Abstract
This study aims to explore the treatment methods for patients with abdominal aortic aneurysms (AAAs) that required occlusion of the openings of the bilateral internal iliac arteries (IIAs) in endovascular aneurysm repair (EVAR) and to evaluate the efficacy of these treatments. Four patients with AAA were treated with endovascular aneurysm repair (EVAR) and the crossover chimney technique in the bilateral internal iliac arteries (IIAs). We inserted and released the abdominal aortic stent as usual and implanted the bypass stent graft simultaneously. The intraoperative immediate angiography showed complete isolation of the AAA and patency of the bypass. One month after surgery, it showed contrast engorgement in the bypass stent in three patients. The IIA on the bypass side and its branches had good developing. Another case in which we utilized a COOK stent, occlusion started at the opening of the bypass stent, with no occurrence of other complications. For patients in whom AAAs involve bilateral iliac arteries and the openings of the bilateral IIAs need to be occluded, EVAR and a crossover chimney technique can protect the unilateral IIA.
Keywords: Abdominal aortic aneurysm (AAA), endovascular aneurysm repair, crossover chimney technique, iinternal iliac artery (IIA)
Introduction
Abdominal aortic aneurysm (AAA) is a serious, life-threatening macrovascular disease. At its onset, AAA is often asymptomatic. However, as it expands, serious consequences may occur. Interventional treatment for patients with appropriate indications is gradually replacing traditional surgical treatment and has become the preferred choice. Endovascular aneurysm repair (EVAR) is increasingly becoming a mainstream method for AAA treatment and is accepted by more and more medical staff and patients [1-5]. However, for some patients with AAA, the bilateral iliac arteries are involved in surgery. In previous reports, EVAR has involved occlusion of the bilateral internal iliac arteries (IIAs), but later, it was found that this approach decreased pelvic blood supply and caused nervous system complications [6,7]. The bypass may solve these problems [8-10], but hybrid surgery or two-phase surgery will increase the burden on patients and medical staff to a different extent. The crossover chimney technique, which was created and named by Wu et al. [11] has a good effect in preserving the IIA in AAA with common iliac artery aneurysms. In this study, EVAR combined with the crossover chimney technique was applied to treat patients with AAA. The objective was to provide a better method for protecting bilateral IIAs in EVAR for patients with AAA.
Methods
Patient selection
As of July 2013, totally 4 AAA patients underwent endovascular repair of their AAA and IIA heparin-coated stent bypass at our medical center. This study was conducted in accordance with the declaration of Helsinki. This study was conducted with approval from the Ethics Committee of Beijing Anzhen Hospital of Capital Medical University. Written informed consent was obtained from all participants.
The key data of the four patients with AAA were as follows: Patient 1 (male, 57 years old): The patient experienced severe lower abdominal pain with no obvious inducing factor 2 weeks before presentation, and the pain was not associated with posture. Computerized tomography (CT) examination revealed an infrarenal AAA with a diameter of about 45 mm. Patient 2 (male, 66 years old): Five years before presentation, a physical examination revealed an infrarenal AAA. Ten days before presentation, a computed tomographic angiography (CTA) examination showed an enlarged AAA with a diameter of about 42 mm, suggesting a high risk of rupture. Patient 3 (male, 65 years old): The patient had three-vessel coronary artery disease previously and abdominal pain in recent days. Abdominal CT showed an infrarenal AAA. The aneurysm diameter was about 41 mm. Patient 4 (male, 73 years old): The patient visited a local hospital because of abdominal discomfort 2 weeks before presentation to our medical center. The ultrasound and CT examination revealed an infrarenal AAA. The aneurysm diameter was about 39 mm.
Study protocol
As CTA of the aorta is an accepted standard procedure to evaluate and plan endovascular treatment for AAA [9], all four patients underwent preoperative CTA examination of the abdominal aorta and were diagnosed with AAA with involvement of both iliac arteries. The sizes of the various arteries were measured precisely to guide the surgery. As the openings of the two IIAs were relatively close to the iliac bifurcation, they needed to be covered together. To ensure quality of life for the patients after surgery, EVAR of the abdominal aorta and reverse IIA heparin-coated stent bypass were performed.
The idea of EVAR plus the crossover chimney technique in the IIA is to perform IIA bypass together with the traditional EVAR surgery. Figure 1 illustrates the surgical procedures using patient 1 as an example. Individual differences are specified. The procedure followed the instructions-for-use in all four patients [10].
Figure 1.
