Abstract
Psychological stress has become a common and important cause of premature ovarian failure (POF). Therefore, it is very important to explore the mechanisms of POF resulting from psychological stress. Sixty SD rats were randomly divided into control and model groups. Biomolecules associated with POF (β-EP, IL-1, NOS, NO, GnRH, CRH, FSH, LH, E2, P, ACTH, and CORT) were measured in the control and psychologically stressed rats. The regulation relationships of the biomolecules were explored in the psychologically stressed state using support vector regression (SVR). The values of β-EP, IL-1, NOS, and GnRH in the hypothalamus decreased significantly, and the value of NO changed slightly, when the values of 3 biomolecules in the hypothalamic-pituitary-adrenal axis decreased. The values of E2 and P in the hypothalamic-pituitary-ovarian axis decreased significantly, while the values of FSH and LH changed slightly, when the values of the biomolecules in the hypothalamus decreased. The values of FSH and LH in the pituitary layer of the hypothalamic-pituitary-ovarian axis changed slightly when the values of E2 and P in the target gland layer of the hypothalamic-pituitary-ovarian axis decreased. An Imbalance in the neuroendocrine-immune bimolecular network, particularly the failure of the feedback action of the target gland layer to pituitary layer in the pituitary-ovarian axis, is possibly one of the pathogenic mechanisms of POF.
Keywords: Psychological stress, premature ovarian failure, biological mechanisms, support vector regression machine
Introduction
Premature ovarian failure (POF), a common gynecological endocrine disease, is characterized by amenorrhea, dysgenesis, hypoestrogenism, and increased levels of gonadotropins resulting from the exhaustion of ovarian follicles before the age of 40 [1]. The causes of POF are complex. Studies have demonstrated that psychological stress, such as chronic anxiety, sadness, fear, and other negative emotions can lead to POF by changing the functioning of the hypothalamic-pituitary-target gland axis, which causes the emergence of the hypothalamic-pituitary-ovarian axis disorder [2].
Many studies on the changes of biomolecules that are associated with POF have been conducted, but only a few studies have revealed some of the biological mechanisms of POF [3-5]. In our previous studies, we reached preliminary conclusions about the mechanisms of POF caused by psychological stress, based on factor analysis. We found that psychological stress decreased the values of biomolecules in the pituitary-adrenal axis, which disrupted the functioning of the hypothalamus-pituitary-ovarian axis through the action of the biomolecules in the hypothalamus, which finally led to POF [6]. The POF-related biomolecules [3-12] were divided into 3 groups: (1) β-endorphin (β-EP), interleukin-1 (IL-1), nitric oxide synthase (NOS), nitric oxide (NO), gonadotropin-releasing hormone (GnRH), and corticotropin-releasing hormone (CRH) in the hypothalamus; (2) follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), and progesterone (P) in the pituitary-ovarian axis; and (3) adrenocorticotropic hormone (ACTH) and cortisol (CORT) in the pituitary-adrenal axis. Support vector machine (SVM) is a new machine learning method created by Vapnik et al [13], which recently has become popular because of its effective learning properties [14]. SVM was first used for classification problems, and then applied to regression problems, forming SVR theory. SVR has a predictive function, so SVR models can be created to forecast changes in biological molecule outputs based on changes in biological molecule inputs. The regulation relationships of POF-related biomolecules in 3 positions were explored by creating bimolecular network models of POF based on SVR, so that the biological mechanisms of POF caused by psychological stress could be revealed further.
Materials and methods
Animals
Female, specific pathogen-free (SPF)-grade, Sprague-Dawley (SD) rats, weighing 200-220 g, on a standard basal diet, were supplied by the Guangdong Medical Laboratory Animal Center. Our animal license number was SCXK 2008-0002. The rats were acclimated to the facility for 3 days, and their estrus cycle was observed every day. Rats with 2 continuous estruses were chosen for the experiment and randomly divided into 2 groups (n = 30 each): the control and model groups.
Reagents and equipment
The 0.9% sodium chloride injection was purchased from Henan Tai-Long Pharmaceutical Co., Ltd (Batch No. 11103015). The TP kit was supplied by BioSino Bio-Technology and Science Inc. The NOS kit was obtained from the Nanjing Jian-Cheng Bioengineering Institute. The CRH, GnRH, β-EP, IL-1, and NO kits were provided by the Beijing Sino-UK Institute of Biological Technology. The E2 and P kits were purchased from Sigma Corporation. A Chemistry Analyzer 7160 was provided by Hitachi (Japan), and the TDL80-2B centrifuge was purchased from Anting Scientific Instrumental Factory (Shanghai, China). The R-911 RIA counter was obtained from the University of Science and Technology of China. The acousto-optical-electric stimulator was made in our laboratory.
