Abstract
This study aims to elucidate the change in pulmonary function in stage 0 COPD patients. A total of 48 stage 0 COPD patients and 46 healthy adults were included in the study. The status of their pulmonary function was determined by an impulse oscillometry (IOS) system, and the spirometric indexes such as forced vital capacity, maximum expiratory flow-volume (MEFV) curve, total respiratory impedance (Zrs) and respiratory resistance (Rrs) between the two groups were compared. Significant decreases in the values of forced expiratory flow (FEF) at both 75% and 50% of the vital capacity of the predicted value (EF75/pre and FEF50/pre) were detected in stage 0 COPD patients compared with those in the control (P < 0.05). Significant increases were found in the resonant frequency (Fres) (14.37±3.63 VS 11.26±2.61), total respiratory impedance (Z5) compared with the prediction (Z5/pre) (135.65±19.37 VS 104.69±20.64), total airway resistance at 5 Hz (R5) compared with prediction (R5/pre) (128.46±20.14 VS 100.60±20.98) and peripheral airway resistance (R5-R20) compared with prediction (R5-R20/pre) (282.34±192.83 VS 109.31±80.05) in the study group compared with those in the control(all P < 0.05). The reactance at 5 Hz (X5) (-0.14±0.05 VS -0.08±0.05) in the stage 0 COPD group was markedly lower than that in the healthy group (P < 0.05). Disturbance in the small airway may be detected by the MEFV curve and IOS, and these indexes would be valuable in diagnosing stage 0 COPD.
Keywords: COPD, pulmonary function test, impulse oscillometry
Introduction
Chronic obstructive pulmonary disease (COPD), which is progressive and associated with abnormal inflammatory response of the lung to toxic particles or gas, is “a diseased state characterized by airflow reduction that is not fully reversible [1].” At present, the diagnosis and staging of COPD mainly rely on the pulmonary function test parameters forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) and FEV1/pre [1]. However, because early pathological changes in COPD is localized within small airways and neither FEV1/FVC nor FEV1/pre is capable of detecting pulmonary function changes in small airways (diameter < 2 mm to 4 mm) or the existence of airflow limitation at early and preliminary stages of COPD, making early diagnosis and treatment on COPD patients is rather difficult. Therefore, the technology and methods of small airway function determination may be more important in observing COPD patients. Regular lung function check-up should be conducted on people without airway obstruction but with respiratory symptoms and are “at risk” for COPD, i.e., GOLD stage 0 COPD, to reveal the airflow reduction at an early stage [2,3]. The existence of airflow limitation can not be detected by routine pulmonary function inspection. Determining whether or not other lung function parameters could help in discovering the phenomenon of airflow reduction in GOLD stage 0 COPD patients has been paid close attention in recent years to provide the foundation for early prevention and treatment of these patients.
Owing to the advantage of being non-invasive and a versatile method and demanding minimal cooperation from the patient, Impulse oscillometry (IOS) is gradually accepted by increasing number of clinicians. In obstructive lung disease, further increase in Rrs and changes in Xrs to more negative values at lower oscillatory frequencies have been reported [4]. Within-breath changes in Xrs5 has also been reported (ΔXrs5: the difference between expiratory and inspiratory reactance) and suggested to represent the overall distribution of expiratory airflow reduction during tidal breathing [5-7]. COPD and asthma studies have shown that IOS measurements are more sensitive than FEV1 in measuring the pulmonary effects of bronchodilator drugs [8-10]. The IOS measurements can also be used to diagnose sensitively obstructive lung disease [11,12].
To determine the lung function parameters that could help discover the phenomenon of airflow limitation in GOLD stage 0 COPD patients, some spirometric indexes such as FVC, maximum expiratory flow-volume (MEFV) curve, respiratory impedance (Zrs) and respiratory resistance (Rrs) of 48 COPD GOLD stage 0 patients were determined and compared with those of healthy controls.
