Abstract
Abnormal proliferation and migration of vascular smooth muscle cells (VSMCs) and the stimulation of platelet-derived growth factor (PDGF)-BB play major pathological processes involved in the development of cardiovascular diseases. As a result, the use of anti-proliferative and anti-migratory agents for VSMCs offers promise for the treatment of vascular disorders. Myricitrin is a naturally occurring phenolic compound which possesses antioxidant and anti-inflammatory activity. In this study, we investigate the inhibitory effect of myricitrin on PDGF-BB-induced VSMCs proliferation and migration. In accordance with these findings, myricitrin induced the arrest of cell cycle progression at G0/G1 phase. Myricitrin also decreased the expressions of G0/G1 specific regulatory proteins including cyclin D1, cyclin-dependent kinases (CDK) 4, cyclin E and CDK2, as well as increased the expression of p21 in PDGF-BB-induced VSMCs. Moreover, myricitrin inhibited PDGF-BB-induced phosphorylation of PDGFRβ, Akt and Erk1/2. These results suggest that myricitrin plays an important role in prevention of VSMCs proliferation and migration through the G0/G1 cell cycle arrest by PDGF signaling pathway. Thus, myricitrin is effective in reducing atherosclerotic process by blocking proliferation of VSMCs.
Keywords: Myricitrin, vascular smooth muscle cells, PDGF-BB, proliferation, migration
Introduction
Cardiovascular diseases (CVDs), along with cancer, are the most common causes of death. The primary underlying pathology of CVD is atherosclerosis, a chronic inflammatory disease of the vessel wall of large-to medium-sized arteries [1]. Atherosclerotic plaques are complex lesions in which repair of tissue injury is associated with vascular smooth muscle cells (VSMCs) proliferation and migration, connective tissue formation and calcium deposition [2]. VSMCs play a critical role in the development of vascular disease. The abnormal proliferation and migration of VSMCs has a pivotal role in the progression of vascular occlusion diseases, such as atherosclerosis and restenosis [3]. As a result, anti-proliferative and anti-migratory agents for VSMCs offer promise for the treatment of vascular disorders.
Myricitrin, a botanical flavone, is abundantly distributed in the root bark of Myrica cerifera, Ampelopsis grossedentata, Myrica esculenta, Chrysobalanus icaco, Nymphaea lotus, and other plants [4,5]. Many investigations have found that myricitrin has numerous pharmacological effects; for example, it can protect a variety of cells from in vitro and in vivo injuries and antioxidant [6], anti-inflammatory [7], as well as a neuroprotective action through the antagonism of 6-OHDA-induced neurotoxicity [8]. Previous studies have shown that myricitrin could attenuate endothelial cell apoptosis to prevent atherosclerosis [9]. Myricitrin has been shown to protect endothelia cells against ROS-induced apoptosis and prevent atherosclerosis formation in ApoE/mice [10]. All of these findings imply that myricitrin may be used for the prevention and treatment of cardiovascular disease. However, its detailed mechanisms require further study to provide more scientific evidence for the clinical treatment of atherosclerosis.
The proliferation of VSMC can be induced by cytokines and growth factors, such as platelet-derived growth factor (PDGF), tumor necrosis factor (TNF)-α and transforming growth factor (TGF)-β [11]. PDGF is a major growth factor and is known to contribute to the development of atherosclerosis through the induction of abnormal VSMCs phenotypes [12]. VSMCs proliferation induced by PDGF-BB has been considered to be crucial for the development of vascular diseases [13]. Therefore, the regulatory mechanism of PDGF-BB signaling in inhibition of VSMCs proliferation is one of the key pharmacological strategies for prevention of atherosclerosis. However, the effects of myricitrin on VSMCs proliferation have not yet been clarified. Thus the aim of this study was to elucidate the effects of myricitrin on PDGF-BB-stimulated VSMCs proliferation and migration, as well as its mechanism.
