Abstract
ER+ and ER- tumors exhibit different histopathological and clinical properties. Receptor determination exists as a marker with predictive value rather than prognostic importance. Patients with invasive breast cancer (n=2849) were investigated retrospectively between 1981 and 2013. Patients were separated to four subgroups, as follows: ER+; non-luminal HER2+; ER-/PR-/HER2-; ER-PR+. We investigated the effects of ER positivity on long-term survival in breast cancers, by considering their pathological properties, surgical method applications, chemotherapy preferences, and combined hormonal treatments with regard to ER, PR and HER2 status. ER+ cases were premenopausal, and they existed with low-grade, small-sized and early stage tumors (P<0.05). One thousand three hundred and eighty five cases (68.6%) were administered chemotherapy, which was followed by hormone therapy. Non-luminal HER2+ tumors were found to exhibit longer survival, when compared to triple negative and ER- tumors (P=0.010). Triple negative cases had the shortest survival rates; survival values determined in the HER2+ and ER-/PR+ cases were found to be between the survivals of ER+ and TN tumors. ER, PR and HER2 positivity was not concomitant with a risk of recurrence (P>0.05). Furthermore, recurrence risk rose significantly when age, tumor stage and tumor grade increased (P<0.05). ER+ tumors are observed in women of advanced age, but have a good clinical response. Currently, receptor determination is still generally preferred as a practical application. ER analysis in the early stage breast cancers for women of advanced ages must be considered as an indicator of anti-estrogenic therapy administration, rather than prognostic importance.
Keywords: Breast cancer subtype, estrogen receptor, survival
Introduction
Breast cancer remains a significant health issue. Surgical procedures, chemotherapy, radiotherapy, and combined hormonal treatment help to improve prognoses. Histopathological properties of the tumor, receptor subtypes, personal properties of the patient and preferences of chemotherapy regimens, play important roles in the recurrence of the disease. Different genetic variations in the tumor biology and cross-reactions between receptors are the main prognostic and predictive factors. Estrogen receptor (ER)-positive breast cancers comprise 80% of all breast cancers. ER is a practical indicator of normal breast development and progress of the cancer [1]. ER positivity is an advantageous response to endocrine therapy [1]. Progesterone receptor (PR) release maintained by estrogen has a weak predictive value, but it has great importance in clinical evaluation [1,2]. PR positivity in particular, affects the prognosis in a positive way [1].
We aimed in the present study to compare the histopathological properties of ER+ breast tumors with those of triple-negative (TN), HER2+ and ER- subgroups, and to investigate the effect of receptor determination on survival and recurrence in the early stage breast cancers, which have been followed-up for ten years.
Materials and methods
Our study included 2849 cases investigated retrospectively between the years 1981 and 2013. Groups were compared with regard to age, menopause, histological grade, tumor size, and lymphovascular invasion (LVI) and lymph node status. Pathological tumor stage was classified according to the American Joint Committee on Cancer (AJCC) 6th Staging System. Breast cancers were considered in four subtypes according to their receptor properties: ER+, non-luminal HER2 positive, ER-PR+, and triple negative (ER-/PR-/HER2-).
HER2/neu was scored between 0 to 3 immunohistochemically, and classified as follows: 0-1: no membrane staining, HER2 negative, 2: weak staining, 3: intensive staining (complete membrane staining in more than 10% of the cells), HER2+. Cases that had a score of 2 were fluorescence in situ hybridization (FISH) tested; by applying the HercepTest DAKO test, HER2/CEP17 values >2.0 were accepted to be positive. Staining in more than 5% of the tumor cells was accepted as ER/PR positivity.
We obtained informed consent from the patients and were given formal approval from the University’s ethnic committee for the study.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 (IBM). The groups were compared using the chi-square test. Clinicopathologic features consisted of age, menopause, grade, size and tumor node and treatment. Survival analysis was determined by Kaplan-Meier and log-rank tests. Analysis of survival was documented for patients with loco-regional recurrence, contralateral recurrence and distant metastasis. Risk factors affecting survival were analyzed using the multivariate cox-regression test and documented as hazard ratios and 95% confidence interval results. Age (≥60/<40), grade, stage, node and receptor status compared to one another. A P<0.05 value was accepted to be statistically significant.
