nodules that were postoperatively diagnosed as PTC by pathological examination, 182 were found to be malignant upon cytology, in which 112 (112/182, 61.5%) harbored the BRAFV600E mutation. However, BRAFV600E mutation test was especially helpful in the subgroup of indeterminate cytology. The questionable FNAC diagnosis for 42 nodules classified as Bethesda category III (AUS/FLUS) and 34 nodules with suspicious of malignancy were finally addressed through surgery. In these nodules, the PPV of BRAFV6000E mutation was 100% (Table 3).
Table 3.
Role of BRAFV6000E mutation for cancer diagnosis in surgically proven thyroid nodules
| Diagnostic Category | BRAFV6000E mutation | No.of malignancy | Cancer Probability (%) |
|---|---|---|---|
| I (n=5) | Mutation positive (n=0) | - | |
| Mutation negative (n=5) | 0 | 0 | |
| II (n=39) | Mutation positive (n=1) | 1 | 100 |
| Mutation negative (n=38) | 3* | 7.9 | |
| III (n=42) | Mutation positive (n=7) | 7 | 100 |
| Mutation negative (n=35) | 5* | 14.2 | |
| IV (n=0) | Mutation positive (n=0) | - | |
| Mutation negative (n=0) | - | ||
| V (n=34) | Mutation positive (n=16) | 16 | 100 |
| Mutation negative (n=18) | 13 | 72.2 | |
| VI (n=192) | Mutation positive (n=112) | 112 | 100 |
| Mutation negative (n=80) | 70 | 87.5 |
**There were two cases witha BRAF mutation in the surgical specimens, but no mutation in the matched FNAC specimens.
Preoperative combined detection of BRAF mutation and p53-Abs predicts poorer PTC clinicopathologic outcomes
The correlation between serum p53-Abs, BRAF mutation and clinicopathologic parameters in PTC patientsis shown in Table 3. There was a higher prevalence of BRAF mutation in elderly men. Although increased serum p53-Abs was also detected in the elderly patient group (≥45 years), this was not statistically significant. There was a significant difference between micropapillary thyroid cancer (tumor size ≤1 cm) and PTC (tumor size >1 cm) in PTC patients with both BRAF mutation and serum p53-Abs (Table 4). The clinicopathologial characteristics that were associated with BRAF mutation were almost the same as those in patients with serum p53-Abs, except for multifocality (P=0.02). BRAF mutation correlated positively with extrathyoidal extension in PTC patients (P=0.01). In our study, 73.2% (30/41) patients were positive serum p53-Abs and harbored a BRAF mutation. In the p53-Abs negative group, only 58.1% (108/186) of the cases had a BRAF mutation, implying that the BRAF mutation occurred more frequently in p53-Abs positive patients (P=0.073). Our study found 31 positive cases of both BRAF mutation and serum p53-Abs and 117 cases that were positive for either BRAF or p53-Abs. Furthermore, we found a higher prevalence of extrathyoidal extension (P=0.003), lymphnode metastasis (P=0.02), multifocality (P=0.04) and advanced TNM stages (P=0.004) in patients with double positive results for detection of BRAF mutation and serum p53-Abs (Figure 1).
Table 4.
Correlation of BRAF mutation and serum p53-Abs with PTC clinicopathologic characteristics
| Characteristics | p53-Abs+ | p53-Abs- | P value | BRAF+ | BRAF- | P value |
|---|---|---|---|---|---|---|
| [n (%)] | [n (%)] | [n (%)] | [n (%)] | |||
| Age (years) | ||||||
| <45 | 18 (43.9) | 11 (59.7) | 0.06 | 76 (55.1) | 53 (59.6) | 0.51 |
| ≥45 | 23 (56.1) | 75 (40.3) | 62 (44.9) | 36 (40.4) | ||
| Gender | ||||||
| Male | 11 (26.8) | 42 (22.6) | 0.56 | 35 (25.4) | 18 (20.2) | 0.37 |
| Female | 30 (73.2) | 144 (77.4) | 103 (74.6) | 71 (79.8) | ||
| Tumor size (cm) | ||||||
| ≤1 | 23 (56.1) | 135 (72.6) | 0.04 | 82 (59.4) | 76 (85.4) | 0 |
| >1 | 18 (43.9) | 51 (27.4) | 56 (40.6) | 13 (14.6) | ||
| Extrathyoidal extension | 11 (26.8) | 41 (22.0) | 0.51 | 40 (29.0) | 12 (13.5) | 0.01 |
| Lymphnode metastasis | 23 (56.1) | 64 (34.4) | 0.01 | 68 (49.3) | 19 (21.3) | 0 |
| TNM stages | ||||||
| I/II | 32 (78.0) | 167 (89.8) | 0.04 | 116 (84.1) | 83 (93.3) | 0.04 |
| III/IV | 9 (22.0) | 19 (10.2) | 22 (15.9) | 6 (6.7) | ||
| Multifocality | 24 (58.5) | 73 (39.2) | 0.02 | 61 (44.2) | 36 (40.4) | 0.58 |
Figure 1.