Materials and methods
Antibodies and reagents
Myricitrin was purchased from purchased from Sigma-Aldrich (Taufkirchen, Germany). Mouse anti-Erk1/2 monoclonal antibody, mouse anti-Akt monoclonal antibody, mouse anti-PDGF-Rβ monoclonal antibody, mouse anti-p-Erk1/2 monoclonal antibody, mouse anti-p-Akt monoclonal antibody, mouse anti-cyclin-dependent kinases (CDK) 2 monoclonal antibody, mouse anti-CDK4 monoclonal antibody, mouse anti-cyclin D1 monoclonal antibody, mouse anti-cyclin E monoclonal antibody and mouse anti-β-actin monoclonal antibody were obtained from Abcam (Cambridge, MA). HRP-conjugated goat anti-mouse IgG was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). The Fluorescein isothiocyanate (FITC)-Annexin V/Propidium iodide (PI) apoptosis assay kit was from Invitrogen (Carlsbad, CA, USA). Dimethyl sulfoxide (DMSO), 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and propidium iodide (PI) were from Hitachi (Tokyo, Japan). The enhanced chemiluminescence Western blot detection reagents were from Gibco (Rockville, MD).
Cell culture
Animal experiments conformed to the guidelines issued by the Institute of Tianjin Hospital for Laboratory Animals. The present study was performed with approval from by the Animal Ethics Committee of the Institute of Tianjin Hospital. All surgery was performed under sodium pentobarbital anesthesia (Sigma, St. Louis, MO), and all efforts were made to minimize suffering. The VSMCs isolated from Sprague-Dawley rats (Shanghai Laboratory Animal Center, the Chinese Academy of Sciences) were prepared as described previously [14], with minor modifications. VSMCs were maintained in DMEM medium supplemented with 10% FBS, 1 mM sodium pyruvate, 2 mM L-glutamine, 100 mg/L streptomycin and 100 units/L penicillin. The cells were kept in a humidified 5% CO2-95% air incubator at 37°C and were seeded in complete medium for 24 h. Then, the cells were changed to fresh serum-free media containing the indicated concentration of myricitrin (10, 20 and 40 μM). After 24 h, the cells were replaced with fresh serum-free media containing the 20 ng/mL of PDGF-BB for 24 h.
Determination of cell proliferation
VSMCs proliferation was measured using the previously described 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetr-razolium bromide (MTT) assay. The VSMCs were seeded in 96-well flat-bottom microtiter plates (Millipore, Boston, MA, USA) at a density of 1.0×104 cells per well, and VSMCs were treated with various concentration of myricitrin for 24 h and then then stimulated with 20 ng/mL PDGF-BB for an additional 24 h. An MTT solution (5 mg/mL) was added at the end of incubation, and the VSMCs were incubated for 4 h at 37°C. The medium was removed, and dimethyl sulfoxide (DMSO) was added to each well. The absorbance value in 96-well plate was read spectrophotometrically at 570 nm on a microtiter plate reader (Gibco, Rockville, MD).
Cell migration assay
VSMCs migration was examined by using a modified Boyden chamber, as previously described [15]. Briefly, VSMCs (1×104 cells/well) were seeded onto the upper surface of an 8-µm pore size chamber (Sigma, St. Louis, MO, USA) in serum-free medium containing 0.2% bovine albumin serum (BSA) and then pretreated with myricitrin (40 µM). PDGF (20 ng/mL) was added only in the lower chamber, and cells were then incubated at 37°C in air containing 5% CO2. After 16 h, cells that had not migrated were removed from the upper chamber, and the cells that had migrated to the lower surface of the filter were fixed with methanol for 10 min at 4°C. Migrated cells were then stained and counted from at least five fields for each well, using a microscope (100× magnification) [16].
Western blot
The harvested cells were lysed with PRO-PREP® protein extraction solution (Bio-Rad, Hercules, CA, USA). Protein extracts (30 μg of total protein/sample) were electrophoresed using 10% SDS-PAGE and then transferred to the polyvinylidene difluoride (PVDF) membranes (Sigma, St. Louis, MO, USA). The blotted membranes were blocked in 5% non-fat milk for 1 h and then incubated overnight at 4°C with the primary antibodies against Erk1/2, Akt, p-Erk1/2, p-Akt, CDK 2, CDK4, cyclin E, cyclin D1. After that, the membranes were further incubated with the horseradish peroxidase-linked secondary antibodies for 1 h, followed by signal visualization using an electrochemical luminescence (ECL) reagent. Images were acquired using the Image Quant LAS4000 gel imager (Hitachi, Tokyo, Japan). Band intensities were quantified by using the Multi Gauge v3.0 software (Hitachi, Tokyo, Japan). The percentage of protein expression was calculated according to the values of the control group as 100% [16].
Statistical analysis
All data were expressed as mean ± SD, and significance of differences was analyzed by one-way ANOVA followed by Dunnett’s Multiple Comparison test (SPSS 16.0 software). A value of P<0.05 were considered statistically significant.