Results
The mean general age and diagnostic age in 2849 cases were 56.32 years and 49.30 years, respectively. Distribution of the cases were as follows: 2012 cases were ER positive (75.4%), 311 cases were TN (11.7%), 220 cases were ER-PR-HER2+ (8.2%), and 126 cases were ER-PR+ (4.7%) (Table 1). Metastasis was present in 505 (17.7%) cases, and it was not detected in 2344 (82.3%) cases. At the end of a mean 125-month follow-up 289 patients (10.1%) existed with relapse, and 97 patients (3.4%) died.
Table 1.
Histopathological features of tumors
| ER+ | ER-PR-HER2- | ER-PR-HER2+ | ER-PR+ | P-value | |
|---|---|---|---|---|---|
| n=2012 (75.4%) | n=311 (11.7%) | n=220 (8.2%) | n=126 (4.7%) | ||
| Age | |||||
| <60 | 1266 (75%) | 204 (12.1%) | 129 (7.6%) | 88 (5.2%) | 0.000 |
| ≥60 | 742 (75.8%) | 107 (11%) | 91 (9.3%) | 38 (3.9%) | |
| Menopause | |||||
| Pre | 1076 (53.5%) | 171 (55%) | 116 (52.7%) | 75 (59.5%) | 0.001 |
| post | 915 (45.5%) | 140 (45%) | 104 (47.3%) | 51 (40.5%) | |
| Grade | |||||
| I/II | 1203 (59.8%) | 66 (21.2%) | 70 (31.8%) | 35 (27.8%) | 0.000 |
| III | 588 (29.2%) | 217 (69.8%) | 132 (60%) | 78 (61.9%) | |
| Tumor size | |||||
| ≤5 cm | 1579 (78.5%) | 245 (78.8%) | 143 (65%) | 91 (72.2%) | 0.000 |
| >5 cm | 358 (17.9%) | 56 (18%) | 67 (30.4%) | 28 (22.2%) | |
| Node | |||||
| N0 | 1472 (73.2%) | 226 (72.7%) | 129 (58.6%) | 87 (69%) | 0.000 |
| N+ | 540 (26.8%) | 85 (27.3%) | 91 (41.4%) | 39 (31%) | |
| Treatment | |||||
| No | 147 (7.3%) | 11 (3.5%) | 13 (5.9%) | 7 (5.6%) | |
| Chemotherapy | 1385 (68.8%) | 293 (94.3%) | 204 (92.7%) | 112 (88.8%) | |
| Only hormone | 441 (21.9%) | 0 | 0 | 7 (5.6%) | |
| Unknown | 39 (1.9%) | 7 (2.2%) | 3 (1.4%) | 0 | |
| RT+ | 1408 (70%) | 221 (71.1%) | 165 (75%) | 95 (75.4%) |
The types of tumors determined in the patients were as follows: 2321 cases (81.6%) of infiltrative ductal carcinoma, 142 cases (5%) of infiltrative lobular carcinoma, 222 cases (7.8%) of mixed type carcinoma, six cases (0.2%) of adenocarcinoma, 102 cases (3.6%) of carcinoma in situ and two cases of cystosarcoma phyllodes.
ER+ tumors exhibited better histopathological and survival properties when compared with TN, HER2+ and ER- subgroups (P=0.010; Table 2; Figure 1).
Table 2.
Survival analyses of subgroups
| Number of patients | Number of case-observed | Percent of case-observed | Mean survival (day) | P-value | |
|---|---|---|---|---|---|
| ER+ | 396 | 57 | 85.6 | 5030,747 | 0.010* |
| ER-PR+ | 78 | 5 | 86.5 | 4718,160 | |
| HER2+ | 37 | 9 | 87.0 | 3149,519 | |
| TN | 69 | 16 | 79.5 | 4150,100 | |
| Total | 580 | 87 | 85% | 4940,640 |
Figure 1.
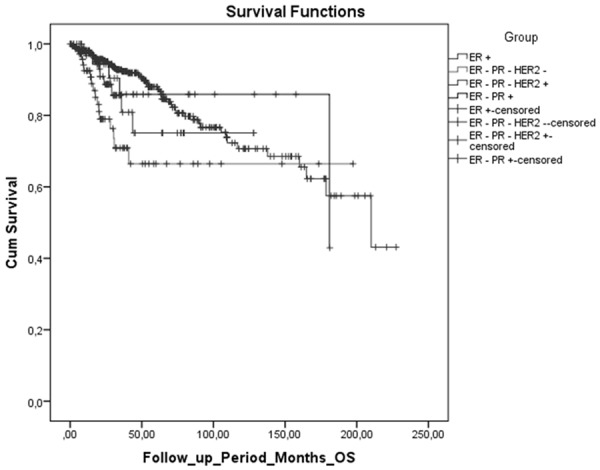
Analysis of overall survival of breast cancer subtypes by Kaplan-Meier. ER-positive cases were determined to have longer survival rates when compared to non-luminal HER2+, ER-PR-HER2- and luminal B (ER-PR+) tumors.
