Abstract
Osteocalcin plays roles in energy, glucose, and lipid metabolism. Consequently, the relationship between osteocalcin level and nonalcoholic fatty liver disease (NAFLD) is of interest. The present study explored the possible correlation between serum osteocalcin levels and NAFLD in patients with CAD. The study enrolled 174 inpatients diagnosed with CAD by coronary angiography (CAG). The presence of fatty liver disease was confirmed by abdominal ultrasonography. NAFLD was diagnosed using the working definition of the revised guidelines for the management of NAFLD published by the Chinese Liver Disease Association. Serum osteocalcin levels were determined using electrochemiluminescent immunoassays. Patients with NAFLD had lower serum osteocalcin levels than those without NAFLD [16.2 (14.2-23.8) vs. 20.7 (15.6-26.2) ng/mL, P<0.05]. After adjustment for gender and age, serum osteocalcin levels correlated with the presence of NAFLD (r=-0.260, P=0.010), fasting plasma glucose level (r=-0.230, P=0.023) and glycated hemoglobin A1c level (r=-0.229, P=0.023). Osteocalcin was an independent factor for the presence of NAFLD (β=-0.097, P=0.025). These data suggested that serum osteocalcin levels were negatively associated with the presence of NAFLD in patients with CAD.
Keywords: Osteocalcin, nonalcoholic fatty liver disease, coronary artery disease
Introduction
Traditionally, bone was considered a relatively inert tissue, providing structural support for the body and participating in mineral metabolism. In the past decade, however, it has become clear that bone is also an active endocrine organ regulating several metabolic processes via the release of bone-derived hormones, including osteocalcin [1], which is a small protein (49 amino acids in humans) synthesized exclusively by osteoblasts. Osteocalcin is initially synthesized as a pre-pro-peptide; several post-translational modifications follow, including excision of the pre-pro-peptide and vitamin K-dependent γ-carboxylation of specific glutamic acid residues. Fully carboxylated osteocalcin has a high affinity for hydroxyapatite and is stored principally in the bone mineral matrix. However, osteocalcin is also present in blood, in both fully and partially carboxylated, and even completely uncarboxylated forms [2]. Osteocalcin was long regarded as a marker of bone formation [3,4], but mounting evidence now suggests that it plays important roles in obesity, metabolic syndrome, and type 2 diabetes. Thus osteocalcin is involved in the cross-talk between bone and energy metabolism [5].
High incidences of cardiovascular disease (CVD) and nonalcoholic fatty liver disease (NAFLD) pose major threats to public health worldwide. An intimate link is evident between CVD and NAFLD, which share a pathophysiological foundation (insulin-resistance, usually accompanied by obesity and disorders of glucose and lipid metabolism) [6,7]. Osteocalcin is involved in glycolipid and energy metabolism, and several human studies have found that serum osteocalcin levels are inversely associated with both the presence and severity of coronary artery disease (CAD) [8-11]. However, any link between serum osteocalcin levels and the presence of NAFLD remains unclear, although several researchers have suggested that such a link exists. This has not been tested in patients with CVD. Therefore, this study was designed to investigate the relevance between serum osteocalcin levels and NAFLD in patients diagnosed with CAD using coronary angiography (CAG).
Materials and methods
Study participants
During July 2008 and January 2010, inpatients of the Cardiology Department of the Sixth People’s Hospital affiliated with Shanghai Jiao Tong University who underwent coronary angiography and were diagnosed with CAD were recruited for this study. Each participant completed a standardized questionnaire exploring previous and present illnesses, medication history, and smoking status. Those with the following conditions were excluded: (1) acute or chronic viral hepatitis, drug-induced or autoimmune liver disease; (2) alcoholism (≥140 g alcohol per week [men] or ≥70 g per week [women]) [12]; (3) a recent myocardial infarction (<3 months prior); (4) acute coronary syndrome; (5) congestive heart failure (New York Heart Association Class III-IV); (6) chronic kidney disease; (7) hyper- or hypo-thyroidism; (8) a need for total parenteral nutrition; (9) cancer; (10) a fracture within 1 year prior; and (11) current therapeutic use of drugs known to affect bone or calcium metabolism, such as corticosteroids.
