Abstract
Kushen (Radix Sophorae Flavescentis) is the dried roots of Sophora Flavescens Ait, alkaloids and flavonoids are the main active constituents of Radix Sophorae Flavescentis. The influence of Radix Sophorae Flavescentis on the activities of CYP450 isoforms CYP2B6, CYP2C19, CYP1A2, CYP2C9, CYP3A4 and CYP2D6 were evaluated by cocktail method. The rats were randomly divided into Radix Sophorae Flavescentis group and control group. The Radix Sophorae Flavescentis group rats were given 5 g/kg Radix Sophorae Flavescentis decoction by intragastric administration. The six probe drugs (bupropion, omeprazole, phenacetin, tolbutamide, midazolam and metroprolol) were given to rats through intragastric administration, and the plasma concentration were determined by UPLC-MS/MS. The result of Radix Sophorae Flavescentis group compared to control group, there were statistical pharmacokinetics difference for omeprazole, phenacetin, tolbutamide and metroprolol. It indicated that the Radix Sophorae Flavescentis may induce the activities of CYP2D6, and inhibit of CYP2C19, CYP1A2 and CYP2C9 of rats. As other drugs are always used after Radix Sophorae Flavescentis, interactions between other drugs and Radix Sophorae Flavescentis undertake the risk of either diminished efficacy or adverse effects. This may give advising for reasonable drug use after Radix Sophorae Flavescentis.
Keywords: CYP450, Radix Sophorae Flavescentis, cocktail, rat
Introduction
Kushen (Radix Sophorae Flavescentis) is the dried roots of Sophora Flavescens Ait, alkaloids and flavonoids are the main active constituents of Radix Sophorae Flavescentis, which mainly include benzoic acids, isoflavone glycosides and alkaloids. Modern pharmacological experiments show that the alkaloids in Kushen (Radix Sophorae Flavescentis) have a variety of pharmacological activities, including anti-tumour, antibacterial, anti-inflammatory and analgesic effects [1,2]. Cytochromes P450 (CYP) constitutes the major drug-metabolizing enzyme system in human beings. A large number of drugs are metabolized by CYP enzymes in the liver, and more than 90% of drug-drug interactions occur at the CYP-catalyzed step [3-5]. The activity of these enzymes is subjected to a great interindividual variability which could cause interindividual differences in plasma drug concentrations and result in therapeutic failure or side effects [6]. To avoid these problems, it is of great importance to evaluate the in vivo CYP activity (phenotyping).
In this paper, six probe drugs are used to evaluate the induction or inhibition effects of Radix Sophorae Flavescentis on the activities of rats CYP450 isoforms such as CYP2B6, CYP2C19, CYP1A2, CYP2C9, CYP3A4 and CYP2D6 in rats. According to the changes of pharmacokinetic parameters of six specific probe drugs, it may provide rational drug guidance use after Radix Sophorae Flavescentis.
Material and methods
Chemicals
Bupropion, omeprazole, phenacetin, tolbutamide, midazolam, metroprolol (purity, all > 98%) and the internal standard diazepam were obtained from Sigma-Aldrich Company (St. Louis, USA). Ultra-pure water was prepared by Millipore Milli-Q purification system (Bedford, USA). Methanol and acetonitrile (HPLC grade) were obtained from Merck Company (Darmstadt, Germany).
Animals
Sprague-Dawley rats (male, 220±20 g) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd. Animals were housed under a natural light-dark cycle conditions with controlled temperature (22°C). All twenty rats were housed at Wenzhou Medical University Laboratory Animal Research Center. All experimental procedures were approved ethically by the Wenzhou Medical University Administration Committee of Experimental Animals.
Radix Sophorae Flavescentis decoction
These raw materials were obtained from the Second Affiliated Hospital & Yuying Children’s Hospital of Wenzhou Medical University, China, and stored in an environment of normal atmospheric pressure and decoction at 100°C for 30 minutes, and then the residues were discarded, the final decoction concentration was fixed at 2.5 g/mL. The decoction was stored at 4°C.