Materials and methods
Patients
The COPD GOLD stage 0 group consisted of 48 cases with 23 males and 25 females whose average age was 47.54±14.21 years old. The course of the disease was from 2 years to 7 years. The diagnosis of all COPD cases complied with the GOLD criteria [2,3]. All patients had COPD-related factors, including 21 smokers with average smoking index of 23.50±100.47 pack-years (18 of which were long-term passive smokers), three cases with history of occupational dust exposure and six patients that has been living in a community with severe air pollution. Data in terms of FEV1/FVC ≥ 70% and FEV1/pre ≥ 80% in lung function tests were obtained from all patients. All of patients’ X-ray has no obvious abnormality, except for three patients that have mild emphysema. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Shanghai Pulmonary Hospital, Tongji University School of Medicine. Written informed consent was obtained from all participants.
No abnormal finding was observed in other accessory examinations related to cardio-pulmonary function. The possibility of cardio-pulmonary disease was excluded in all cases as well as the history of respiratory tract infection in the past two months. The control group was composed of 46 healthy adults with the same age as the study group. Among them, 22 were males and 24 were females. Their average age was 46.71±10.09 years. None of them had ever smoked or had COPD-related factors. No abnormal finding was observed in other accessory examinations related to cardio-pulmonary function. The possibility of cardio-pulmonary disease was excluded in all cases, as well as the history of respiratory tract infection in the past two months. The differences in the sex composition, age, weight or height between the two groups were not statistically significant (P > 0.05) (Table 1).
Table 1.
Characteristics of the study group and control (x̅±s)
| Control | COPD GOLD stage 0 | |
|---|---|---|
| n | 46 | 48 |
| Sex (male/female) | 22/24 | 23/25 |
| Age (yr) | 46.71±10.09 | 47.54±14.21 |
| Height (cm) | 163.2±6.0 | 165.0±7.2 |
| Weight (kg) | 60.2±9.8 | 64.2±11.2 |
Measurements
Each patient performed pulmonary function tests in the following order: impulse oscillometry system (IOS), body plethysmography (including measurement of lung volumes) and spirometry.
Pulmonary function measurements IOS (Masterscreen IOS, Erich Jaeger, Hoechberg, Germany) measurements were performed as previously described of [10] and the actual values of respiratory resistance at 5 and 20 Hz (R5, R20 and R5-R20, respectively), reactance at 5 Hz (X5) and the resonant frequency (Fres) were recorded. FEF75, FEF50 and FEF25 were measured in a constant volume plethysmograph (Sensormedics Vmax 6200). IOS and body plethysmograph measurements were repeated thrice and the mean value was used for further analysis. FEV1 and FVC were measured using the spirometry system on the Masterscreen. Readings were taken thrice and the highest FEV1 and FVC recorded.
Statistical analysis
T test was adopted to compare the general data and the pulmonary function parameters between the COPD stage 0 group and the control. The diagnostic value of the different parameters was analyzed by ROC curve analysis. All statistical analyses were performed using the SPSS10.0 statistical software package.
Results
Determination results
Our study found that none of the differences in the FVC/pre, FEV1/pre or FEV1/FVC between the two groups was statistically significant (P > 0.05). Neither the difference on the PEF/pre nor the difference on the FEF25/pre between the two groups was statistically significant. However, both FEF50/pre and FEF75/pre were markedly lower than those of the control, and the differences were significant (P < 0.05). Among the IOS determination parameters, Fres, Z5/pre, R5/pre and R5-R20/pre of the COPD stage 0 group were notably higher than those of the control, and the differences were statistically significant (P < 0.05). X5 of the COPD stage 0 group was markedly lower than that of the control, and the difference was statistically significant (P < 0.05). R20/pre of the COPD stage 0 group was not different compared with that of the control (P > 0.05) (Table 2).
Table 2.