The study was approved by the Ethics Committee of Sixth People’s Hospital and complied with the Declaration of Helsinki. All subjects provided written informed consent.
Anthropometric evaluations and laboratory measurements
Weight (in kg) and height (in m) were recorded and used to calculate the Body Mass Index (BMI; kg/m2). Waist circumference (W) was measured at the midpoint between the inferior border of the lowest rib and the upper margin of the iliac crest, in the mid-axillary line. Blood pressure (BP) was measured with a sphygmomanometer. Patients were instructed to fast overnight (10 h), then fasting and 2-h-postprandial venous blood samples were collected. Fasting plasma glucose (FPG) and 2-h-postprandial glucose (2 h PG) levels were measured using the glucose oxidase method. A Bio-Rad Variant II high-pressure liquid chromatographic platform (Hercules, CA, USA) was used to detect glycated hemoglobin A1c (HbA1c). Fasting serum insulin (FINS) levels were determined by radioimmunoassay (Linco Research, St. Charles, MO, USA) and used to calculate Homeostasis Model of Assessment-Insulin Resistance (HOMA-IR), as follows: [FPG (mmol/L) × FINS (mU/L)]/22.5. A Hitachi 7600-020 auto-analyzer (Tokyo, Japan) was used to measure the levels of the liver function markers aspartate aminotransferase (AST), alanine aminotransferase (ALT), and gamma-glutamyl transpeptidase (GGT) (by enzymatic methods); and lipid levels including those of triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C) (again by enzymatic methods). C-reactive protein (CRP) levels were determined via particle-enhanced immunonephelometry using the Cardiophase hs-CRP Reagent (Siemens Healthcare Diagnostics, Newark, NJ, USA). Serum adiponectin levels were measured with a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) (Antibody and Immunoassay Services, The University of Hong Kong); the intra- and inter-assay coefficients of variation were 7.34% and 8.64%, respectively. Total serum osteocalcin levels were quantified via electrochemiluminescent immunoassay (Roche Diagnostics GmbH, Mannheim, Germany); the intra- and inter-assay coefficients of variation were 1.2-4.0% and 1.7-6.5%, respectively.
Coronary arteriography and CAD diagnosis
Coronary arteriography was performed using the standard Judkins technique [13]. All major coronary arteries were examined on at least two orthogonal views. Angiograms were evaluated by two experienced cardiologists blinded to study design and clinical information. CAD was diagnosed when stenosis was evident in ≥50% of the luminal diameter of a major coronary artery, i.e., the left main coronary artery, left anterior descending artery or its first diagonal branch, left circumflex artery or its first obtuse marginal branch, or right coronary artery.
Diagnostic criteria for NAFLD
As it was impractical to perform liver biopsies on all of the study participants, fatty liver disease was diagnosed by B ultrasonography of the liver. The ultrasonographic criteria included a diffusely increased near-field ultrasound echo (a ‘bright liver’); a liver echo greater than that of the kidney; vascular blurring; and gradual attenuation of the far-field ultrasound echo [12]. NAFLD was diagnosed using the working definition of the revised guidelines for the management of NAFLD published by the Chinese Liver Disease Association [12].
Statistical analysis
All statistical analyses were performed with SPSS ver. 19.0 (SPSS, Chicago, USA). The one-sample Kolmogorov-Smirnov test was used to explore the normality of data distribution. Normally distributed data were expressed as means ± standard deviations, and skewed data as medians (with inter-quartile ranges). Clinical data were compared between the two groups using the unpaired Student’s t-test (for normally distributed data) or Mann-Whitney U-test (for skewed data), and the chi-square test was used to compare categorical variables. Spearman correlation coefficients between serum osteocalcin levels and various clinical parameters were calculated in simple and partial correlation analyses. Multivariate logistic regression analysis was used to identify factors that were independently associated with the presence of NAFLD. All P-values were two-tailed and the threshold for statistical significance was set at 0.05.