UPLC-MS/MS determination of probe drugs
The concentration of bupropion, omeprazole, phenacetin, testosterone, tolbutamide and metroprolol in rat plasma were simultaneously determined by a sensitive and simple UPLC-MS/MS method [7]. UPLC-MS/MS with ACQUITY I-Class UPLC and a XEVO TQD triple quadrupole mass spectrometer equipped with an electrospray ionization (ESI) interface (Waters Corp., Milford, MA, USA) were used to analyze the compounds. The UPLC system was comprised of a Sample Manager with Flow-Through Needle (SM-FTN) and a Binary Solvent Manager (BSM). The Masslynx 4.1 software was used for data acquisition and instrument control (Waters Corp., Milford, MA, USA).
Pharmacokinetics
Twenty rats (220±20 g) were randomly divided to Radix Sophorae Flavescentis group and control group. Radix Sophorae Flavescentis group were give Radix Sophorae Flavescentis decoction (5 g/kg) by intragastric administration. Control group were give saline by intragastric administration. After 7 days, the Radix Sophorae Flavescentis and control group intragastric administration of six probe drugs (bupropion, omeprazole, phenacetin, tolbutamide, midazolam and metroprolol, 10, 10, 10, 1, 10 and 10 mg/kg).
Blood (0.2 mL) samples were collected at 0.0833, 0.5, 1, 2, 3, 4, 6, 8, 12, 24, 36 h from the tail vein into heparinized 1.5 mL polythene tubes after intragastric administration of six probe drugs. The 50 μL plasma was obtained from blood sample after centrifuged at 4000 g for 10 min. In a 1.5 mL centrifuge tube, 50 μL of collected plasma sample followed by the addition of 150 μL of acetonitrile (containing 50 ng/mL IS). After vortex-mixed for 1.0 min, the sample was centrifuged at 13000 g for 15 min. Then the 2 µL supernatant was injected into the UPLC-MS/MS system for analysis.
Plasma probe drugs concentration versus time was analyzed by Version 3.0 Data Analysis System (Wenzhou Medical University, China). The main pharmacokinetic parameters of the Radix Sophorae Flavescentis group and control group were analyzed by SPSS l8.0 statistical software, statistical significance was assessed by t-test (P<0.05 was considered as statistically significant).
Results
UPLC-MS/MS method
The LLOQ for each probe drug in plasma was 2 ng/mL. The RSD of the six probe drugs were less than 10%. The calibration plot of the probe drugs is in the range of 2-2000 ng/mL (r>0.995). The intra-day and inter-day accuracy ranged from 85% to 110%. The matrix effects were more than 85% or less than 112%. The extraction recoveries were better than 83%. To this effect, the established method is suitable for pharmacokinetic study.
Pharmacokinetics
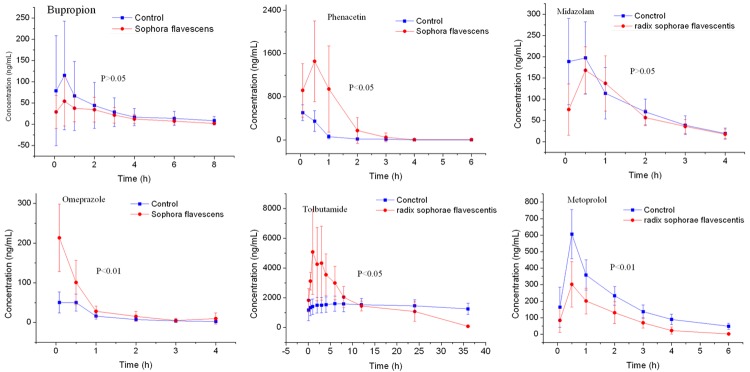
The main pharmacokinetic parameters of bupropion, omeprazole, phenacetin, tolbutamide, midazolam and metroprolol were summarized from non-compartment model analysis in Tables 1, 2 and 3. The representative bupropion, omeprazole, phenacetin, tolbutamide, midazolam and metroprolol concentration vs. time profiles were presented in Figure 1. As could be seen from Figure 1, the Cmax and AUC of omeprazole, phenacetin, tolbutamide in Radix Sophorae Flavescentis group is higher than the control group, while the Cmax and AUC of bupropion, metroprolol is lower than the control group.
Table 1.