Animal model preparation
Acousto-optical-electric stimulation (acousto-optical stimulation for 10 s, acousto-optical-electric stimulation for 60 s, and electrical stimulation for 5 s) was applied to prepare the model for 20 days, 5 times per day, at random intervals [15]. Each procedure was completed within 1 h. The acousto, optical, and electrical stimuli used the following constant parameters: 1) a sound intensity of 65 dB; 2) an illumination intensity of 500 lux with shining and frequency of 1/s, 500 lux with lights on, and 300 lux with lights off; and 3) a voltage of 24~36 V.
Detection index
Blood was collected from the femoral artery and centrifuged at 3,000 rpm for 20 min. Supernatants were transferred to new tubes. The hypothalami were separated, cleaned with filter paper, and then homogenized by grinding in 1 mL of sodium chloride solution (containing 20 μL of 0.05 mol/L acetic acid). The samples were centrifuged at 3000 rpm for 10 min, and the supernatants were transferred to separate tubes. Precipitates were homogenized in 0.5 mL sodium chloride solution (containing 20 μL of 0.05 mol/L acetic acid) and centrifuged at 3000 rpm for 10 min. The supernatant was collected and combined with that obtained from the first centrifuge. Then, 25 μL of 0.05 mol/L NaOH was added to adjust the pH to about 7.4. Ten microliters were used to determine the protein concentration. Radioimmunoassays were performed to detect E2, P, LH, FSH, CORT, ACTH, and NO in serum, and CRF, IL-1, β-EP, and GnRH in the hypothalamus. NOS in the hypothalamus was examined by immunohistochemical methods.
Data normalization and elimination of extreme values
Indices obtained from various experimental biological molecules were normalized according to their different units and ranges. During an animal experiment and the detection of biological molecules, multiple external and subjective conditions can result in unexpected changes, leading to the detection of extreme values. In this study, outliers were excluded according to the method described by Grubbs [16].
Student’s t-tests for independent samples
The difference of the values of the biomolecules in the hypothalamus-pituitary-adrenal axis between the control and model groups were analyzed by Student’s t-tests for independent samples using SPSS software (SPSS Statistics 20, IBM, New York, USA).
Basic model of SVR forecasting
SVR is a new machine learning method with a predictive function. We used the basic model of SVR as previously described [17]. SVR seeks the optimal functional relationship of a reactive sample datum, y = f(x), according to the given sample collection {(xi,yi) i = 1,2,…,k}, among which, xi is an input vector, and yi is an expected output. A proper core function is adopted K (xi,xj) to determine the regression model: Equation 1. In the regression model, when αi is not zero or αi * is not zero, the corresponding sample is the support vector. Commonly used core functions include the linear core, the polynomial core, and the radial basis function (RBF) core.
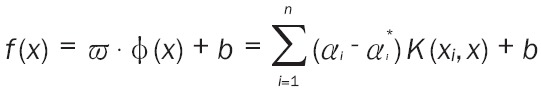 |
Bimolecular network models of the POF based on SVR
Our previous study found that the values of biomolecules in hypothalamus-pituitary-adrenal axis were decreased by psychological stress, then the functioning of the hypothalamus-pituitary-ovarian axis was disrupted by the biomolecules in the hypothalamus [6]. The bimolecular network of the POF was created containing the biomolecules in the hypothalamus, hypothalamus-pituitary-adrenal axis and hypothalamus-pituitary-ovarian axis. This is shown in Figure 1.
Figure 1.