Results
Myricitrin inhibits PDGF-BB-induced VSMCs proliferation
We first examined the effect of myricitrin on PDGF-BB-induced proliferation of VSMCs using the MTT assay. The number of surviving cells is directly proportional to the level of formazan product. As shown in Figure 1A, the OD570 was significantly higher in the PDGF-BB group than in the control group (P<0.05). However, a significant down-regulation of OD570 was observed in the various concentration of myricitrin + PDGF-BB groups compared with the PDGF-BB group (P<0.05) in a dose dependent manner. Moreover, myricitrin treatment in the absence of PDGF-BB did not decrease the viability of the VSMCs compared with the control group (Figure 1B). These results indicated that myricitrin appeared to inhibit PDGF-BB-induced VSMCs proliferation.
Figure 1.
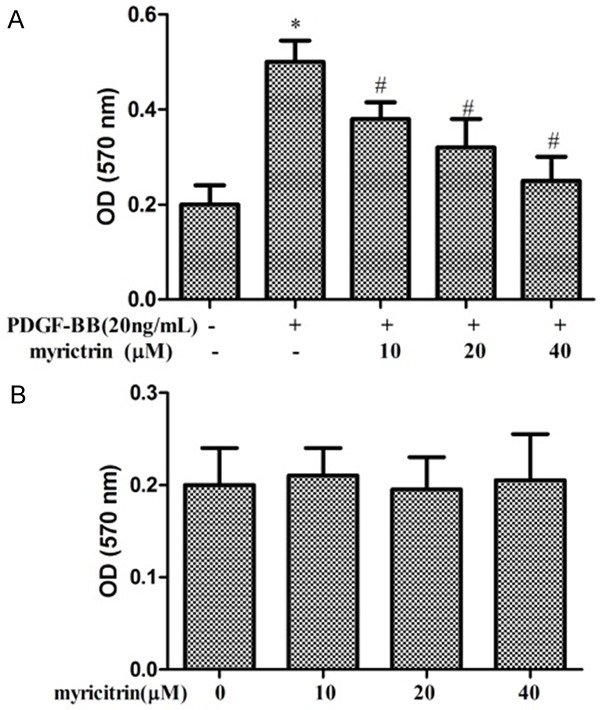
Effects of myricitrin on PDGF-BB-induced proliferation of VSMCs. A. Cell proliferation was measured with the MTT assay. VSMCs were pre-cultured in serum-free medium in the presence or absence of myricitrin (10, 20 and 40 μM) for 24 h, and then stimulated with 20 ng/mL PDGF-BB for a further 24 h (n=3). B. VSMCs were treated with 10, 20 and 40 μM of myricitrin in serum-free medium for 24 h (n=3). *P<0.05 compared to control group. #P<0.05 compared to PDGF-BB treatment group.
Myricitrin inhibits PDGF-BB-induced VSMC migration
The transwell Boyden chamber experiment was performed to confirm the inhibitory effect of myricitrin on VSMCs migration. As shown in Figure 2, treatment with PDGF-BB for 24 h markedly increased the number of VSMCs that migrated through transwell chamber. However, myricitrin significantly reduced the number of migrated cells by PDGF-BB stimulation. These results indicated that myricitrin appeared to inhibit PDGF-BB-induced VSMCs migration.
Figure 2.
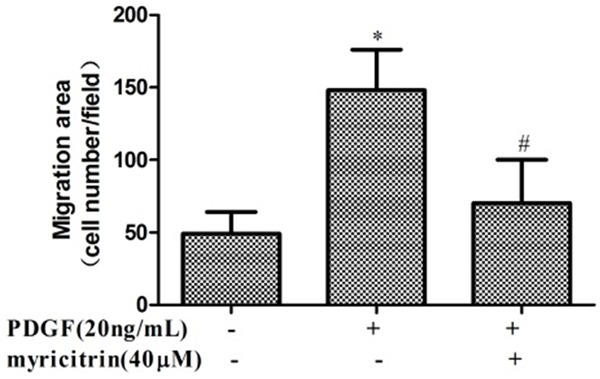
Effects of myricitrin on PDGF-BB-induced migration of VSMCs. Cell migration was measured with the transwell assay. Cells were seeded at 1×104 cells per well in the upper chamber and pretreated with myricitrin (40 μM). PDGF (20 ng/mL) was added to the lower chamber as a chemoattractant, and migration was allowed to proceed for 16 h before measurement. All experiments were repeated at least three times. *P<0.05 compared to control group. #P<0.05 compared to PDGF-BB treatment group.