Pharmacokinetic parameters of bupropion and omeprazole in control-group and Radix sophorae flavescentis-group rats (mean ± SD, n=10)
| Parameters | AUC(0-t) | AUC(0-∞) | t1/2 | CL | V | Cmax | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| ng/mL*h | ng/mL*h | h | L/h/kg | L/kg | ng/mL | ||
| Bupropion | Control | 255.7±297.7 | 284.7±333.7 | 2.5±0.5 | 88.6±65.7 | 337.0±326.7 | 131.4±146.2 |
| Radix sophorae flavescentis | 151.1±133.9 | 156.8±135.9 | 1.6±0.7* | 93.2±44.2 | 225.5±154.5 | 62.1±59.1 | |
| Omeprazole | Control | 58.8±13.6 | 62.9±16.4 | 0.8±0.7 | 169.0±46.6 | 182.6±114.9 | 63.0±22.2 |
| Radix sophorae flavescentis | 144.5±59.4** | 147.4±61.5** | 0.6±0.1 | 86.5±59.9* | 77.9±61.3 | 213.4±84.8** | |
Compared Radix sophorae flavescentis group with the control group;
P<0.05;
P<0.01.
Table 2.
Pharmacokinetic parameters of and phenacetin and tolbutamide in control-group and Radix sophorae flavescentis -group rats (Mean ± SD, n=10)
| Parameters | AUC(0-t) | AUC(0-∞) | t1/2 | CL | V | Cmax | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| ng/mL*h | ng/mL*h | h | L/h/kg | L/kg | ng/mL | ||
| Phenacetin | Control | 376.3±130.5 | 380.1±127.1 | 1.4±0.9 | 28.9±9.6 | 66.4±57.5 | 546.5±109.0 |
| Radix sophorae flavescentis | 1849.5±1129.7* | 1851.1±1129.6* | 0.7±0.3 | 7.5±4.4** | 7.2±5.8* | 1530.9±658.8* | |
| Tolbutamide | Control | 52607.0±13518.7 | 193505.7±148512.1 | 65.6±50.2 | 0.008±0.008 | 0.535±0.260 | 1893.6±560.8 |
| Radix sophorae flavescentis | 56765.5±21022.1 | 57620.5±21275.2* | 5.7±1.7* | 0.020±0.007* | 0.157±0.059** | 5336.6±2942.6** | |
Compared Radix sophorae flavescentis group with the control group;
P<0.05;
P<0.01.
Table 3.
Pharmacokinetic parameters of midazolam and metroprolol in control-group and Radix sophorae flavescentis-group rats (Mean ± SD, n=10)
| Parameters | AUC(0-t) | AUC(0-∞) | t1/2 | CL | V | Cmax | |
|---|---|---|---|---|---|---|---|
|
| |||||||
| ng/mL*h | ng/mL*h | h | L/h/kg | L/kg | ng/mL | ||
| Midazolam | Control | 342.1±154.4 | 375.1±173.4 | 1.1±0.2 | 37.7±32.1 | 53.3±36.8 | 214.9±89.3 |
| Radix sophorae flavescentis | 300.9±78.1 | 332.1±89.2 | 1.1±0.3 | 31.9±8.7 | 51.0±21.9 | 180.6±59.6 | |
| Metroprolol | Control | 1139.5±249.1 | 1241.6±293.0 | 1.6±0.2 | 8.4±1.9 | 19.2±4.4 | 606.0±149.2 |
| Radix sophorae flavescentis | 538.2±234.0** | 551.6±226.8** | 0.8±0.2** | 22.9±15.0* | 31.6±30.9 | 304.2±133.2** | |
Compared Radix sophorae flavescentis group with the control group;
P<0.05;
P<0.01.
Figure 1.
The pharmacokinetics profiles of bupropion, omeprazole, phenacetin, tolbutamide, midazolam and metroprolol in control-group and Radix Sophorae Flavescentis-grouprats (n=10).
Discussion
As other drugs are always used after Radix Sophorae Flavescentis, interactions between other drugs and Radix Sophorae Flavescentis undertake the risk of either diminished efficacy or adverse effects. Drug-drug interactions often occur at the active site of these enzymes since CYP450 enzymes play a key role in the phase I metabolism of the majority of all marketed drugs.