Discussion
Expression levels of ER and PR are also related with prognostic factors such as menopausal status, stage, tumor size, nodal involvement, and histological type and the grade of the tumor [3]. 25% of malign tumors exist with ER positivity, and 30% of them exhibit PR positivity [3]. ER expression does not exist in tumor types like medullary carcinoma, metaplastic breast cancer, and cystic adenoid carcinoma [3]. The staining ratio of estrogen receptor affects the response to therapy. The response to therapy was reported to be 20% in intermediate and densely receptor-positive tumors that exhibit ER membrane staining of 1% to 33%; however, response to hormonal therapy has been found to be 50% to 75% in tumors that show more than 34% of staining [3]. ER positivity exists with response to antiestrogen treatment, but it has less prognostic effect [3].
In our study, ER-positive cases were determined to have longer survival rates when compared to non-luminal HER2+, TN and luminal B (ER-PR+) tumors (P=0.010, Table 2). The clinical course showed differences, even though tumors existed with similar histopathological properties. Contrary to some other studies, our cases with ER+ tumors that were applied chemotherapy followed by singular or alternating hormonal therapy, did not exhibit similar survival analyses with those of ER- cases [4,5]. Prognostic factors that cause recurrence were determined to be age (P=0.000), grade (P=0.039) and stage of tumor (P=0.002). A one-unit increase in the patient’s age, grade or stage risked an increase in the possibility of recurrence 1.03 fold (RR=1.03, SE=0.01, P=0.001), 8.4 fold (RR=8.4, SE=1.03, P=0.039), and 8.2 fold (RR=8.2, SE=0.57, P=0.002), respectively. ER, PR or HER2 positivity did not cause a risk of recurrence (RR) (P>0.05, Table 3). Cases with TN tumors existed with the shortest survivals [6,7]. ER+ tumors exhibited low grade, smaller tumor size, early stage, and N0-N1 nodal status; it was found that statistical differences were caused by non-luminal HER2+ or triple negative status, rather than ER negativity (P=0.000). In a study by Moise M. et al., the expression of ER or PR was not found to be significantly related to the tumor stage, menopause, lymphatic invasion, tumor size, or histological type [3]. In similar studies [8,9], luminal A tumors were found to have lower RR when compared to TN and non-luminal HER2 types. TN tumors exhibited higher risks for loco-regional recurrence when compared with ER+ tumors, except for non-luminal HER2 tumors [8]. In a study by Bessonova et al. [10], risk for local recurrence was determined in hormone receptor-negative tumors, which was unrelated with HER2 status.
Table 3.
Univariate Cox-regresyon analysis of factors associated with recurrence in patients with subgroups
| RR | 95% CI | P-value | |
|---|---|---|---|
| Age | |||
| >60/<40 | 1.0 | 0.3-0.6 | 0.000 |
| Stage (2, 3, 4)/1 | |||
| 3/1 | 8.2 | 0.3-15 | 0.002 |
| 2/1 | 2.3 | 0.2-0.9 | 0.328 |
| Grade (III/II)/I | |||
| III/I | 8.4 | 1.1-2.6 | 0.039 |
| II/I | 1.2 | 0.8-1.9 | 0.311 |
| Node | |||
| N+/N0 | 0.9 | 1.4-2.3 | 0.959 |
| ER positive | 0.5 | 1.3-1.9 | 0.194 |
| PR positive | 0.8 | 1.2-1.8 | 0.704 |
| HER2 positive | 0.6 | 0.6-0.9 | 0.194 |
ER positivity was detected in 2012 cases (75.4%) in our study, and this result is in accordance with those in the literature [2,11]; additionally, 126 cases (4.7%) were determined to possess ER-PR+ status. However, ER determination by immunohistochemical methods is considered to be a weak marker in determining the clinical course of breast cancer, but it is a strong marker in predicting the response for hormonal therapy [12]. ER status also affects the tumor grade and histology. ER+ tumors are frequently lobular, colloid or tubular carcinomas with low grades [12]. The estrogen receptor is related with a long disease-free survival and increased hormonal response. They are frequently small-sized tumors of low grade [13]. The prognosis is good in HER2-tumors with high levels of estrogen receptor [4]. In addition to these, we determined less nodal invasion in the cases with ER+ tumors, and this result is in accordance with that in the literature.