Results
Clinical characteristics of study participants
This study enrolled 174 inpatients (mean age 66.9±10.1 (range 38-86) years; 123 men, 51 postmenopausal women), who were confirmed by CAG to have CAD. The subjects were subdivided into two groups by NAFLD status. As shown in Table 1, compared with non-NAFLD subjects, those with NAFLD had significantly higher BMI, W, blood pressure, FPG, 2 h PG, HbA1c, HOMA-IR, TC, TG, CRP, ALT, and GGT levels. These patients also used more anti-hypertensive drugs, but had significantly lower HDL-C levels (P<0.05). The subjects with NAFLD also had significantly lower serum osteocalcin levels than those without NAFLD [16.2 (14.2-23.8) vs. 20.7 (15.6-26.2) ng/mL, P<0.05; Figure 1].
Table 1.
Subject characteristics (Data were expressed as mean ± SD or median (interquartile range))
| Variables | Non-NAFLD | NAFLD | P |
|---|---|---|---|
| 130 | 44 | ||
| Men/women | 89/41 | 34/10 | 0.267 |
| Age (years) | 67.7±9.8 | 64.5±11.0 | 0.070 |
| BMI (kg/m2) | 23.4±2.7 | 26.5±2.9 | <0.001 |
| Waist circumference (cm) | 87.8±8.9 | 95.5±7.7 | <0.001 |
| SBP (mmHg) | 130 (120-144) | 140 (130-153) | 0.003 |
| DBP (mmHg) | 75 (70-80) | 80 (70-90) | 0.006 |
| FPG (mmol/L) | 5.3 (4.9-6.1) | 6.4 (5.4-7.7) | <0.001 |
| 2 h PG (mmol/L) | 7.8 (6.4-11.4) | 11.5 (8.6-15.3) | <0.001 |
| HbA1c (%) | 6.1 (5.7-6.7) | 6.9 (6.2-8.0) | <0.001 |
| HOMA-IR | 3.8 (2.5-5.0) | 5.7 (4.2-9.2) | <0.001 |
| TC (mmol/L) | 4.1±1.0 | 4.7±1.2 | 0.002 |
| TG (mmol/L) | 1.4 (1.0-1.7) | 2.3 (1.6-3.7) | <0.001 |
| HDL-c (mmol/L) | 1.1 (0.9-1.3) | 0.9 (0.8-1.1) | <0.001 |
| LDL-c (mmol/L) | 2.8±0.9 | 3.1±1.0 | 0.103 |
| CRP (mg/L) | 0.9 (0.5-2.7) | 2.1 (1.1-4.5) | 0.001 |
| ALT (UI/L) | 16.0 (11.0-25.3) | 26.0 (18.0-36.5) | <0.001 |
| AST (UI/L) | 20.0 (16.0-25.0) | 22.0 (19.0-25.8) | 0.080 |
| GGT (UI/L) | 21.0 (14.0-35.0) | 35.0 (23.3-49.5) | <0.001 |
| Adiponectin (mg/L) | 8.2 (5.1-13.5) | 5.6 (4.2-9.6) | 0.001 |
| Anti-diabetic drugs, N (%) | 31 (23.8) | 15 (34.1) | 0.183 |
| Anti-hypertensives, N (%) | 81 (62.3) | 36 (81.8) | 0.017 |
| Statins therapy, N (%) | 39 (30.0) | 17 (38.6) | 0.289 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; 2 h PG, 2 h postprandial plasma glucose; HbA1c, glycosylated haemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase.
Figure 1.