In general, changes in pharmacokinetics are thought to be caused by drug-drug or drug-food interactions [8]. In pharmacokinetic interactions, approximately 65% of drug-drug interactions occur in metabolic sites, and drug metabolic enzymes are considered to be the most important interactive sites. Similarly, supplement-drug interactions involving CYP activity are occasionally found to cause considerable adverse events. For these reasons, we evaluated the effects of Radix Sophorae Flavescentis on the activity of CYP enzymes in vivo. We selected CYP isoforms CYP2B6, CYP2C19, CYP1A2, CYP2C9, CYP3A4 and CYP2D6, which more than 90% of drugs are known to be metabolized by these 6 CYP enzymes [9-13].
As can be seen from Table 1, the pharmacokinetic parameters of bupropion and omeprazole have changed, AUC(0-t) decreased (P>0.05), CL increased (P>0.05), Cmax decreased (P>0.05) for bupropion, compared Radix Sophorae Flavescentis group with the control group; AUC(0-t) increased (P<0.01), CL decreased (P<0.05), Cmax increased (P<0.01) for omeprazole. It indicates that the Radix Sophorae Flavescentis on rats may not inhibit or induce the activity of CYP2B6 enzyme and inhibit CYP2C19 enzyme of rats. The above results showed that when Radix Sophorae Flavescentis used incombination with other drugs which metabolized by the CYP2C19, the potential herb-drug interactions would be pay more attention so as to reduce some adverse reactions due to high plasma concentration.
As can be seen from Table 2, the pharmacokinetic parameters of phenacetin and tolbutamide have changed, AUC(0-t) increased (P<0.05), CL decreased (P<0.01), Cmax increased (P<0.05) for phenacetin, compared Radix Sophorae Flavescentis group with the control group; AUC(0-∞) increased (P<0.05), CL decreased (P<0.05), Cmax increased (P<0.01) for tolbutamide. It indicates that the Radix Sophorae Flavescentis may inhibit the activity of CYP1A2 and CYP2C9 enzyme of rats. The above results showed that when Radix Sophorae Flavescentis used incombination with other drugs which metabolized by the CYP1A2 and CYP2C9, the potential herb-drug interactions would be pay more attention so as to reduce some adverse reactions due to high plasma concentration.
As can be seen from Table 3, the pharmacokinetic parameters of midazolam and metroprolol have changed, AUC(0-t) decreased (P>0.05), CL decreased (P>0.05), Cmax decreased (P>0.05) for midazolam, compared Radix Sophorae Flavescentis group with the control group; AUC(0-t) decreased (P<0.01), CL increased (P<0.05), Cmax increased (P<0.01) for metroprolol. It indicates that the Radix Sophorae Flavescentis could not influence the activity of CYP3A4 and may induce the activity of CYP2D6 enzyme in rats. The above results showed that when Radix Sophorae Flavescentis used in combination with other drugs which metabolized by the CYP2D6, the potential herb-drug interactions would be pay more attention so as to the failure in treatment due to low plasma concentration.
Conclusion
In our study, Radix Sophorae Flavescentis (5 g/kg) may induce the activities of CYP450 isoforms CYP2D6 of rats, and may inhibit of CYP2C19, CYP1A2 and CYP2C9 of rats. These results would give us valuable information regarding the interactions of Radix Sophorae Flavescentis with drugs, drugs used after Radix Sophorae Flavescentis might cause pharmacokinetic interactions, which required dose adjustment to avoid over dosage or reduced plasma concentration.
Acknowledgements
This study was supported by grants from the Wenzhou Municipal Science and Technology Bureau project Funding, Y20140223; the Youth Talent Program Foundation of The First Affiliated Hospital of Wenzhou Medical University (qnyc043); the incubator project of The First Affiliated Hospital of Wenzhou Medical University (FHY2014023).
Disclosure of conflict of interest
None.