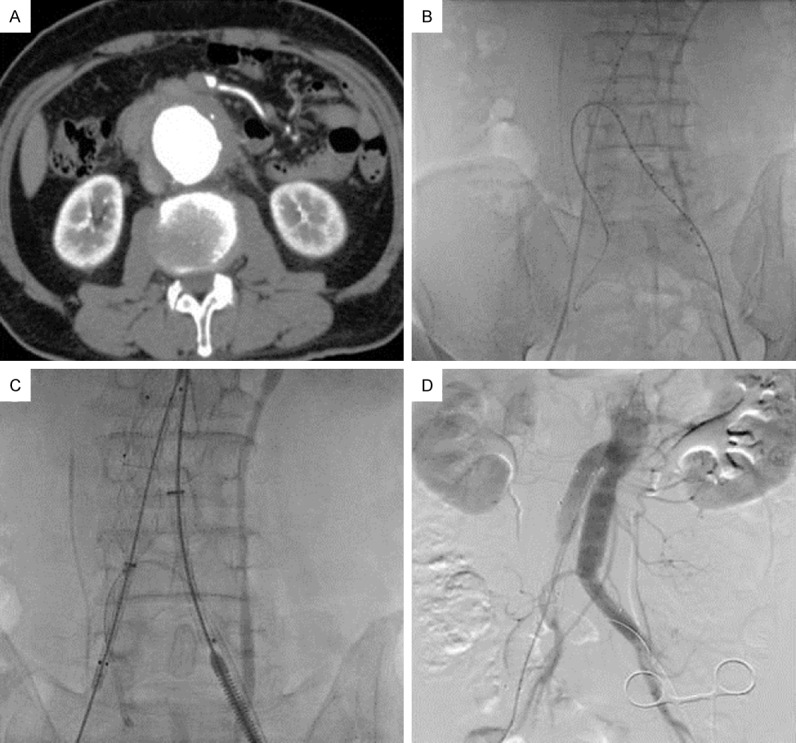
A. AAA formation; B. Super-slip guide wire inserted into the IIA on the bypass side to establish a track for the bypass stent graft; C. Release of the bypass stent graft and the iliac artery stent; D. Postoperative angiography showed clear images of the bypass stent graft, whereas the right femoral artery was occluded and the image was poor (The image in this figure was involved in Patient 1).
For the patient 1 shown in Figure 1, comprehensive preoperative examinations showed a relatively minor degree of right IIA atherosclerosis. As the vascular condition of the right IIA was satisfactory, it was set to be the bypass side. After the patient was sent to the operating room, local anesthesia and general anesthesia were initiated. The bilateral femoral arteries were first exposed and isolated. Because the range of the left femoral artery exposure was larger than that of the right, the bypass stent graft would be inserted from the left side. The bilateral femoral arteries were punctured. The puncture point on the left femoral artery was in the upper part of the exposed artery. A 5-French (5F) femoral arterial sheath was inserted, and a marker catheter was guided by a super-slip guide wire to the upper abdominal aorta. Digital subtraction angiography of the abdominal aorta was performed. The diameters of different arteries were measured again before bypass tracks were established. Based on our experience, we inserted the 5F femoral arterial sheath approximately 8-10 mm below the first puncture point, ideally toward the medial side. The super-slip guide wire and an ultra-election catheter were placed into the right IIA and retained there. When crossing the iliac bifurcation to the contralateral side, a Cordis 5F JR 3.5 catheter was used for assistance. When the guide wire reached the IIA on the bypass side, it was inserted as far as possible. Then, the ultra-election catheter was inserted into the bypass IIA for 2 cm or more, before being replaced by a stiff guide wire (Boston Scientific V-18TM Control WireTM or Boston Scientific Amplatz Super StiffTM, U.S.A.). The guide wire was retained, whereas the ultra-election catheter was rapidly replaced by an 8F sheath (COOK, U.S.A.), and the distal end of the 8F sheath was also retained at 2 cm within the bypass IIA. The bypass track was established at this point. Attention was paid so that no migration of the sheath and guide wire occurred.
Next, stiff guide wires were inserted through the bilateral femoral arterial sheaths, and the femoral arteries were temporarily clamped. The marker catheter and the 5F femoral arterial sheath were removed, and the left femoral artery was cut open for insertion of the stent delivery system. The main branch stent was inserted from the left side to the predetermined position, which was confirmed by angiography, as was the fact that the renal artery was not blocked. The abdominal aortic segment of the main branch stent was first opened, and later, the iliac side branch segment of the main branch stent was opened. The iliac side branch stent was inserted from the right side, adjoined to and partially overlapping the main branch. Then, the bypass stent graft was inserted in the bypass IIA along the track, which had been established previously and also needed to be approximately 2 cm into the IIA. The bypass stent graft used in this patient was the VIABAHN stent (GORE, U.S.A.). If there were lesions at the proximal end of the IIA, the bypass stent graft was inserted as far distal as possible to cross the lesion. The bypass stent graft was secured and temporarily not released. The side branch stent in the right iliac artery was partially released, and then the VIABAHN was released. At the end, the iliac branch of the main stent in the abdominal aorta and the side branch stent in the right iliac artery were fully released.