Effects of myricitrin on PDGF-BB-induced cell cycle progression
We analyzed the effect of myricitrin treatment on the cell cycle stage distribution of PDGF-BB-stimulated VSMCs using flow cytometry analysis. As shown in Figure 3, Serum-deprivation of primary cultured VSMCs for 24 h led to 85.87% synchronization in the G0/G1 phase of cell cycle progression. Treatment with PDGF-BB caused an increase in the percentage of cells into the S phase (from 6.37% to 13.64%) and a decrease of cells in the G0/G1 phase, indicating that the cell proliferation may be activated by PDGF-BB. Treatment with myricitrin reduced the cell population in S phase to about 7.93% and increased the fraction of G0/G1 phase cells in PDGF-BB-treated cells. These results indicate that myricitrin induced an arrest at G0/G1 phase of the cell cycle and inhibited progression of the cell cycle to later (S, G2, and M) phases for the proliferation of VSMCs.
Figure 3.
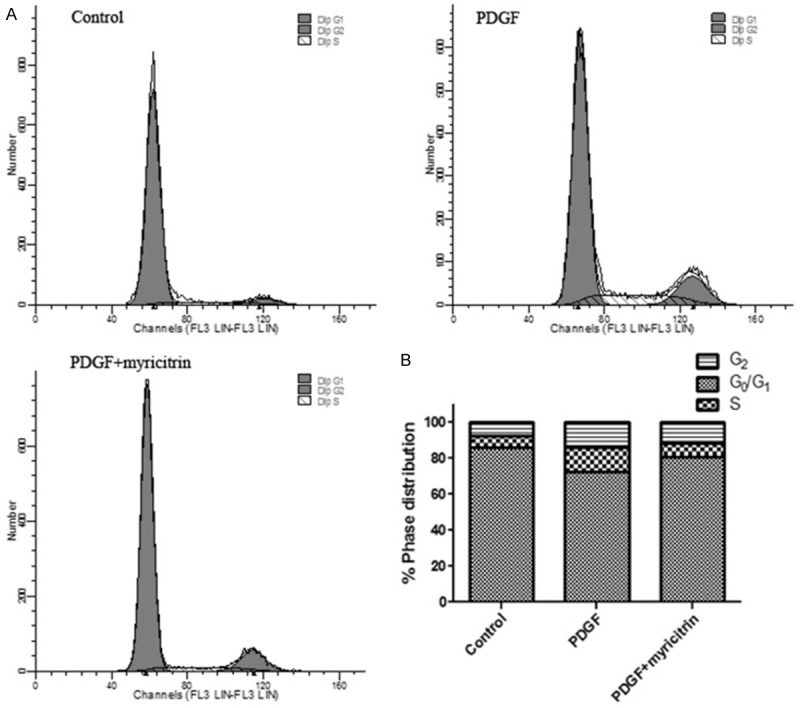
Effects of myricitrin on PDGF-BB-induced cell cycle progression in VSMCs. A. Cell cycle progression was evaluated by flow cytometry. The cells were pre-cultured in the presence or absence of myricitrin in serum-free medium for 24 h and then stimulated with 20 ng/mL PDGF-BB. After 24 h, cell cycle progression was evaluated by flow cytometry. B. Percentages of cell cycle distribution among different groups are presented as a histogram graph. The experiments were repeated three times and the data are shown as means ± SD. *P<0.05 compared to control group. #P<0.05 compared to PDGF-BB treatment group.
Effects of myricitrin on the expression of cell cycle regulatory proteins
It is known that cyclins, cyclin-dependent kinases (CDKs) and their inhibitors regulate cell cycle progression. To clarify whether inhibition by myricitrin involves the regulation of cell cycle-related proteins, the effects of myricitrin treatment on the expression of cell cycle regulatory proteins in VSMCs were examined. In the absence of myricitrin, the expression levels of cyclin D1, CDK4, cyclin E and CDK2 were significantly increased by PDGF-BB stimulation compared with the untreated control cells. However, pretreatment with myricitrin suppressed the PDGF-BB-induced the expression of cyclin D1, CDK4, cyclin E and CDK2 (Figure 4A-E). Taken together, this observation indicates that myricitrin inhibits cell cycle progression from G0/G1 to S phase by inhibiting the expression of cyclin D1, CDK4, cyclin E and CDK2 in PDGF-BB-stimulated VSMCs.
Figure 4.