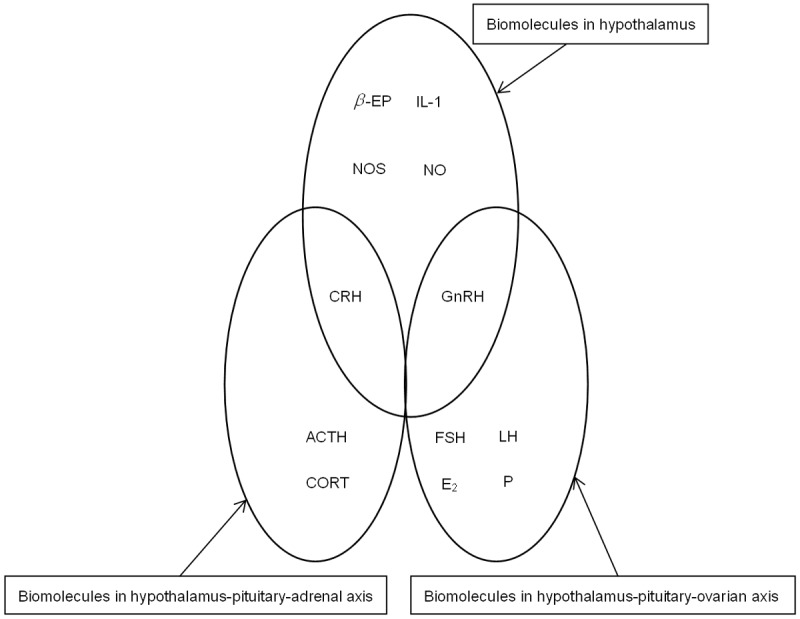
The bimolecular network of the POF.
The bimolecular network models of POF based on SVR were created as follows: the biomolecules in the hypothalamus-pituitary-adrenal axis (CRH, ACTH and, CORT) were used as input variables xi,i = 1,2,3. The biomolecules in the hypothalamus (β-EP, IL-1, NOS, NO, and GnRH) were used as output variables f(x), to create five SVR models, labeled models 1-5. Then β-EP, IL-1, NOS, NO, CRH, and GnRH were used as input variables xi, i = 1,2,3,4,5,6, and FSH, LH, E2, and P were used as output variables f(x), to create four SVR models of the same type, labeled 6-9. To further examine the feedback action of the biomolecules of the target gland layer on the biomolecules of the pituitary layer in hypothalamus-pituitary-ovarian axis, E2 and P, the biomolecules of target gland layer in hypothalamus-pituitary-ovarian axis, were used as input variables xi,i = 1,2, while the biomolecules of the pituitary layer in hypothalamus-pituitary-ovarian axis, FSH and LH, were used as output variables f(x), to create two SVR models, labeled models 10-11 Core functions K (xi,xj) were all selected as the RBF core: Equation 2. Parameter γ and punishment coefficient C of each model required optimization and determination. In this study, the data collection was shortened and coded as [0, 1], and the network method was adopted for searching the parameters, making the mean square errors (MSE) of the jackknife test the minimum.
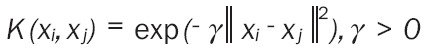 |
The programs were self-edited with Scilab software (vers 5.4.1, INRIA, Paris, France), which was used to create the eleven SVR models of POF caused by psychological stress.
Results
Changes in the weights of the ovarian pituitary, ovaries, and adrenals, and changes in the ovarian morphology of rats with POF caused by psychological stress
Our previous studies showed that the weights of the ovarian pituitary, ovaries, and the adrenal glands of the rats with POF caused by psychological stress were significantly lighter, and the ovarian tissue was showed significant injury compared with the normal rats [15]. These findings showed that our animal experimental protocol was successful. Furthermore, the regulation relationships of the biomolecules network of POF caused by psychological stress were explored with SVR models based on the success of the animal model.
Test of the SVR models of POF caused by psychological stress
First, the eleven SVR models were tested with the jackknife test. The SVR models were created using 30 samples. The jackknife test was applied to one sample as the predictive sample, and the other 29 samples were used as training samples. Therefore, 30 predictive errors and 30 predictive multiple correlation coefficients were obtained for each model. The 30 predictive errors of each model were all less than 10%, which indicated the feasibility of the models. Moreover, the 30 multiple correlation coefficients of each bimolecular network model of POF based on SVR were all larger than 0.98, showing that the predictive efficacy of these eleven models were reliable.