Postoperative angiography showed that the AAA exclusion was complete. In addition, the abdominal aortic stent and the drainage stent were patent, and the image of the drainage stents appeared before that of the right IIA (Figure 1).
Of the four patients we treated, the chimney technique was applied to protect the lower renal artery in two patients because of the short proximal aneurysm neck. A path was established from the left brachial artery, and a renal artery stent (INVTAEC, Italy) was inserted to a specified location and temporarily not released. After the main branch stent was placed in the predetermined position of the abdominal aorta and its proximal segment was released, the renal artery stent was released. Patient 3 suffered from coronary heart disease at the same time; therefore, complex surgery was performed in the hybrid operating room. First, a cardiothoracic surgeon performed off-pump coronary artery bypass grafting, and then the abdominal aorta EVAR and IIA bypass were performed. Because of the formation of an internal iliac aneurysm, migration occurred during bypass stent graft release; therefore, three VIABAHN stents were used (Figure 2). Postoperative follow-up was carried out. Non-contrast-enhanced volumetric computed tomography (NCT) has been used for follow-up after EVAR [12]; however, CTA was selected in this study in order to obtain clear imaging of the bypass stent.
Figure 2.
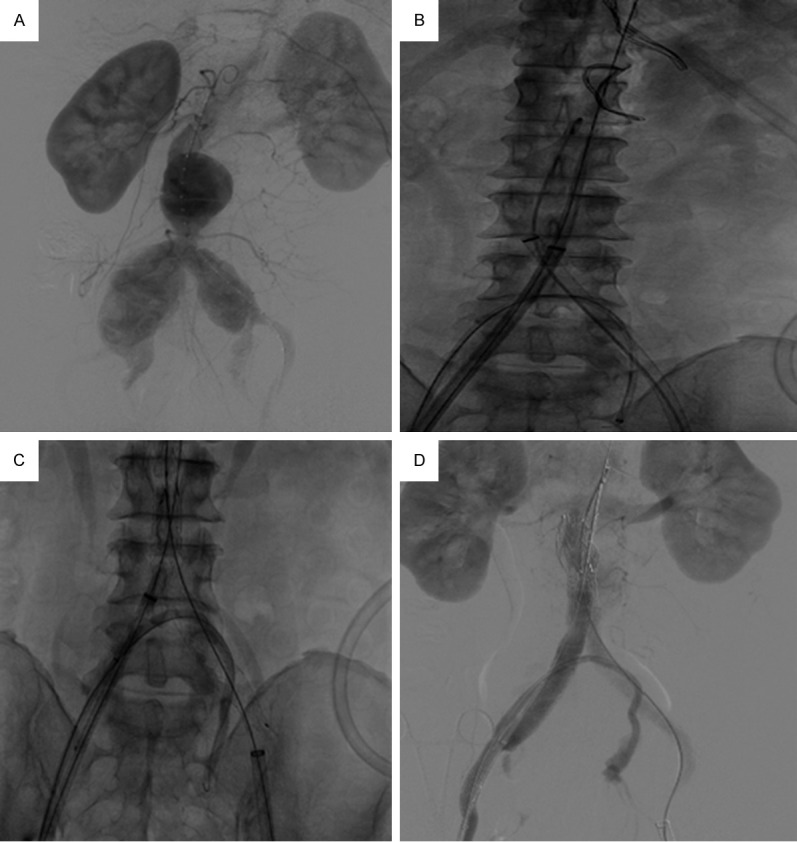
A. AAA formation, bilateral iliac arteries involved; left IIA was chosen as the bypass side as its condition was better. B. The three stent delivery tracks were established; prevention of migration of the catheter and guide wire that set the tracks for the bypass was key to a successful operation. C. Bypass stent graft was patent and fit the iliac bifurcation rather tightly. D. Postoperative angiography showed that the AAA endovascular repair was successful; the bypass stent graft was patent; the small amount of contrast agent in the aneurysm cavity was caused by leakage of contrast agent through the coating of the main branch stent (The image in this figure was involved in Patient 3).