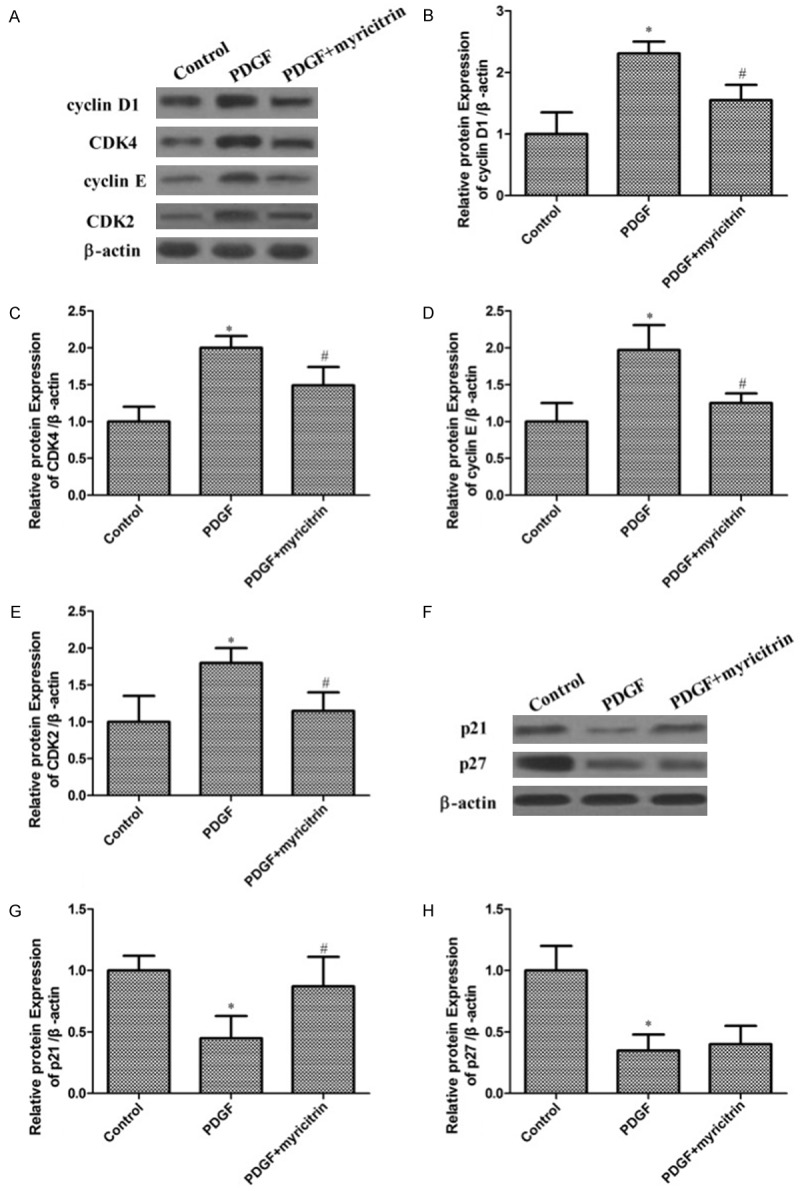
Effects of myricitrin on PDGF-BB-induced cell cycle related-proteins in VSMCs. A. The proteins cyclin D1, CDK4, cyclin E and CDK2 were determined using western blotting with corresponding antibodies. β-actin was used as an internal control. B-E. Data are expressed as mean ± SD; *P<0.05 compared to control group. #P<0.05 compared to PDGF-BB treatment group. F. The proteins p21 and p27 were determined using western blotting with corresponding antibodies. β-actin was used as an internal control. G, H. Data are expressed as mean ± SD; *P<0.05 compared to control group. #P<0.05 compared to PDGF-BB treatment group.
The effects of myricitrin on the expression of CDK inhibitors were further examined. Figure 4F showed that PDGF-BB markedly decreased the expression of p21and p27 in VSMCs. In contrast, myricitrin treatments increased the expression of p21 in PDGF-BB-stimulated VSMCs. While myricitrin does not effect on the p27 expression in PDGF-BB-induced VSMCs. Thus, these findings verify that myricitrin mediated cell cycle arrest in the G0/G1 phase of PDGF-BB-treated cells through regulation of p21.