References
- 1.Jin JH, Kim JS, Kang SS, Son KH, Chang HW, Kim HP. Anti-inflammatory and anti-arthritic activity of total flavonoids of the roots of Sophora flavescens. J Ethnopharmacol. 2010;127:589–595. doi: 10.1016/j.jep.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Hwang GB, Lee JE, Nho CW, Lee BU, Lee SJ, Jung JH, Bae GN. Short-term effect of humid airflow on antimicrobial air filters using Sophora flavescens nanoparticles. Sci Total Environ. 2012;421-422:273–279. doi: 10.1016/j.scitotenv.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 3.Lin GY, Ma JS, Xu RA, Hu LF, Wang Z, Wang XQ. Effects of Ougan juice on P450 activities using a cocktail method. Pharmazie. 2012;67:242–246. [PubMed] [Google Scholar]
- 4.Qin CZ, Ren X, Tan ZR, Chen Y, Yin JY, Yu J, Qu J, Zhou HH, Liu ZQ. A high-throughput inhibition screening of major human cytochrome P450 enzymes using an in vitro cocktail and liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2014;28:197–203. doi: 10.1002/bmc.3003. [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Chen X, Chen M, Hu G, Ma J, Pan J, Hu L, Lin G. Assessment of effects of chronic hydrogen sulfide poisoning on cytochrome P450 isoforms activity of rats by cocktail approach. Biol Pharm Bull. 2013;36:1627–1633. doi: 10.1248/bpb.b13-00502. [DOI] [PubMed] [Google Scholar]
- 6.Bosilkovska M, Clement M, Dayer P, Desmeules J, Daali Y. Incorporation of flurbiprofen in a 4-drug cytochrome p450 phenotyping cocktail. Basic Clin Pharmacol Toxicol. 2014;115:465–466. doi: 10.1111/bcpt.12231. [DOI] [PubMed] [Google Scholar]
- 7.Ma J, Wang S, Zhang M, Zhang Q, Zhou Y, Lin C, Lin G, Wang X. Simultaneous determination of bupropion, metroprolol, midazolam, phenacetin, omeprazole and tolbutamide in rat plasma by UPLC-MS/MS and its application to cytochrome P450 activity study in rats. Biomed Chromatogr. 2015;29:1203–12. doi: 10.1002/bmc.3409. [DOI] [PubMed] [Google Scholar]
- 8.Naramoto K, Kato M, Ichihara K. Effects of an Ethanol Extract of Brazilian Green Propolis on Human Cytochrome P450 Enzyme Activities in Vitro. J Agric Food Chem. 2014;62:11296–11302. doi: 10.1021/jf504034u. [DOI] [PubMed] [Google Scholar]
- 9.Borkar RM, Bhandi MM, Dubey AP, Nandekar PP, Sangamwar AT, Banerjee SK, Srinivas R. Plasma protein binding, pharmacokinetics, tissue distribution and CYP450 biotransformation studies of fidarestat by ultra high performance liquid chromatography-high resolution mass spectrometry. J Pharm Biomed Anal. 2014;102C:386–399. doi: 10.1016/j.jpba.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Tan ML, Lim LE. The effects of Andrographis paniculata (Burm. f.) Nees extract and diterpenoids on the CYP450 isoforms’ activities, a review of possible herb-drug interaction risks. Drug Chem Toxicol. 2015;38:241–53. doi: 10.3109/01480545.2014.947504. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Chen M, Chen X, Ma J, Wen C, Pan J, Hu L, Lin G. The effects of acute hydrogen sulfide poisoning on cytochrome P450 isoforms activity in rats. Biomed Res Int. 2014;2014:209393. doi: 10.1155/2014/209393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu RA, Xu ZS, Lin GY, Hu LF, Wang XQ, Ma JS. Effect of Repeated Wuniu Early Tea Administration on the CYP450 Activity Using a Cocktail Method. Indian J Pharm Sci. 2013;75:94–98. doi: 10.4103/0250-474X.113536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Han A, Wen C, Chen M, Chen X, Yang X, Ma J, Lin G. The effects of H2S on the activities of CYP2B6, CYP2D6, CYP3A4, CYP2C19 and CYP2C9 in vivo in rat. Int J Mol Sci. 2013;14:24055–24063. doi: 10.3390/ijms141224055. [DOI] [PMC free article] [PubMed] [Google Scholar]