Results
In all four patients, an endovascular heparin-coated stent bypass in the IIA was performed in addition to the conventional AAA endovascular repair. Intraoperative angiography showed clear images of the IIA on the bypass side, and the blood flow velocity was not notably decreased. After the operation, the patients were given anticoagulant drugs orally, and after a month, CTA examinations of the abdominal aorta were performed. In all cases, the AAA endovascular repair was successful, and there was no clear contrast agent within the original aneurysm. In patients 1, 3, and 4, contrast agent filling was found in the bypass stent graft, and the images of the IIA on the bypass side as well as its branches were clear (Figure 3). In patient 2 (Figure 4), who reported to be feeling well and had symmetrical muscle strength in both lower extremities and normal skin temperature and color on physical examinations, CTA revealed thrombosis within the bypass stent graft.
Figure 3.
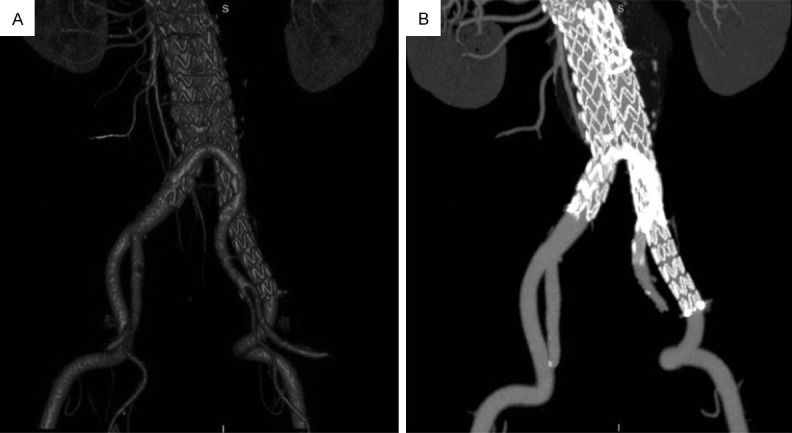
One month after the operation, CTA showed successful AAA exclusion and bypass stent graft patency (Imaging in this figure was involved in Patient 1).
Figure 4.
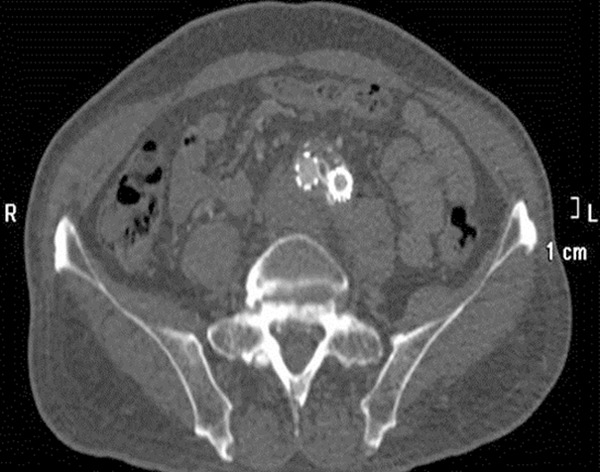
Bypass stent graft at the level of iliac bifurcation and the proximal end of the left common iliac artery were completely compressed (Imaging in this figure was involved in Patient 2).
Discussion
AAA endovascular repair and the crossover chimney technique
AAA is the third leading cause of sudden death [13]. The incidence of aneurysm in women is four times that in men, so it is more deadly in women [14]. Patients with AAA in China are aged 50-70 years, which is younger than patients in other countries, and most of them have bilateral iliac artery involvement. In the past, endovascular repair often involved occlusion of the bilateral IIAs. With the increased awareness of the importance of the IIA, it is generally considered that one side of the IIA blood supply should be protected to prevent pelvic ischemia or neurological symptoms [15-17]. Therefore, how to protect the IIA blood supply and improve the quality of life for patients has become the focus of clinical study. To retain the patency of at least one IIA to the maximum limit, we have a new alternative to using contralateral external iliac artery-IIA bypass. The reverse endovascular stent bypass no longer requires the hybrid surgery room and can be performed completely in the conventional cardiac catheterization room. This can prevent the risks associated with general anesthesia, which is more conducive to postoperative recovery.
For protecting the IIA blood supply during EVAR, the current methods include the sandwich technique and iliac branch device technique. The sandwich technique requires the brachial artery and subclavian artery to be in good condition to pass the stent, but the patients often have arteriosclerosis and extensive aortic tortuosity. The excessive intra-arterial operation can increase the risk of stroke or embolism of other artery branches and even form an endoleak. The IIA also requires a longer anchor area [18,19]. For the iliac branch device technique, the stent has not been further applied in China, so our experience is limited. There is a certain difficulty in positioning and selecting fenestration [20].