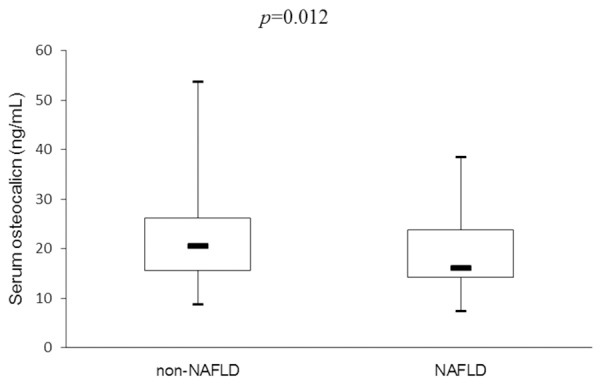
Comparison of serum osteocalcin levels between non-NAFLD and NAFLD groups.
Association between serum osteocalcin levels and NAFLD
We first sought correlations between serum osteocalcin levels and anthropomorphic and biochemical variables. We found that serum osteocalcin levels were negatively associated with male gender (r=-0.239, P=0.002), the presence of NAFLD (r=-0.192, P=0.011), and FPG (r=-0.215, P=0.004). After adjustment for gender and age, the latter two associations remained (NAFLD: r=-0.260, P=0.010 and FPG: r=-0.230, P=0.023), and serum osteocalcin levels correlated with HbA1c also (r=-0.229, P=0.023) (Table 2).
Table 2.
Association between serum osteocalcin levels and anthropometric parameters and biochemical indices
| Variable | Osteocalcin | Adjusted for sex and age | ||
|---|---|---|---|---|
|
| ||||
| r | P | r | P | |
| Male gender | -0.239 | 0.002 | ||
| Age | -0.028 | 0.715 | ||
| NAFLD | -0.192 | 0.011 | -0.260 | 0.010 |
| BMI | -0.048 | 0.530 | -0.063 | 0.539 |
| Waist circumference | -0.144 | 0.058 | -0.037 | 0.716 |
| SBP | 0.109 | 0.153 | 0.081 | 0.425 |
| DBP | -0.009 | 0.906 | -0.088 | 0.391 |
| FPG | -0.215 | 0.004 | -0.230 | 0.023 |
| 2 h PG | -0.076 | 0.320 | -0.141 | 0.166 |
| HbA1c | -0.122 | 0.111 | -0.229 | 0.023 |
| HOMA-IR | -0.083 | 0.293 | -0.099 | 0.334 |
| CRP | 0.041 | 0.602 | -0.182 | 0.073 |
| ALT | -0.012 | 0.874 | -0.018 | 0.862 |
| AST | -0.038 | 0.618 | 0.006 | 0.955 |
| GGT | 0.016 | 0.836 | 0.156 | 0.126 |
| TC | 0.030 | 0.751 | -0.010 | 0.919 |
| TG | 0.097 | 0.294 | -0.050 | 0.623 |
| HDL-C | -0.037 | 0.687 | -0.063 | 0.535 |
| LDL-C | 0.110 | 0.235 | 0.047 | 0.647 |
| Adiponectin | 0.017 | 0.828 | -0.030 | 0.772 |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; FPG, fasting plasma glucose; 2 h PG, 2 h postprandial plasma glucose; HbA1c, glycosylated haemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol.
We next performed multivariate logistic regression analysis using the independent variables osteocalcin, gender, age, BMI, W, blood pressure, HbA1c, HOMA-IR, lipid, CRP, liver enzyme, adiponectin, use of therapeutic statins, use of anti-hypertensives, and use of anti-diabetic drugs. We found that BMI (β=0.345, P<0.001), HbA1c (β=0.641, P=0.004), and TG (β=1.002, P<0.001) were independent positive factors for the presence of NAFLD, whereas the only independent negative factor was serum osteocalcin levels (β=-0.097, P=0.025) (Table 3).
Table 3.