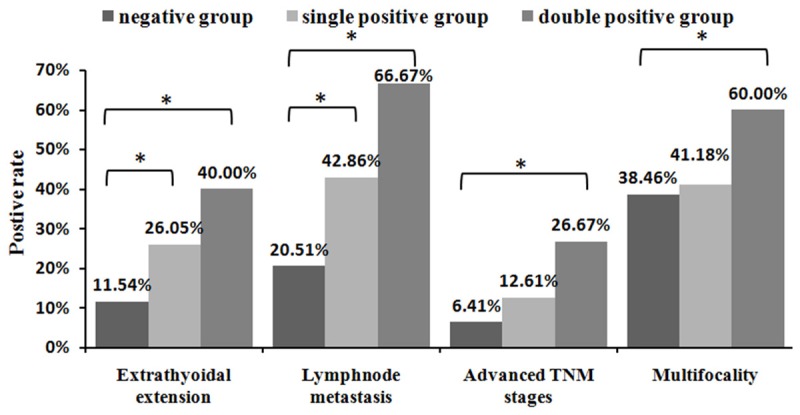
Comparison of the differences of PTC clinicopathologic characteristics between negative, single positive and double positive. groups. *Significance of the difference (P<0.05) between 2 groups determined by chi-square test.
Discussion
To improve the sensitivity of the detection assays of serum p53-Abs, we developed a phage-ELISA that displays a peptide that belongs to the immunodominant regions of the amino-terminal part of p53 protein on the surface of phage. This phage-ELISA was selected for our study. Results showed that the efficiency of phage-ELISA to detect serum p53-Abs is better than p53-ELISA. This finding is consistent with previously published studies [18,19]. It is known that the peptides displayed on the surface of phage could effectively simulate natural epitope, and the phage display system allows better surface-exposure of the displayed peptides [13]. In our study, serum p53-Abs were detected by two ELISA methods in 41 (18.6%) PTC patients. The percentage of p53-Abs positive cases in the PTC group was significantly higher than that in the benign group. Our study confirmed that phage display technology improves detection of serum p53-Abs.
Our results also showed that, as a biomarker, the sensitivity, specificity and PPV of p53-Abs were 18.06%, 91.76% and 85.42%, respectively. Detection of serum p53-Abs in PTC was less sensitive than FNAC (18.06% vs 80.18%), but the specificity of serum p53-Abs in PTC was higher than FNAC (91.76% vs 88.24%). We also found that the sensitivity and specificity of BRAF mutation was superior to serump53-Abs in PTC. PTC patients with a high PPV (100%) of BRAFV6000E mutation and an indeterminate FNAC may be particularly benefited by surgical treatment. The sensitivity, NPV and accuracy of BRAFV6000E mutation analysis alone were 59.91% 48.30%, and 70.83%, respectively. The combination of cytological diagnosis and BRAFV6000E mutation analysis could significantly improve sensitivity, NPV and accuracy. Although incorporation of p53-Abdetection in BRAF mutation analysis and FNAC could improve diagnose of PTC, the combination of these three parameters was not significant compared to the combination of BRAF mutation analysis and FNAC. In the management of PTC, the diagnostic value of p53-Abs might be less than the value of BRAFV6000E mutation analysis, which is a useful adjunct to cytology for cancer diagnosis [20-24]. These results indicate that serum p53-Abs alone is not a useful marker for PTC diagnosis. The application of BRAFV6000E mutation analysis in US-FNAC may optimize the diagnostic accuracy of thyroid nodules.
Several clinical factors have been shown to be associated with a poorer PTC prognosis, such as advanced age, male sex, tumor size, extrathyoidal extension, lymphnode metastasis, multifocality and advanced TNM stages [6,14,25-29]. Our study also demonstrated a close association of BRAF mutation with extrathyroidal extension, lymph node metastasis, and advanced TNM stages III/IV of PTC. We also found that serum p53-Abs was associated with aggressive pathological outcomes in PTC, such as multifocality, lymphnode metastasis, and high TNM stages. Finally, serum p53-Abs and BRAFV6000E mutation were positively correlated with poor PTC clinicopathologic outcomes. Therefore, both have prognostic value for PTC patients. As compared to those with single abnormality, PTC patients with both a BRAF mutation and serum p53-Abs may have a higher prevalence of clinicopathologic parameters, especially lymphnode metastasis (P=0.02). Preoperative detection of these two markers may be useful for making a surgical decision of total thyroidectomy or prophylactic central neck dissection. Therefore, the BRAFV600E mutation and serum p53-Abs may be important molecular markers for optimizing PTC therapeutic strategies.