Comparison of FEV value, MEFV curve and IOS between the study group and the control (x̅±s)
| Control | COPD GOLD stage 0 | |
|---|---|---|
| FVC/pre (%) | 96.14±9.72 | 95.33±8.41 |
| FEV1/pre (%) | 95.53±10.46 | 94.3±9.02 |
| FEV1/FVC (%) | 81.38±3.86 | 80.55±5.88 |
| PEF/pre (%) | 88.65±15.98 | 85.24±15.70 |
| FEF25/pre (%) | 89.46±12.40 | 84.68±10.46 |
| FEF50/pre (%) | 87.62±14.53 | 67.36±13.44* |
| FEF75/pre (%) | 83.55±12.47 | 55.40±14.52* |
| Fres (Hz) | 11.26±2.61 | 14.37±3.63* |
| Z5/pre (%) | 104.69±20.64 | 135.65±19.37* |
| R5/pre (%) | 100.60±20.98 | 128.46±20.14* |
| R20/pre (%) | 96.73±17.95 | 101.51±16.07 |
| R5-R20/pre (%) | 109.31±80.05 | 282.34±192.83* |
| X5 (kPa·L-1·S-1) | -0.08±0.05 | -0.14±0.05* |
P < 0.05 when compared with the control.
ROC curve analysis
Our study also revealed that all the MEFV curve parameters (FEF50/pre and FEF75/pre) and IOS parameters (Fres, Z5/pre, R5/pre, R5-R20/pre and X5 COPD) could differentiate the stage 0 group and the control (area under the ROC curve Az > 0.5; the difference was significantly different when compared with that in Az = 0.5) (see Figure 1 and Table 3 for the ROC curves and the areas under the curves). The Youden indexes were calculated based on the sensitivity and specificity of the point coordinates. FEF50/pre and FEF75/pre reached their maximum Youden indexes at approximately 70% The highest Youden index of Fres was at approximately 13 Hz. The Youden indexes of Z5/pre and R5/pre achieved their highest values near 120%.
Figure 1.
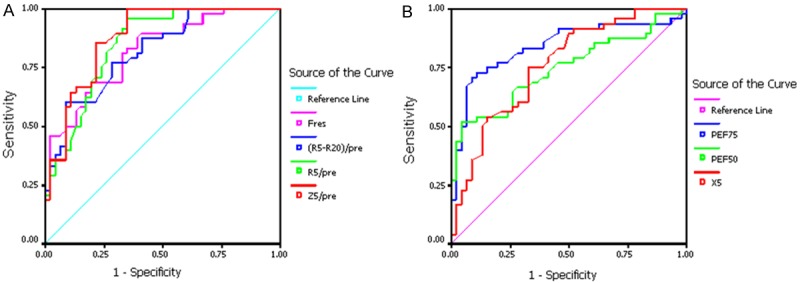
(A) bigger value was ROC curve of the abnormal parameter. (B) smaller value was ROC curve of the abnormal parameter. (A) represent the sensitivity and specificity of Fres, (R5-R20)/pre, R5/pre and Z5/pre. (B) represent the sensitivity and specificity of PEF75, PEF50 and X5.
Table 3.
AUC (area under curve) of MEFV curve and ROC curve of IOS parameters
| Z5/pre | R5/pre | R5-R20/pre | X5 | Fres | FEF50/pre | FEF75/pre | |
|---|---|---|---|---|---|---|---|
| Az | 0.879 | 0.848 | 0.819 | 0.760 | 0.820 | 0.737 | 0.841 |
Our study also revealed that the sensitivities, rates of missed diagnosis, specificities, rates of misdiagnosis, positive likelihood ratios, negative likelihood ratios and Youden indexes in diagnosing COPD stage 0 using either FEF75/pre < 70%, FEF50/pre < 70%, Fres > 13 Hz, Z5/pre > 120% and R5/pre > 120% or FEF75/pre < 80%, FEF50/pre < 80%, Fres > 15 Hz, Z5/pre > 150% and R5/pre > 150% were distinctively different. The former was considered superior to the latter (Table 4).