Forecasting of the SVR models of POF caused by psychological stress
According to the multiple correlation coefficients of the jackknife test of the SVR, the predictive efficacy of the eleven models were reliable. Hence, the changes in the biomolecules in the hypothalamus could be forecasted according to models 1-5, when the biomolecules in the hypothalamus-pituitary-adrenal axis changed under psychological stress. Thus, the feedback action of the hypothalamus-pituitary-adrenal axis on the hypothalamus under psychological stress could be explored. Based on the same principle, the changes of the biomolecules in the hypothalamus-pituitary-ovarian axis could be forecasted according to models 6-9, when the biomolecules in the hypothalamus changed. Finally the changes in the biomolecules of the pituitary layer in the hypothalamus-pituitary-ovarian axis could be forecasted according to models 10-11, when the biomolecules of the target gland layer in the hypothalamus-pituitary-ovarian axis changed.
The values of the biomolecules in hypothalamus-pituitary-adrenal axis of the rats with POF caused by psychological stress all decreased, as shown in Table 1. This result fits with the theory of “Fright Impairing Kidney-qi” in TCM [18,19].
Table 1.
Changes of the values of the biomolecules in the hypothalamus-pituitary-adrenal axis (x̅ ± s)
| Group | Number of cases(n) | CRH (ng/mL) | ACTH (pg/mL) | CORT (ng/mL) |
|---|---|---|---|---|
| Control group | 30 | 1.76 ± 0.37 | 27.05 ± 5.39 | 309.74 ± 20.27 |
| Model group | 30 | 1.38 ± 0.15ΔΔ | 19.99 ± 0.64ΔΔ | 294.39 ± 9.14Δ |
Notes: Compared with the control group;
P ≤ 0.05;
P ≤ 0.01.
Therefore, all 30 samples were used as training samples. First, the values of the biomolecules in the hypothalamus-pituitary-adrenal axis (CRH, ACTH, and CORT) were multiplied by 0.9 as forecasting samples, and models 1-5 were used, respectively, to forecast changes in β-EP, IL-1, NOS, NO, and GnRH. Next, the values of the biomolecules in the hypothalamus (β-EP, IL-1, NOS, NO, and GnRH) were multiplied by 0.9 as forecasting samples, and models 6-9 were applied to forecast changes in FSH, LH, E2, and P, respectively. Then, the values of the biomolecules of the target gland layer in the hypothalamus-pituitary-ovarian axis (E2 and P) were multiplied by 0.9 as forecasting samples, and models 10 and 11 were used to forecast volume changes in the biomolecules of the pituitary layer in the hypothalamus-pituitary-ovarian axis, FSH and LH, respectively. The relative change rate of the biomolecules was defined as: (predictive value-original experimental value)/(original experimental value) × 100%. The average relative change rates of the index values of the predictive biomolecules were calculated. The values of the biomolecules in the hypothalamus-pituitary-adrenal axis of the rats with POF decreased under psychological stress, followed by decreases in the biomolecules involved in the hypothalamus-pituitary-adrenal axis feedback regulating the hypothalamus, the hypothalamic regulation of the pituitary-ovarian axis, and the target gland layer feedback regulating the pituitary layer in the hypothalamus-pituitary-ovarian axis. The results are shown in this order in Tables 2, 3 and 4.
Table 2.
Relative change rates of the biomolecules in the hypothalamus when the values of the biomolecules in the hypothalamus-pituitary-adrenal axis were multiplied by 0.9
| Biomolecules | Relative change rates | Multiple correlation coefficient |
|---|---|---|
| β-EP | -8.37% | 0.8270 |
| IL-1 | -15.49% | 0.9098 |
| NOS | -3.12% | 0.9184 |
| NO | -0.20% | 0.8165 |
| GnRH | -5.01% | 0.8095 |
Note: Multiple correlation coefficients here refer to the multiple correlation coefficient of the prediction model.
Table 3.
Relative change rates of the biomolecules in the pituitary-ovarian axis when the values of the biomolecules in the hypothalamus were multiplied by 0.9
| Biomolecules | Relative change rates | Multiple correlation coefficient |
|---|---|---|
| FSH | -0.19% | 0.9872 |
| LH | -0.70% | 0.8971 |
| E2 | -8.27% | 0.9828 |
| P | -3.44% | 0.9809 |
Note: Multiple correlation coefficients here refer to the multiple correlation coefficient of the prediction model.
Table 4.
Relative change rates of the biomolecules of the pituitary layer in the hypothalamus-pituitary-ovarian axis when the values of the biomolecules in the target gland layer were multiplied by 0.9
| Biomolecules | Relative change rates | Multiple correlation coefficient |
|---|---|---|
| FSH | 0.23% | 0.9272 |
| LH | 0.25% | 0.8187 |
Note: Multiple correlation coefficients here refer to the multiple correlation coefficient of the prediction model.