Additionally, the cross-talk between the BRAF mutation and p53-Abs was also observed in our study. We found that BRAFV6000E mutation frequency in the p53 positive nodules was higher than the frequency in p53 negative nodules, although there was no significant correlation of BRAFV6000E mutation and p53 Abs.
In summary, our study revealed: (1) Detection of serum p53-Abs using a combination of two ELISA methods could identify more p53-Abs positive cases in PTC patients. (2) Serum p53-Abs alone is not useful preoperative biomarker for PTC diagnosis, but US-FNAC plus BRAFV600E mutation analysis might significantly optimize the diagnostic accuracy of thyroid nodules. (3) Preoperative combined detection of serum p53-Abs and FNAC plus BRAF mutation analysis may be useful for optimizing surgical therapy of PTC and predicting poorer PTC clinicopathologic outcomes in patients. Therefore, serum p53-Abs and BRAFV6000E mutation could be considered complementary biomarkers for PTC diagnosis and prognosis.
Acknowledgements
This study was supported by Grant 20140414063GH and 20150520149JH.
Disclosure of conflict of interest
None.
References
- 1.Leenhardt L, Grosclaude P, Chérié-Challine L. Increased incidence of thyroid carcinoma in France: a true epidemic or thyroid nodule management effects? Report from the French Thyroid Cancer Committee. Thyroid. 2004;14:1056–1060. doi: 10.1089/thy.2004.14.1056. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the US, 1985-1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association (ATA) guidelines taskforce on thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Ranjbari N, Almasi S, Mohammadi-Asl J, Rahim F. BRAF mutations in Iranian patients with papillary thyroid carcinoma. Asian Pac J Cancer Prev. 2013;14:2521–2523. doi: 10.7314/apjcp.2013.14.4.2521. [DOI] [PubMed] [Google Scholar]
- 6.Xing M. Prognostic utility of BRAF mutation in papillary thyroid cancer. Mol Cell Endocrinol. 2010;321:86–93. doi: 10.1016/j.mce.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen CT, Qiu ZL, Luo QY. Efficacy and safety of selumetinib compared with current therapies for advanced cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:2369–2374. doi: 10.7314/apjcp.2014.15.5.2369. [DOI] [PubMed] [Google Scholar]
- 8.Zhou YL, Zhang W, Gao EL, Dai XX, Yang H, Zhang XH, Wang OC. Preoperative BRAF mutation is predictive of occult contralateral carcinoma in patients with unilateral papillary thyroid microcarcinoma. Asian Pac J Cancer Prev. 2012;13:1267–1272. doi: 10.7314/apjcp.2012.13.4.1267. [DOI] [PubMed] [Google Scholar]
- 9.Soussi T. p53 Antibodies in the sera of patients with various types of cancer: a review. Cancer Res. 2000;60:1777–1788. [PubMed] [Google Scholar]
- 10.Lubin R, Zalcman G, Bouchet L, Trédanel J, Legros Y, Cazals D, Hirsch A, Soussi T. Serum p53 antibodies as early markers of lung cancer. Nat Med. 1995;1:701–702. doi: 10.1038/nm0795-701. [DOI] [PubMed] [Google Scholar]
- 11.Zalcman G, Schlichtholz B, Trédaniel J, Urban T, Lubin R, Dubois I, Milleron B, Hirsch A, Soussi T. Monitoring of p53 autoantibodies in lung cancer during therapy: relationship to response to treatment. Clin Cancer Res. 1998;4:1359–1366. [PubMed] [Google Scholar]
- 12.Quiros RM, Ding HG, Gattuso P, Prinz RA, Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 13.Pan P, Han X, Li F, Fu Q, Gao X, Sun H, Wang L. Detection of serum p53 antibodies from Chinese patients with papillary thyroid carcinoma using phage-SP-ELISA: correlation with clinical parameters. Endocrine. 2014;47:543–549. doi: 10.1007/s12020-014-0243-9. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 15.Cibas ES, Ali SZ. The Bethesda System For Reporting Thyroid Cytopathology. Am J Clin Pathol. 2009;132:658–665. doi: 10.1309/AJCPPHLWMI3JV4LA. [DOI] [PubMed] [Google Scholar]