Table 4.
Sensitivity, specificity, positive likelihood ratio, negative likelihood ratio and the corresponding Youden Index
| Sensitivity | Rate of missed diagnosis | Specificity | Erroneous diagnosis rate | Positive likelihood ratio | Negative likelihood ratio | Youden Index | |
|---|---|---|---|---|---|---|---|
| FEF50/pre < 70% | 0.50 | 0.5 | 0.96 | 0.04 | 11.99 | 0.52 | 0.46 |
| FEF50/pre < 80% | 0.67 | 0.33 | 0.67 | 0.33 | 2.04 | 0.49 | 0.34 |
| FEF75/pre < 70% | 0.77 | 0.23 | 0.78 | 0.12 | 3.55 | 0.29 | 0.55 |
| FEF75/pre < 80% | 0.92 | 0.08 | 0.43 | 0.57 | 1.62 | 0.19 | 0.35 |
| Fres > 15 Hz | 0.48 | 0.52 | 0.91 | 0.09 | 5.51 | 0.57 | 0.39 |
| Fres > 13 Hz | 0.69 | 0.31 | 0.80 | 0.2 | 3.51 | 0.39 | 0.49 |
| Z5/pre > 120% | 0.83 | 0.17 | 0.74 | 0.26 | 3.19 | 0.23 | 0.57 |
| Z5/pre > 150% | 0.21 | 0.79 | 0.98 | 0.02 | 9.60 | 0.81 | 0.19 |
| R5/pre > 120% | 0.63 | 0.37 | 0.72 | 0.28 | 2.21 | 0.52 | 0.34 |
| R5/pre > 150% | 0.19 | 0.81 | 0.98 | 0.02 | 9.50 | 0.81 | 0.19 |
Discussion
Our study revealed that MEFV curve parameters used in the small airway functional testing, including FEF50/pre and FEF75/pre, of the COPD stage 0 group were significantly lower than those of the control. Meanwhile, the IOS detection indexes Fres, Z5/pre, R5/pre and R5-R20/pre were significantly higher in the COPD stage 0 patients than those in the control. X5 was lower in the COPD stage 0 subjects than that in the control, but no significant difference was found with R20/pre between the two groups. The above results indicated that in COPD stage 0 patients, the pulmonary function was only relatively normal, as opposed to absolutely normal. Airflow limitation in the small airway may already have existed in the COPD stage 0 patients. At the preliminary stage of COPD, inflammation induces a repeated cycle of small airway wall damage and repair, remodelling of the airway wall structure and increased airway resistance. The result is a small airway airflow limitation. Our finding on the high sensitivity of the IOS technology-related parameters in detecting the slightly increased airway resistance that reflects a small airway airflow limitation is completely in accordance with previous study results by international researchers of the extreme sensitivity of the IOS parameters to variations in the airway resistance [13-15]. Oppenheimer et al [16] found that IOS provided a non-invasive tool for assessment of distal airway function when spirometry was normal, which can be applied to various clinical settings including early diagnosis of COPD (GOLD stage 0). Frantz et al [17] found IOS might have the potential to detect pathology associated with COPD earlier than spirometry. These finding are consist with our research. IOS determination may reflect the extent of airway limitation [9,10]. The values of the IOS parameters in the COPD patients are closely related to the parameters used in routine pulmonary function tests and reflect the airflow limitation [18,19].
To crystallize the diagnostic value of the MEFV curve and IOS technique in diagnosing COPD stage 0, we performed ROC curve analyses on the parameters with statistically significant difference between the two groups. These parameters included FEF50/pre and FEF75/pre and the IOS parameters Fres, Z5/pre, R5/pre, R5-R20/pre and X5 We found that all these parameters can discriminate the COPD stage 0 subjects and the control (the area under the ROC curve Az > 0.5; the difference was significant compared with Az = 0.5). When Az is 0.5 to 0.7, the diagnostic accuracy is usually considered low. The accuracy is medium when Az is 0.7 to 0.9. The diagnostic accuracy is considered relatively high if Az is larger than 0.9. Therefore, based on these criteria, because the Az value of the above parameters in our study were all within the range of 0.7 to 0.9, the corresponding diagnostic accuracy was considered moderate. Because most of the curves were interdigitated and the differences among the areas under the curves were modest, no diversity test on the area under the ROC curve among the abovementioned parameters was performed to compare the diagnostic values of the parameters. Comprehensive analysis of these parameters is favourable in increasing the diagnostic accuracy.