In some studies, PR positivity in the cases with ER+ tumors led to better clinical outcomes; however in several studies, recurrence and mortality rates independent of PR status were determined in ER+ cases that took hormone therapy [1,14]. ER- breast cancers have poor prognosis because of chromosomal instability [15]; however, they respond well to chemotherapy [16].
In a study by Li Anqi et al., ER+/PR- tumors occurred with advanced age (55 years), high grade, large tumor size (>5 cm), N+ involvement, and high proliferation index [1]. Ki67 index is low in ER+ tumors that exhibit low PR expression, and they therefore show good responses to chemotherapy and the subsequent hormonal treatment. However, in some studies, chemotherapy and additional hormonal therapy were not found to be any more helpful with regard to expected risks in ER+ tumors with low grade and proliferation index [17,18]. Therefore, a preference for chemotherapy in these tumors is still a matter of debate.
Our cases were administered singular or combined chemotherapy by using cyclophosphamide, taxane, doxorubicin, epirubicin, fluorouracil, gemcitabine, herceptin, platinum and methotrexate. One thousand eight hundred and ninety six (1,896) cases (66.7%) were applied mastectomy, and 781 cases (27.4%) had undergone breast-protecting surgery (BPS). All BPS cases were administered local RT. 1679 of the ER+ cases (83.4%), 253 of the TN cases (81.3%), 165 of the HER2+ cases (75%), and 95 of the ER- cases (75.4%) were administered combined adjuvant chemotherapy regimens followed by antiestrogenic treatment (tamoxifen, aromatase inhibitors or ‘switch’ treatment) alternatives. Although defined in the breast cancer subgroups, luminal A and luminal B types were observed not to differ obviously at the cellular level [16]; therefore, this patient group was classified as ‘luminal’. Gene sequence is more complex in ER+ tumors when compared to ER- ones, and they therefore possess more genetic sequences responsible for the reaction to chemotherapy. However, the response to chemotherapy is maintained by metabolic pathways in ER- tumors. Chemotherapeutic effect exists with multiple drug interactions in different pathways [16]. Therefore, the determination of the histopathological properties of the tumor, rather than the receptor analysis, is important when considering therapeutic preferences; recurrence score (21-gene assay) determination is important when predicting the future course of the disease. These considerations will lead to elimination of excess chemotherapeutic applications and prolonged hormonal therapy alternatives.
Conclusion
Despite similarities in the practical determinations of histopathological and intrinsic subtypes, breast cancers exhibit different prognostic and therapeutic responses due to the heterogeneity existing in their gene expression patterns. Therefore, the histopathological properties of the tumor provide noticeably useful information in early stage breast cancers. Intrinsic properties of the tumor, rather than ER, play an important role in the response to therapy. Determination of the estrogen receptor may only indicate whether the tumor is a good candidate for antiestrogenic therapy.
Disclosure of conflict of interest
None.