Independent factors of NAFLD identified by multivariate logistic regression analysis
| Independent variable | β | S.E. | OR | P | 95% C.I |
|---|---|---|---|---|---|
| Osteocalcin | -0.097 | 0.044 | 0.907 | 0.025 | 0.833-0.988 |
| BMI | 0.345 | 0.098 | 1.412 | <0.001 | 1.166-1.710 |
| HbA1c | 0.641 | 0.220 | 1.898 | 0.004 | 1.233-2.923 |
| TG | 1.002 | 0.277 | 2.723 | <0.001 | 1.584-4.682 |
Variables included in the original model were osteocalcin, gender, age, BMI, W, SBP, DBP, HbA1c, HOMA-IR, TC, TG, HDL-C, LDL-C, CRP, ALT, AST, GGT, adiponectin; use of therapeutic statins, use of anti-hypertensives, and use of anti-diabetic drugs. Abbreviations: BMI, body mass index; W, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HbA1c, glycosylated haemoglobin A1c; HOMA-IR, homeostasis model assessment of insulin resistance; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; CRP, C-reactive protein; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase.
Discussion
Since it was realized that osteocalcin regulates glycolipid and energy metabolism, many studies have focused on the relationship between osteocalcin and NAFLD, in efforts to find new ways to prevent and treat the condition. A case-control study found that serum osteocalcin levels were significantly lower in patients with biopsy-proven NAFLD than in healthy controls; serum osteocalcin levels were inversely associated with both ALT and AST concentrations and were the only independent predictor of the extent of hepatocyte ballooning in NALFD patients [14]. Large-scale population-based studies found that, in men, serum osteocalcin levels were lower in those with NAFLD than without NAFLD; a lower level was associated with the presence of NAFLD [15]. In women, serum osteocalcin levels were independently and inversely associated with the presence of NAFLD in both pre- and post-menopausal women without osteopenia or osteoporosis [16]. Animal studies showed that subcutaneous infusion or intermittent intraperitoneal injection of osteocalcin into wild-type mice fed high-fat diets improved hepatic steatosis, degeneration caused by ballooning, and fibrosis [17-19]. Osteocalcin attenuated endoplasmic reticulum stress via the NF-κB signaling pathway, and robustly reduced the liver expression levels of pro-inflammatory and pro-fibrotic genes, alleviating NAFLD [18,19]. Such findings suggested that osteocalcin influences the development of NAFLD not only phenomenologically, but also mechanistically. Similarly, in this study, we found that serum osteocalcin levels were significantly lower in CAD patients with NAFLD than in those without NAFLD; furthermore, serum osteocalcin levels were independently associated with the presence of NAFLD in CAD patients. Therefore, osteocalcin may influence the development of NAFLD in patients with CAD. Although several studies described associations between serum osteocalcin levels and NAFLD, other studies yielded contradictory results. Previously, we found that although serum osteocalcin levels were inversely correlated with the presence of NAFLD (P<0.01), the association did not remain significant upon logistic regression analysis [20]. Separately, Rubén et al. [21] and Sinn et al. [16] reported that serum osteocalcin levels were not associated with NAFLD in severely obese patients or in pre- and post-menopausal women with osteopenia or osteoporosis. These findings suggest that the relationship between serum osteocalcin levels and NAFLD may be complex, influenced by race, bone mineral density, weight, and concomitant diseases.
Recent work in the mouse has shown that osteocalcin are closely associated with glucose and lipid metabolism [17,22], and it is widely accepted that perturbations in these aspects of metabolism are common causes of NAFLD. Compared to wild-type mice, osteocalcin-deficient animals exhibited reduced insulin secretion, glucose tolerance, and insulin sensitivity; but increased body weight, fat mass, and adipocyte numbers. Mice with osteocalcin gain-of-function had a near-opposite phenotype [22]. Human epidemiological studies have yielded similar findings; serum osteocalcin levels were inversely associated with body mass, blood glucose level, and insulin-resistance [23-25]. Likewise, in the present study, serum osteocalcin levels were linked to the FPG levels and HbA1c levels. In addition, body mass, TG and HbA1c levels were independently associated with the presence of NAFLD in CAD patients, suggesting that osteocalcin influences disease development by regulating glucose metabolism.