Effects of myricitrin on PDGFRβ/ERK signaling pathway
A previous study showed that the Akt and Erk1/2 signaling pathway plays an important role in proliferation and migration of PDGF-BB-stimulated VSMCs [15]. We examined whether myricitrin affected on the levels of phosphorylated PDGF receptor β (PDGFRβ) and mitogens downstream of PDGFRβ signaling pathways in PDGF-BB-stimulated VSMCs. As shown in Figure 5, PDGF-BB increased the levels of phosphorylated PDGF-Rβ, Akt and Erk1/2, which were inhibited by treatment of myricitrin. The level of PDGFRβ, Akt and Erk1/2 protein was statistically similar in all groups. Thus, these results demonstrated that myricitrin inhibited the PDGF-BB stimulated proliferation and migration of VSMCs through blocking of PDGFRβ, Akt and Erk1/2 phosphorylation.
Figure 5.
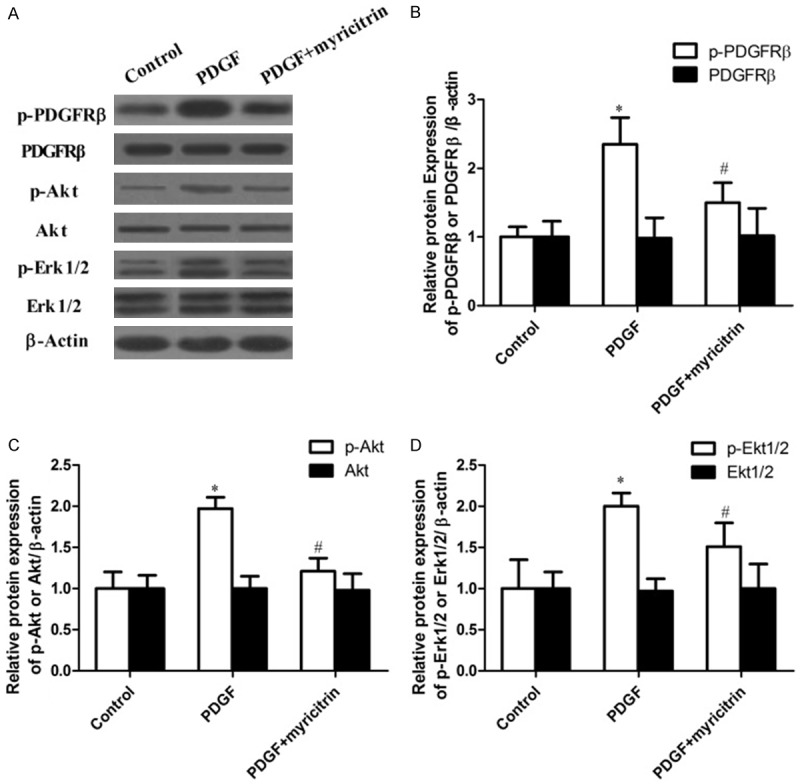
Myricitrin suppresses PDGFRβ, Akt and Erk1/2 signaling pathways activated by PDGF-BB in VSMCs. A. The proteins PDGFRβ, Akt, Erk1/2, p-Akt and p-Erk1/2 were determined using western blotting with corresponding antibodies. β-actin was used as an internal control. B-D. Data are expressed as mean ± SD; *P<0.05 compared to control group. #P<0.05 compared to PDGF-BB treatment group.
Discussion
The major finding of this study is that myricitrin inhibits PDGF-BB-induced VSMCs proliferation by blocking the autophosphorylation of PDGF-Rβ and subsequently suppressing PDGF-Rβ-mediated signaling pathways. Thus, our results suggest that myricitrin may be an effective tool to control PDGF-Rβ activity and the PDGF-Rβ-mediated signaling transduction pathway for the management of abnormal VSMCs proliferation and migration in vascular disorders.
The importance of VSMCs proliferation and migration in the initial formation of arterial plaques and also in advanced stages in the process of atherosclerosis has been well documented [17]. Thus, inhibition of VSMCs proliferation may have a beneficial effect in retarding the development of atherosclerotic disease. Among many known growth factors, PDGF is important regulators of VSMCs behavior with well-defined activities as potent chemoattractants and potent mitogens [18]. Pharmacological inhibition of PDGF signaling reduces VSMCs proliferation, migration, and occupancy in the neointima [19,20].
Myricitrin is a naturally occurring flavonoid derived from Chinese bayberry bark and fruit as well as other medicinal plants, which has been reported to have anti-inflammatory, anti-oxidative and antinociceptive effect [21]. Therefore, the aim in the present study is to investigate the protective effect of myricitrin in the development of atherosclerosis. However, the underlying signaling pathway involved in this process remains largely unclear.