- 16.Gao RJ, Bao HZ, Yang Q, Cong Q, Song JN, Wang L. The presence of serum anti-p53 antibodies from patients with invasive ductal carcinoma of breast: correlation to other clinical and biological parameters. Breast Cancer Res Treat. 2005;93:111–115. doi: 10.1007/s10549-005-4321-9. [DOI] [PubMed] [Google Scholar]
- 17.Qiu LL, Hua PY, Ye LL, Wang YC, Qiu T, Bao HZ, Wang L. The detection of serum anti-p53 antibodies from patients with gastric carcinoma in China. Cancer Detect Prev. 2007;31:45–49. doi: 10.1016/j.cdp.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Schlichtholz B, Trédaniel J, Lubin R, Zalcman G, Hirsch A, Soussi T. Analyses of p53 antibodies in sera of patients with lung carcinoma define immunodominant regions in the p53 protein. Br J Cancer. 1994;69:809–816. doi: 10.1038/bjc.1994.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubin R, Schlichtholz B, Bengoufa D, Zalcman G, Trédaniel J, Hirsch A, Fromentel C, Preudhomme C, Fenaux P, Fournier G, Mangin P, Laurent-Puig P, Pelletier G, Schlumberger M, Desgrandchamps F, Duc AL, Peyrat JP, Janin N, Bressac B, Soussi T. Analysis of p53 antibodies in patients with various cancers define B-cell epitopes of human p53: distribution on primary structure and exposure on protein surface. Cancer Res. 1993;53:5872–5876. [PubMed] [Google Scholar]
- 20.Di Benedetto G. Thyroid fine-needle aspiration: the relevance of BRAF mutation testing. Endocrine. 2014;47:351–353. doi: 10.1007/s12020-014-0222-1. [DOI] [PubMed] [Google Scholar]
- 21.Xing M, Clark D, Guan H, Ji M, Dackiw A, Carson KA, Kim M, Tufaro A, Ladenson P, Zeiger M, Tufano R. BRAF mutation testing of thyroid fine-needle aspiration biopsy specimens for preoperative risk stratification in papillary thyroid cancer. J. Clin. Oncol. 2009;27:2977–2982. doi: 10.1200/JCO.2008.20.1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra A, Di Stasi V, Zeppa P, Faggiano A, Marotta V, Vitale M. BRAF(V600E) assessment by pyrosequencing in fine needle aspirates of thyroid nodules with concurrent Hashimoto’s thyroiditis is a reliable assay. Endocrine. 2014;45:249–255. doi: 10.1007/s12020-013-9994-y. [DOI] [PubMed] [Google Scholar]
- 23.Marchetti I, Iervasi G, Mazzanti CM, Lessi F, Tomei S, Naccarato AG, Aretini P, Alberti B, Di Coscio G, Bevilacqua G. Detection of the BRAF(V600E) mutation in fine needle aspiration cytology of thyroid papillary microcarcinoma cells selected by manual macrodissection: an easy tool to improve the preoperative diagnosis. Thyroid. 2012;22:292–298. doi: 10.1089/thy.2011.0107. [DOI] [PubMed] [Google Scholar]
- 24.Kumagai A, Namba H, Akanov Z, Saenko VA, Meirmanov S, Ohtsuru A, Yano H, Maeda S, Anami M, Hayashi T, Ito M, Sagandikova S, Eleubaeva Z, Mussinov D, Espenbetova M, Yamashita S. Clinical implications of pre-operative rapid BRAF analysis for papillary thyroid cancer. Endocr J. 2007;54:399–405. doi: 10.1507/endocrj.k06-194. [DOI] [PubMed] [Google Scholar]
- 25.Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. doi: 10.1210/jc.2003-030838. [DOI] [PubMed] [Google Scholar]
- 26.Namba H, Nakashima M, Hayashi T, Hayashida N, Maeda S, Rogounovitch TI, Ohtsuru A, Saenko VA, Kanematsu T, Yamashita S. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab. 2003;88:4393–4397. doi: 10.1210/jc.2003-030305. [DOI] [PubMed] [Google Scholar]
- 27.Kim J, Giuliano AE, Turner RR, Gaffney RE, Umetani N, Kitago M, Elashoff D, Hoon DS. Lymphatic mapping establishes the role of BRAF gene mutation in papillary thyroid carcinoma. Ann Surg. 2006;244:799–804. doi: 10.1097/01.sla.0000224751.80858.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim KH, Kang DW, Kim SH, Seong IO, Kang DY. Mutations of the BRAF gene in papillary thyroid carcinoma in a Korean population. Yonsei Med J. 2004;45:818–821. doi: 10.3349/ymj.2004.45.5.818. [DOI] [PubMed] [Google Scholar]
- 29.Lee JH, Lee ES, Kim YS, Won NH, Chae YS. BRAF mutation and AKAP9 expression in sporadic papillary thyroid carcinomas. Pathology. 2006;38:201–204. doi: 10.1080/00313020600696264. [DOI] [PubMed] [Google Scholar]