Several limitations of this study should be noted. The study was cross-sectional in nature, the sample size relatively small, and some inherent bias may have been masked. In addition, we did not perform any liver biopsies, which remain the “gold standard” for NAFLD diagnosis. Rather, we used ultrasonography to this end; this technique is of low sensitivity when used to detect hepatic steatosis.
In conclusion, we found that serum osteocalcin levels were independently and negatively associated with the presence of NAFLD in CAD patients. As the study was cross-sectional in nature, we do not know whether osteocalcin influences the incidence of NAFLD in CAD patients, or whether NAFLD developing in such patients changes serum osteocalcin levels. Further large-scale prospective clinical studies are required.
Acknowledgements
We are grateful to all medical staff and nursing at the Department of Cardiology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital for their dedication in the present study. This work was funded by 973 Program of China (2012CB524906), Grant from Shanghai Health and Family Planning Commission (2013ZYJB1001), and Project of National Natural Science Foundation of China (31571212).
Disclosure of conflict of interest
None.
References
- 1.Musso G, Paschetta E, Gambino R, Cassader M, Molinaro F. Interactions among bone, liver, and adipose tissue predisposing to diabesity and fatty liver. Trends Mol Med. 2013;19:522–535. doi: 10.1016/j.molmed.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Cairns JR, Price PA. Direct demonstration that the vitamin K-dependent bone Gla protein is incompletely gamma-carboxylated in humans. J Bone Miner Res. 1994;9:1989–1997. doi: 10.1002/jbmr.5650091220. [DOI] [PubMed] [Google Scholar]
- 3.Brown JP, Delmas PD, Malaval L, Edouard C, Chapuy MC, Meunier PJ. Serum bone Gla-protein: a specific marker for bone formation in postmenopausal osteoporosis. Lancet. 1984;1:1091–1093. doi: 10.1016/s0140-6736(84)92506-6. [DOI] [PubMed] [Google Scholar]
- 4.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 5.Ducy P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia. 2011;54:1291–1297. doi: 10.1007/s00125-011-2155-z. [DOI] [PubMed] [Google Scholar]
- 6.Bonora E, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Cacciatori V, Santi L, Targher G, Bonadonna R, Muggeo M. HOMA-estimated insulin resistance is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabetes Care. 2002;25:1135–1141. doi: 10.2337/diacare.25.7.1135. [DOI] [PubMed] [Google Scholar]
- 7.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 8.Holvik K, van Schoor NM, Eekhoff EM, den Heijer M, Deeg DJ, Lips P, de Jongh R. Plasma osteocalcin levels as a predictor of cardiovascular disease in older men and women: a population-based cohort study. Eur J Endocrinol. 2014;171:161–170. doi: 10.1530/EJE-13-1044. [DOI] [PubMed] [Google Scholar]
- 9.Bao Y, Zhou M, Lu Z, Li H, Wang Y, Sun L, Gao M, Wei M, Jia W. Serum levels of osteocalcin are inversely associated with the metabolic syndrome and the severity of coronary artery disease in Chinese men. Clin Endocrinol (Oxf) 2011;75:196–201. doi: 10.1111/j.1365-2265.2011.04065.x. [DOI] [PubMed] [Google Scholar]
- 10.Polgreen LE, Jacobs DR, Nathan BM, Steinberger J, Moran A, Sinaiko AR. Association of osteocalcin with obesity, insulin resistance, and cardiovascular risk factors in young adults. Obesity. 2012;20:2194–2201. doi: 10.1038/oby.2012.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lerchbaum E, Schwetz V, Pilz S, Grammer TB, Look M, Boehm BO, Obermayer-Pietsch B, März W. Association of bone turnover markers with mortality in men referred to coronary angiography. Osteoporos Int. 2013;24:1321–1332. doi: 10.1007/s00198-012-2076-9. [DOI] [PubMed] [Google Scholar]