In the present study, we evaluated the effects of myricitrin on the inhibition of PDGF-BB-induced proliferation, migration and cell cycle arrest in VSMCs, along with its mechanism of action. Our studies showed that myricitrin could both inhibit PDGF-BB-induced VSMCs proliferation and migration. Furthermore, we showed that PDGF-BB led to changes in the proportion of cells in the G0/G1 stages of the cell cycle in VSMCs. However, this change was reversed by treatment with myricitrin. The G1 phase of the cell cycle is a major point of control for cell proliferation in mammalian cells [22] and the cyclin D/CDK 4 and cyclin E/CDK 2 complexes are important for the G1-S phase transition [23]. We found that myricitrin inhibited up-regulation of cyclin D1/CDK4 and cyclinE/CDK2. Additionally, myricitrin induced p21expression in PDGF-BB-stimulated VSMCs. Thus, our results indicate that the anti-proliferative actions of myricitrin in PDGF-BB-stimulated VSMCs are mediated through inhibiting the expression of both cyclin D1/CDK4 and cyclin E/CDK2 as well as inducing the expression of p21, leading to a G0/G1 cell cycle arrest.
A previous study showed that the Erk-MAPK and Akt pathway regulated cell proliferation and cell migration in response to growth factors and cytokines [24]. In this study, Akt and Erk1/2 kinase phosphorylation were increased by PDGF-BB, and myricitrin inhibited PDGF-BB-induced phosphorylation of Akt and Erk1/2. These results indicate that inhibiting PDGF-BB-induced activation of PDGF signaling pathway may have contributed to the inhibition of VSMCs proliferation and migration exerted by myricitrin.
In conclusion, the present study showed myricitrin inhibited PDGF-BB-induced VSMCs proliferation and migration, which are critical in atherosclerotic. Moreover, these protective effects were shown to be associated with the cell cycle arrest, the downregulated activity of the PDGF signaling pathway. Our findings may provide new clues regarding the potential function of myricitrin to prevent or treat vascular diseases.
Disclosure of conflict of interest
None.
References
- 1.Lagraauw HM, Kuiper J, Bot I. Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain Behav Immun. 2015;50:18–30. doi: 10.1016/j.bbi.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Singh RB, Mengi SA, Xu YJ, Arneja AS, Dhalla NS. Pathogenesis of atherosclerosis: A multifactorial process. Exp Clin Cardiol. 2002;7:40–53. [PMC free article] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 4.Paul BD, Rao GS, Kapadia GJ. Isolation of myricadiol, myricitrin, taraxerol, and taraxerone from Myrica cerifera L. root bark. J Pharm Sci. 1974;63:958–959. doi: 10.1002/jps.2600630638. [DOI] [PubMed] [Google Scholar]
- 5.Meotti FC, Senthilmohan R, Harwood DT, Missau FC, Pizzolatti MG, Kettle AJ. Myricitrin as a substrate and inhibitor of myeloperoxidase: implications for the pharmacological effects of flavonoids. Free Radic Biol Med. 2008;44:109–120. doi: 10.1016/j.freeradbiomed.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Domitrović R, Rashed K, Cvijanović O, Vladimir-Knežević S, Škoda M, Višnić A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem Biol Interact. 2015;230:21–29. doi: 10.1016/j.cbi.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Winekenstädde D, Angelis A, Waltenberger B, Schwaiger S, Tchoumtchoua J, König S, Werz O, Aligiannis N, Skaltsounis AL, Stuppner H. Phytochemical profile of the aerial parts of Sedum sediforme and anti-inflammatory activity of myricitrin. Nat Prod Commun. 2015;10:83–88. [PubMed] [Google Scholar]