Lower FEF50-75 and FEF50-75/FVC were observed in the early onset of COPD [20]. However, for years, judgment and analysis of the functional testing result of the small airway have been controversial. Some researchers considered FEF50/pre < 80% and FEF75/pre < 80% as the cut-off abnormality, whereas others insisted on FEF50/pre < 70% and FEF75/pre < 70%. Currently, the judgment and analysis of the IOS detection results mostly rely on international diagnostic criteria, i.e., Fres > 15 Hz, Z5/pre > 150% and R5/pre > 150% are considered abnormal. The Youden indexes of the abovementioned parameters were calculated based on the sensitivity and specificity of the coordinate points on the ROC curves (sensitivity/1-specificity). Generally, the higher the Youden index is, the higher is the diagnostic accuracy. Our study found that FEF50/pre and FEF75/pre reached their maximum Youden indexes at approximately 70% and 70%, respectively, which indicated that considering FEF75/pre < 70% and FEF50/pre < 70% as the discriminating criteria for abnormality could be reasonable. The findings that the highest Youden index value of Fres was at approximately 13 Hz and that the Youden indexes of Z5/pre and R5/pre achieved their highest values near 120% indicated that the criteria for abnormality of the Chinese people may be lower than that of the foreigners. By considering either Fres > 15 Hz, Z5/pre > 150% and R5/pre > 150% or Fres > 13 Hz, Z5/pre > 120% and R5/pre > 120% as criteria for abnormality, the sensitivities, rates of missed diagnosis, specificities, rates of misdiagnosis, positive likelihood ratios negative likelihood ratio and Youden indexes in diagnosing COPD stage 0 were calculated. The result of the comprehensive analysis indicated that the former was apparently superior to the latter. Therefore, considering Fres > 13 Hz, Z5/pre > 120% and R5/pre > 120% as the criteria for abnormality of Chinese people may be more appropriate. Similarly, the discrepancy in the criteria for the abnormality of the IOS parameters affects the evaluation of the diagnostic value of the parameters. For the COPD stage 0, the international criteria for abnormality are Fres > 15 Hz, Z5/pre > 150% and R5/pre > 150%, which make the sensitivity of Fres higher than that of Z5/pre or R5/pre. Based on the results of our study, however, Fres > 13 Hz, Z5/pre > 120% and R5/pre > 120% appeared to be reasonable criteria for abnormality, which make the sensitivity of Z5/pre higher than that of Fres. Therefore, the determination of the criteria for abnormality of the IOS-related parameters and the comparison and evaluation of the diagnostic value of certain parameters remain to be substantiated by further studies.
In our study, the detection rates for the abnormality, i.e., sensitivity and rate of misdiagnosis of both the COPD stage 0 group and the control (Table 4), were calculated by considering FEF50/pre < 70% and FEF75/pre < 70% in the MEFV curve parameters and Fres > 13 Hz, Z5/pre > 120% and R5/pre > 120% in the IOS parameters as the criteria for abnormality. The detection rate of the COPD stage 0 group calculated based on the above parameters was markedly higher than that of the control group. Taking the above criteria for abnormality as the criteria for exclusion, the ratios of normal subjects in the COPD stage 0 group divided by those in the control group, i.e., the rate of missed diagnosis and the specificity, were calculated (Table 4). The former is notably lower than the latter. Thus, the MEFV curve and IOS parameters may have some value and significance in the diagnosis and differential diagnosis of COPD stage 0.