Discussion
Tables 2, 3 and 4 show that the multiple correlation coefficients of the eleven predictive models forecasting the predictive samples all were larger than 0.80, indicating that the predictive results are reliable. Decreased values of the biomolecules in the hypothalamus-pituitary-adrenal axis caused obvious decreases in the values of the biomolecules (β-EP, IL-1, NOS, and GnRH) in the hypothalamus (Table 2). Decreased values of the biomolecules in the hypothalamus caused obvious decreases in the values of the biomolecules of the target gland layer (E2 and P) in the pituitary-ovarian axis, whereas there was little change in the biomolecules of the pituitary layer (FSH and LH) in the pituitary-ovarian axis (Table 3). Changes in the biomolecules of the target gland layer (E2 and P) in pituitary-ovarian axis led to very few changes of the biomolecules of the pituitary layer (FSH and LH) in the pituitary-ovarian axis (Table 4), which indicates that the feedback regulation of the target gland layer to the pituitary layer in the hypothalamus-pituitary-ovarian axis declined. This result is consistent with previous research on POF [20].
As seen in Tables 1, 2, 3 and 4, the development of POF caused by psychological stress is as follows: First, the values of the biomolecules of the hypothalamus-pituitary-adrenal axis of POF all decreased due to psychological stress. Next, the volume changes of the biomolecules in the hypothalamus-pituitary-adrenal axis that feedback to the hypothalamus led to decreases of the biomolecules in the hypothalamus, and decreases of the biomolecules in the hypothalamus caused decreases of the biomolecules in the hypothalamus-pituitary-ovarian axis. The decreases of the biomolecules associated with POF, particularly the failure of the feedback regulatory action of the target gland layer to the pituitary layer in the hypothalamus-pituitary-ovarian axis destroyed the balance of the neuroendocrine-immune biomolecular networks, eventually led to POF.
In conclusion, psychological stress can cause changes in reproductive endocrinology. The reproductive endocrine system is not only important because it is involved in the stress response, but because it also is vulnerable to harm from psychological stress, which can impair the neuroendocrine-immune network. Psychological stress has become a common and important cause of reproductive endocrine disorders [21,22]. It is very important to study the biological mechanisms of POF caused by psychological stress for the development of multi-target drugs for regulating POF. In this study, SVR models were used to analyze non-linear relationships among the neuroendocrine-immune network in rats with POF caused by psychological stress. These results enhance our understanding of POF, and are significant in revealing the biological mechanisms of POF. The results also show that SVR modeling can effectively predict non-linear relationships in the neuroendocrine-immune network. If more biomolecules are involved, POF can be comprehensively and clearly understood. This study provides an example of a methodological approach for the study of complex diseases from the perspective of systems biology.
Acknowledgements
This work was supported by National Natural Science Foundation for Young Scholars of China (Grant No. 81403153) and National Natural Science Foundation of China (General Program) (Grant No. 81073073).
Disclosure of conflict of interest
None.