- 8.Meotti FC, Posser T, Missau FC, Pizzolatti MG, Leal RB, Santos AR. Involvement of p38MAPK on the antinociceptive action of myricitrin in mice. Biochem Pharmacol. 2007;74:924–931. doi: 10.1016/j.bcp.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 9.Qin M, Luo Y, Meng XB, Wang M, Wang HW, Song SY, Ye JX, Pan RL, Yao F, Wu P, Sun GB, Sun XB. Myricitrin attenuates endothelial cell apoptosis to prevent atherosclerosis: An insight into PI3K/Akt activation and STAT3 signaling pathways. Vascul Pharmacol. 2015;70:23–34. doi: 10.1016/j.vph.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Sun GB, Qin M, Ye JX, Pan RL, Meng XB, Wang M, Luo Y, Li ZY, Wang HW, Sun XB. Inhibitory effects of myricitrin on oxidative stress-induced endothelial damage and early atherosclerosis in ApoE/mice. Toxicol Appl Pharmacol. 2013;271:114–126. doi: 10.1016/j.taap.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Ross R. Atherosclerosis is an inflammatory disease. Am Heart J. 1999;138:S419–S420. doi: 10.1016/s0002-8703(99)70266-8. [DOI] [PubMed] [Google Scholar]
- 12.Glass CK, Witztum JL. Atherosclerosis. the road ahead. Cell. 2001;104:503–16. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 13.Wanjare M, Agarwal N, Gerecht S. Biomechanical strain induces elastin and collagen production in human pluripotent stem cell-derived vascular smooth muscle cells. Am J Physiol Cell Physiol. 2015;309:C271–C281. doi: 10.1152/ajpcell.00366.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee WR, Kim A, Kim KS, Park YY, Park JH, Kim KH, Kim SJ, Park KK. Alpha-lipoic acid attenuates atherosclerotic lesions and inhibits proliferation of vascular smooth muscle cells through targeting of the Ras/MEK/ERK signaling pathway. Mol Biol Rep. 2012;39:6857–6866. doi: 10.1007/s11033-012-1511-5. [DOI] [PubMed] [Google Scholar]
- 15.Chen YC, Wen ZH, Lee YH, Chen CL, Hung HC, Chen CH, Chen WF, Tsai MC. Dihydroaustrasulfone alcohol inhibits PDGF-induced proliferation and migration of human aortic smooth muscle cells through inhibition of the cell cycle. Mar Drugs. 2015;13:2390–2406. doi: 10.3390/md13042390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li PC, Sheu MJ, Ma WF, Pan CH, Sheu JH, Wu CH. Anti-Restenotic Roles of Dihydroaustrasulfone Alcohol Involved in Inhibiting PDGF-BBStimulated Proliferation and Migration of Vascular Smooth Muscle Cells. Mar Drugs. 2015;13:3046–3060. doi: 10.3390/md13053046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cersosimo E, Xu X, Upala S, Triplitt C, Musi N. Acute insulin resistance stimulates and insulin sensitization attenuates vascular smooth muscle cell migration and proliferation. Physiol Rep. 2014:2. doi: 10.14814/phy2.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu W, Kong H, Zeng X, Wang J, Wang Z, Yan X, Wang Y, Xie W, Wang H. Iptakalim inhibits PDGF-BB-induced human airway smooth muscle cells proliferation and migration. Exp Cell Res. 2015;336:204–210. doi: 10.1016/j.yexcr.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 19.Myllärniemi M, Calderon L, Lemström K, Buchdunger E, Häyry P. Inhibition of platelet-derived growth factor receptor tyrosine kinase inhibits vascular smooth muscle cell migration and proliferation. FASEB J. 1997;11:1119–26. doi: 10.1096/fasebj.11.13.9367346. [DOI] [PubMed] [Google Scholar]
- 20.Yamasaki Y, Miyoshi K, Oda N, Watanabe M, Miyake H, Chan J, Wang X, Sun L, Tang C, McMahon G, Lipson KE. Weekly dosing with the platelet-derived growth factor receptor tyrosine kinase inhibitor SU9518 significantly inhibits arterial stenosis. Circ Res. 2001;88:630–636. doi: 10.1161/01.res.88.6.630. [DOI] [PubMed] [Google Scholar]
- 21.Huang Q, Gao B, Wang L, Hu YQ, Lu WG, Yang L, Luo ZJ, Liu J. Protective effects of myricitrin against osteoporosis via reducing reactive oxygen species and bone-resorbing cytokines. Toxicol Appl Pharmacol. 2014;280:550–560. doi: 10.1016/j.taap.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Fang F, Newport JW. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991;66:731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- 23.Moore GD, Lear SC, Wills-Frank LA, Martin AW, Snyder JW, Helm CW. Differential expression of cdk inhibitors p16, p21cip1, p27kip1, and cyclin E in cervical cytological smears prepared by the ThinPrep method. Diagn Cytopathol. 2005;32:82–87. doi: 10.1002/dc.20186. [DOI] [PubMed] [Google Scholar]
- 24.Corradetti MN, Guan KL. Upstream of the mammalian target of rapamycin: do all roads pass through mTOR? Oncogene. 2006;25:6347–6360. doi: 10.1038/sj.onc.1209885. [DOI] [PubMed] [Google Scholar]