Although most of the parameters in our study were ratios of the measured to the predicted values and the result of the pulmonary function testing is under the influence of the predicted values, no unified criteria have been presently set yet for the predicted values in the lung function test in China. In our study, the Knudson predicted value was used in the FVC parameters, the EKGS 93 predicted value was used in the MEFV curve indexes and the Vogel Smith predicted value was adopted in the IOS parameters. Presently, the diagnostic criteria for COPD stage 0 include COPD high-risk factor, chronic cough and expectoration and normal lung function are not applicable to COPD stage 0. All the above factors may affect the result of our study. Compared with other large-scale clinical studies, the sample size in our study was relatively small. However, the sample size requirement for the statistical method was still satisfied. Therefore, our study result may reflect the pulmonary function status and trend of the COPD stage 0 patients. However, further intensive studies may be needed to clarify the exact diagnostic value of the IOS determination of the COPD stage 0 patients as well as to establish a rational threshold value as the criteria for abnormality.
The key features of COPD are characterized by a slowly progressive condition characterized by marked airway obstruction, which does not change markedly over time. The existence of airflow limitation cannot be detected by routine pulmonary function inspection. Therefore, close attention has been paid on whether or not other lung function parameters could help in discovering the phenomenon of airflow limitation in GOLD stage 0 COPD patients and thus provide foundation for early prevention and treatment of these patients. Moreover, the study is age matched single center study, and the result may deviate because of small sample size. Therefore, further studies should focus on multicenter, random and larger sample size with prospective direction. Simultaneously, the less IOS parameters are used in the study, and the more sensitive parameters should be further found and applied in future.
Conclusion
Because the symptoms of COPD stage 0 patients are not specific and their lung function parameters such FEV1/FVC and FEV1 are normal, establishing the diagnosis without objective concrete evidence is difficult. In addition, even the patients themselves do not take much concern on COPD at stage 0. These reasons make the early diagnosis and treatment of COPD stage 0 a rather tough job. Our findings, namely, the MEFV curve- and the IOS-related parameters may be used to reveal the airflow limitation in the small airway of COPD stage 0 patients and provide a basis for early diagnosis and treatment. The moderate accuracy of the proposed parameters need for further studies.
Acknowledgements
We would like to thank all the study participants. This work was supported by Youth Research Foundation from Shanghai Pulmonary Hospital.
Disclosure of conflict of interest
None.
References
- 1.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, Fukuchi Y, Jenkins C, Rodriguez-Roisin R, van Weel C, Zielinski J. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 2.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease(GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- 3.Fabbri LM, Hurd SS. Global Strategy for the Diagnosis, Management and Prevention of COPD: 2003 update. Eur Respir J. 2003;22:1–2. doi: 10.1183/09031936.03.00063703. [DOI] [PubMed] [Google Scholar]
- 4.Van Noord JA, Clement J, van de Woestijne KP, Demedts M. Total respiratory resistance and reactance in patients with asthma, chronic bronchitis, and emphysema. Am Rev Respir Dis. 1991;143:922–927. doi: 10.1164/ajrccm/143.5_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- 5.Dellacà RL, Santus P, Aliverti A, Stevenson N, Centanni S, Macklem PT, Pedotti A, Calverley PM. Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J. 2004;23:232–240. doi: 10.1183/09031936.04.00046804. [DOI] [PubMed] [Google Scholar]