- 12.Jian-Gao F. Guidelines for management of nonalcoholic fatty liver disease: an updated and revised edition. Zhonghua Gan Zang Bing Za Zhi. 2010;18:163–166. [PubMed] [Google Scholar]
- 13.Judkins MP. Percutaneous transfemoral selective coronary arteriography. Radiol Clin North Am. 1968;6:467–492. [PubMed] [Google Scholar]
- 14.Yilmaz Y, Kurt R, Eren F, Imeryuz N. Serum osteocalcin levels in patients with nonalcoholic fatty liver disease: association with ballooning degeneration. Scand J Clin Lab Invest. 2011;71:631–636. doi: 10.3109/00365513.2011.604427. [DOI] [PubMed] [Google Scholar]
- 15.Liu JJ, Chen YY, Mo ZN, Tian GX, Tan AH, Gao Y, Yang XB, Zhang HY, Li ZX. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in adult males, South China. Int J Mol Sci. 2013;14:19782–19791. doi: 10.3390/ijms141019782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinn DH, Gwak GY, Rhee SY, Cho J, Son HJ, Paik YH, Choi MS, Lee JH, Koh KC, Yoo BC, Paik SW. Association between serum osteocalcin levels and non-alcoholic fatty liver disease in women. Digestion. 2015;91:150–157. doi: 10.1159/000369789. [DOI] [PubMed] [Google Scholar]
- 17.Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–575. doi: 10.1016/j.bone.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B, Li H, Xu L, Zang W, Wu S, Sun H. Osteocalcin reverses endoplasmic reticulum stress and improves impaired insulin sensitivity secondary to diet-induced obesity through nuclear factor-κB signaling pathway. Endocrinology. 2013;154:1055–1068. doi: 10.1210/en.2012-2144. [DOI] [PubMed] [Google Scholar]
- 19.Gupte AA, Sabek OM, Fraga D, Minze LJ, Nishimoto SK, Liu JZ, Afshar S, Gaber L, Lyon CJ, Gaber AO, Hsueh WA. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology. 2014;155:4697–4705. doi: 10.1210/en.2014-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dou J, Ma X, Fang Q, Hao Y, Yang R, Wang F, Zhu J, Bao Y, Jia W. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in Chinese men. Clin Exp Pharmacol Physiol. 2013;40:282–288. doi: 10.1111/1440-1681.12063. [DOI] [PubMed] [Google Scholar]
- 21.Díez Rodríguez R, Ballesteros Pomar MD, Calleja Fernández A, Calleja Antolin S, Cano Rodríguez I, Linares Torres P, Jorquera Plaza F, Olcoz Goñi JL. Vitamin D levels and bone turnover markers are not related to non-alcoholic fatty liver disease in severely obese patients. Nutr Hosp. 2014;30:1256–1262. doi: 10.3305/nh.2014.30.6.7948. [DOI] [PubMed] [Google Scholar]
- 22.Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou M, Ma X, Li H, Pan X, Tang J, Gao Y, Hou X, Lu H, Bao Y, Jia W. Serum osteocalcin concentrations in relation to glucose and lipid metabolism in Chinese individuals. Eur J Endocrinol. 2009;161:723–729. doi: 10.1530/EJE-09-0585. [DOI] [PubMed] [Google Scholar]
- 24.Saleem U, Mosley TH, Kullo IJ. Serum osteocalcin is associated with measures of insulin resistance, adipokine levels, and the presence of metabolic syndrome. Arterioscler Thromb Vasc Biol. 2010;30:1474–1478. doi: 10.1161/ATVBAHA.110.204859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeap BB, Chubb SA, Flicker L, McCaul KA, Ebeling PR, Beilby JP, Norman PE. Reduced serum total osteocalcin is associated with metabolic syndrome in older men via waist circumference, hyperglycemia, and triglyceride levels. Eur J Endocrinol. 2010;163:265–272. doi: 10.1530/EJE-10-0414. [DOI] [PubMed] [Google Scholar]