References
- 1.Li AQ, Zhou SL, Li M, Xu Y, Shui RH, Yu BH, Yang WT. Clinicopathologic Characteristics of Oestrogen Receptor-Positive/Progesterone Receptor-Negative/Her2-Negative Breast Cancer According to a Novel Definition of Negative Progesterone Receptor Status: A Large Population-Based Study from China. PLoS One. 2015;10:e0125067. doi: 10.1371/journal.pone.0125067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou XL, Fan W, Yang G, Yu MX. The clinical significance of PR, ER, NF-kappa B, and TNFalpha in breast cancer. Dis Markers. 2014;2014:494581. doi: 10.1155/2014/494581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moise M, Buruian MM, Ilie C, Zamfir CL, Folescu R, Motoc AG. Estrogen and progesterone receptor expression in the mammary gland tumors. Rom J Morphol Embryol. 2013;54:961–968. [PubMed] [Google Scholar]
- 4.Lonning PE. Poor-prognosis estrogen receptorpositive disease: present and future clinical solutions. Therap Advan Med Oncol. 2012;4:127–137. doi: 10.1177/1758834012439338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C, Wang YC, Bergh J, Di Leo A, Albain K, Swain S, Piccart M, Pritchard K. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bulut N, Aksoy S, Dizdar O, Dede DS, Arslan C, Dogan E, Gullu I, Ozisik Y, Altundag K. Demographic and clinico-pathological characteristics in patients with triple-negative and non-triple-negative breast cancer. Med Oncol. 2011;28(Suppl 1):S75–79. doi: 10.1007/s12032-010-9715-9. [DOI] [PubMed] [Google Scholar]
- 7.Guo L, Zhu Q, Aisimutuola M, Yilamu D, Liu S, Jakulin A. Expression and prognostic value of estrogen receptor beta in patients with triplenegative and triple-positive breast cancer. Exp Therap Med. 2015;9:2147–2150. doi: 10.3892/etm.2015.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim HJ, Kim SH, Kang BJ, Choi BG, Kim HS, Cha ES, Song BJ. Breast cancer recurrence according to molecular subtype. Asian Pac J Cancer Prev. 2014;15:5539–5544. doi: 10.7314/apjcp.2014.15.14.5539. [DOI] [PubMed] [Google Scholar]
- 9.Najafi B, Anvari S, Roshan ZA. Disease free survival among molecular subtypes of early stage breast cancer between 2001 and 2010 in Iran. Asian Pac J Cancer Prev. 2013;14:5811–5816. doi: 10.7314/apjcp.2013.14.10.5811. [DOI] [PubMed] [Google Scholar]
- 10.Bessonova L, Taylor TH, Mehta RS, Zell JA, Anton-Culver H. Risk of a second breast cancer associated with hormone-receptor and HER2/neu status of the first breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20:389–396. doi: 10.1158/1055-9965.EPI-10-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dowsett M, Houghton J, Iden C, Salter J, Farndon J, A’Hern R, Sainsbury R, Baum M. Benefit from adjuvant tamoxifen therapy in primary breast cancer patients according oestrogen receptor, progesterone receptor, EGF receptor and HER2 status. Ann Oncol. 2006;17:818–826. doi: 10.1093/annonc/mdl016. [DOI] [PubMed] [Google Scholar]
- 12.Maleki Z, Shariat S, Mokri M, Atri M. ERnegative/PR-positive breast carcinomas or technical artifacts in immunohistochemistry? Arch Iran med. 2012;15:366–369. [PubMed] [Google Scholar]
- 13.Sofi GN, Sofi JN, Nadeem R, Shiekh RY, Khan FA, Sofi AA, Bhat HA, Bhat RA. Estrogen receptor and progesterone receptor status in breast cancer in relation to age, histological grade, size of lesion and lymph node involvement. Asian Pac J Cancer Prev. 2012;13:5047–5052. doi: 10.7314/apjcp.2012.13.10.5047. [DOI] [PubMed] [Google Scholar]
- 14.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, Dowsett M, Ingle J, Peto R. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamal-Hanjani M, A’Hern R, Birkbak NJ, Gorman P, Gronroos E, Ngang S, Nicola P, Rahman L, Thanopoulou E, Kelly G, Ellis P, Barrett-Lee P, Johnston SR, Bliss J, Roylance R, Swanton C. Extreme chromosomal instability forecasts improved outcome in ER-negative breast cancer: a prospective validation cohort study from the TACT trial. Ann Oncol. 2015;26:1340–1346. doi: 10.1093/annonc/mdv178. [DOI] [PubMed] [Google Scholar]
- 16.Shen K, Rice SD, Gingrich DA, Wang D, Mi Z, Tian C, Ding Z, Brower SL, Ervin PR Jr, Gabrin MJ, Tseng G, Song N. Distinct genes related to drug response identified in ER positive and ER negative breast cancer cell lines. PLoS One. 2012;7:e40900. doi: 10.1371/journal.pone.0040900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, Cronin M, Baehner FL, Watson D, Bryant J, Costantino JP, Geyer CE Jr, Wickerham DL, Wolmark N. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J. Clin. Oncol. 2006;24:3726–3734. doi: 10.1200/JCO.2005.04.7985. [DOI] [PubMed] [Google Scholar]
- 18.Jessup JM, Lively TG, Taube SE. Program for the Assessment of Clinical Cancer Tests (PACCT): implementing promising assays into clinical practice. Expert Rev Mol Diagn. 2005;5:271–273. doi: 10.1586/14737159.5.3.271. [DOI] [PubMed] [Google Scholar]