- 6.Dellaca RL, Rotger M, Aliverti A, Navajas D, Pedotti A, Farre R. Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J. 2006;27:983–991. doi: 10.1183/09031936.06.00080005. [DOI] [PubMed] [Google Scholar]
- 7.Dellacà RL, Duffy N, Pompilio PP, Aliverti A, Koulouris NG, Pedotti A, Calverley PM. Expiratory flow limitation detected by forced oscillation and negative expiratory pressure. Eur Respir J. 2007;29:363–374. doi: 10.1183/09031936.00038006. [DOI] [PubMed] [Google Scholar]
- 8.Houghton CM, Woodcock AA, Singh D. A comparison of lung function methods for assessing dose-response effects of salbutamol. Br J Clin Pharmacol. 2004;58:134–141. doi: 10.1111/j.1365-2125.2004.02105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Houghton CM, Woodcock AA, Singh D. A comparison of plethysmography, spirometry and oscillometry for assessing the pulmonary effects of inhaled ipratropium bromide in healthy subjects and patients with asthma. Br J Clin Pharmacol. 2005;59:152–159. doi: 10.1111/j.1365-2125.2004.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrill ZL, Houghton CM, Woodcock AA, Vestbo J, Singh D. Measuring bronchodilation in COPD clinical trials. Br J Clin Pharmacol. 2005;59:379–384. doi: 10.1111/j.1365-2125.2004.02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Mutairi SS, Sharma PN, Al-Alawi A, Al-Deen JS. Impulse oscillometry: An alternative modality to the conventional pulmonary function test to categorise obstructive pulmonary disorders. Clin Exp Med. 2007;7:56–64. doi: 10.1007/s10238-007-0126-y. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Lee YW, Jung YH, Park SE, Hong CS. Impulse oscillometry for estimation of airway obstruction and bronchodilation in adults with mild obstructive asthma. Ann Allergy Asthma Immunol. 2007;98:546–552. doi: 10.1016/S1081-1206(10)60733-8. [DOI] [PubMed] [Google Scholar]
- 13.Hellinckx J, Cauberghs M, De Boeck K, Demedts M. Evaluation of impulse oscillation system: comparison with forced oscillation technique and body plethysmography. Eur Respir J. 2001;18:564–570. doi: 10.1183/09031936.01.00046401. [DOI] [PubMed] [Google Scholar]
- 14.Kastelik JA, Aziz I, Ojoo JC, Morice AH. Evaluation of impulse oscillation system: Comparison with forced oscillation technique and body plethysmography. Eur Respir J. 2002;19:1214–1215. doi: 10.1183/09031936.02.01922001. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod D, Birch M. Respiratory input impedance measurement: forced oscillation methods. Med Biol Eng Comput. 2001;39:505–516. doi: 10.1007/BF02345140. [DOI] [PubMed] [Google Scholar]
- 16.Oppenheimer BW, Goldring RM, Berger KI. Distal airway function assessed by oscillometry at varying respiratory rate: Comparison with dynamic compliance. COPD. 2009;6:162–170. doi: 10.1080/15412550902918410. [DOI] [PubMed] [Google Scholar]
- 17.Frantz S, Nihlén U, Dencker M, Engström G, Löfdahl CG, Wollmer P. Impulse oscillometry may be of value in detecting early manifestations of COPD. Respiratory Medicine. 2012;106:1116–1123. doi: 10.1016/j.rmed.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Kolsum U, Borrill Z, Roy K, Starkey C, Vestbo J, Houghton C, Singh D. Impulse oscillometry in COPD: identification of measurements related to airway obstruction, airway conductance and lungvolumes. Respir Med. 2009;103:136–143. doi: 10.1016/j.rmed.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Kanda S, Fujimoto K, Komatsu Y, Yasuo M, Hanaoka M, Kubo K. Evaluation of respiratory impedance in asthma and COPD by an impulse oscillation system. Intern Med. 2010;49:23–30. doi: 10.2169/internalmedicine.49.2191. [DOI] [PubMed] [Google Scholar]
- 20.DeMeo DL, Carey VJ, Chapman HA, Reilly JJ, Ginns LC, Speizer FE, Weiss ST, Silverman EK. Familial aggregation of FEF(25-75) and FEF(25-75)/FVC in families with severe, early onset COPD. Thorax. 2004;59:396–400. doi: 10.1136/thx.2003.012856. [DOI] [PMC free article] [PubMed] [Google Scholar]