References
- 1.Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113:1355–1363. doi: 10.1097/AOG.0b013e3181a66843. [DOI] [PubMed] [Google Scholar]
- 2.Yue LW. Progress in the study of the causes of premature ovarian failure. Medical Overview. 2009;15:410–412. [Google Scholar]
- 3.Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin subunit mRNA levels. Mol Cell Endocrinol. 2006;256:40–48. doi: 10.1016/j.mce.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JC, Li XF, Breen L, Thalabard JC, O’Byrne KT. The role of the locus coeruleus in corticotropin-releasing hormone and stress-induced suppression of pulsatile luteinizing hormone secretion in the female rat. Endocrinology. 2005;146:323–331. doi: 10.1210/en.2004-1053. [DOI] [PubMed] [Google Scholar]
- 5.Kawauchi H, Sower SA. The dawn and evolution of hormones in the adenohypophysis. Gen Comp Endocrinol. 2006;148:3–14. doi: 10.1016/j.ygcen.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhang L, Wang XF, Deng SY, Zhang Y, Luo LC. Study on the Biological Mechanisms of the Premature Ovarian Failure in Rats with Psychological Stress Based on Factor Analysis. Journal of Liaoning TCM. 2013;40:2191–2194. [Google Scholar]
- 7.Zhen HY, Wang L, Su JL, Zhao X, Wu ZX, Cui YY. Studies on the regulation of nitric oxide to the reproductive axis in female rats. Chinese Journal of Histochemistry and Cytochemistry. 2009;4:492–492. [Google Scholar]
- 8.Kadekaro M. Nitric oxide modulation of the hypothalamo-neuro-hypophyseal system. Braz J Med Biol Res. 2004;37:441–450. doi: 10.1590/s0100-879x2004000400001. [DOI] [PubMed] [Google Scholar]
- 9.Dufourny L, Skinner DC. Influence of estradiol on NADPH diaphorase/neuronal nitric oxide synthase activity and colocalization with progesterone or type II glucocorticoid receptors in ovine hypothalamus. Biol Reprod. 2002;67:829–836. doi: 10.1095/biolreprod.102.004648. [DOI] [PubMed] [Google Scholar]
- 10.Tong Y, Zou J, Ni LQ, Liang SH, Cao BY, Chen HJ. Impact of phlegm, yang method on gene expression of IL-1β, IL-2 and hypothalamus CRHmRNA, pituitary ACTHmRNA in chronic stress in rats. Chinese Journal of Basic Medicine of in TCM. 2005;11:501–502. [Google Scholar]
- 11.Raga F, Casañ EM, Bonilla-Musoles F. Gonadotropin-releasing hormone (GnRH)-I regulation of interleukin (IL)-1b and IL-1 receptor antagonist expression in cultured human endometrial stromal cells. J Obstet Gynaecol Res. 2008;34:464–472. doi: 10.1111/j.1447-0756.2008.00737.x. [DOI] [PubMed] [Google Scholar]
- 12.Waschul B, Herforth A, Stiller-Winkler R, Idel H, Granrath N, Deinzer R. Effects of plaque, psychological stress and gender on crevicular Il-1beta and Il-1ra secretion. J Clin Periodontol. 2003;30:238–248. doi: 10.1034/j.1600-051x.2003.00270.x. [DOI] [PubMed] [Google Scholar]
- 13.Cortes C, Vapnik V. Support vector networks. Machine Learning. 1995;20:273–297. [Google Scholar]
- 14.Chou KC. Progress in protein structural class prediction and its impact to bioinformatics and proteomics. Curr Protein Peptide Sci. 2005;6:423–436. doi: 10.2174/138920305774329368. [DOI] [PubMed] [Google Scholar]
- 15.Wang JH, Wang MZ, Wu QH, Min JX, Chen XF, Ou YD. Experimental study on the establishment of sub-functional model of ovarian endocrine patterns in female rats exposed to psychologic stress. Chinese Journal of Laboratory Animal Science. 2002;12:204–206. [Google Scholar]
- 16.Zhang X, Cheng MX, Xiao FP. Origin used in comparison the methods of eliminating the excrescent data. Experiment Science and Technology. 2012;10:74–76. [Google Scholar]
- 17.Yan PF, Zhang CS. Artificial Nerve Network and Analogous Evolution Calculation. Beijing, China: Tsinghua University Publishing House; 2005. pp. 161–163. [Google Scholar]
- 18.Zhang XG, Wang HY. Regulation of the 5-hydroxytryptamine of mice model with ‘terror leading to disorder of kidney’ by Jinkui Shenqi Wan. China Journal of Traditional Chinese Medicine and Pharmacy. 2014;29:608–610. [Google Scholar]
- 19.Gu ZY, Guan HY. Significance of “fright impairing kidney-qi” during the development process of psychosomatic disease. Journal of Liaoning University of Traditional Chinese Medicine. 2011;13:113–114. [Google Scholar]
- 20.Wu LP. Diagnostic value of hormone detection of premature ovarian failure. Journal of Radioimmunology. 2012;25:350–351. [Google Scholar]
- 21.Luo LC, Wang JH, Ma N, Liu HY, Wang MZ. Factor analysis model for studying the influence of psychological stress on the endocrine network of reproduction axis. Journal of Biomedical Engineering. 2008;25:1368–1371. [PubMed] [Google Scholar]
- 22.Jia KR, Guo G, Liu KY, Shi Y, Liu XF, Zou QM. Establishment of an animal model with pure psychological stress and its effects on behaviour and endocrine-immune function. Immunological Journal. 2009;25:329–332. [Google Scholar]