As mentioned previously, to maximally ensure the patency of the IIA on at least one side, the crossover chimney technique has become a new option in addition to conventional open surgery for contralateral external iliac artery-IIA bypass. With this new option, the operation no longer has to be performed in a hybrid operating room and can be completed in the traditional catheterization laboratory. This prevents the risks associated with general anesthesia in open surgeries and is more conducive to postoperative recovery.
Key points and main difficulties of the crossover chimney technique in the IIA
The method used in the present report involves adding the reverse endovascular bypass technique to the AAA endovascular repair. This requires the surgeon to be capable of skillfully completing an AAA endovascular repair. Based on our experience, the key points and difficulties involved in this procedure are as follows:
First, when establishing the bypass track, an appropriate puncture site needs to be chosen. As two femoral arterial sheaths need to be inserted in one femoral artery, the femoral artery exposure on this side should be relatively large. The first puncture point (corresponding to the track for the abdominal aortic stent) should be selected at the proximal end, whereas the second puncture point (corresponding to the track for the bypass stent graft) should be selected at the distal end. The distance between these two points should be 8-10 mm. Meanwhile, to prevent friction between the sheaths, the catheter, and the delivery system, the two points need to be punctured along different axes. We typically puncture the second point at approximately 15-30° inward.
Second, during and after establishing the bypass track, a guide wire or catheter of sufficient length needs to be retained within the IIA on the bypass side. We believe that this length should be no shorter than 2 cm to prevent migration caused by inaccurate operation or other operations and to provide adequate support during insertion of the bypass stent graft. This is especially important for patients with severe iliac atherosclerosis and a sharp iliac bifurcation angle. If migration occurs during the surgery, reestablishment of the guide wire and stents will be quite difficult.
Third, precautions should be taken when feeding the bypass stent graft through the iliac artery bifurcation. As mentioned previously, in some patients, the iliac bifurcation angle is relatively sharp and the iliac artery arteriosclerosis is severe. This may cause the bypass stent graft to bend and curve toward the proximal abdominal aorta because of too much resistance when passing through the iliac bifurcation. Under such conditions, aggressive advancement should be strictly avoided. Instead, the stent should be fed gently with repeated pushes. If a curve forms, the delivery system should be retracted properly. When the bypass stent graft reaches the predetermined position in the IIA, the part of the stent next to the iliac bifurcation should be as close as possible to the iliac bifurcation to reduce the length of the bypass route and the bending angle and to prevent compression between the bypass stent graft and the main branch stent.
Fourth, the order of releasing the abdominal aortic main branch stent and bypass stent graft needs to be appropriate. Our method is to release the abdominal aortic segment of the main branch stent first, i.e., to stop releasing the stent when the contralateral iliac artery branch is just about to be released. Next, the contralateral iliac side branch is fed in to adjoin with and partially overlap the main branch stent. Then, the bypass stent graft is fed into the predetermined position. The contralateral iliac artery side branch stent is partially released until the point close to the IIA opening, ensuring that no migration of the bypass stent graft occurs. Then, the bypass stent graft is released. The advantage of this order of release is that the iliac artery side branch stent that has been released early can secure the bypass stent graft and prevent its migration. Finally, the contralateral iliac artery side branch stent and the main branch stent are fully released.
Fifth, the bypass stent graft needs to be carefully chosen. For the only patient who had bypass stent graft occlusion, one VIABAHN stent was used. The postoperative CTA revealed that the bypass stent graft at the level of the iliac bifurcation was almost completely depressed. In patient 4, one VIABAHN stent was also used, and the postoperative CTA showed bypass stent graft patency. We deduced that there might be two reasons for this. One is that the bypass stent graft might bend on itself because of the large iliac bifurcation angle, causing occlusion. The other reason may be that the occlusion might arise from the abdominal aortic main branch stent pressing the bypass stent graft. For the first factor, although the flexibility of the VIABAHN stent is substantially better than that of other traditional coated stents, it is more cautious to use two VIABAHN stents instead of one. The two stents overlap at the iliac bifurcation and thus can provide stronger support. In the remaining two patients, this method was adopted, and the postoperative CTA showed bypass stent graft patency. For the second factor, the abdominal main branch stent used in the patient who had postoperative occlusion of the bypass stent graft was a COOK Zenith series. This stent provides very strong support and may also oppress the bypass stent graft and deform it, eventually leading to hemodynamic changes. Therefore, the appropriate choice of the main branch stent is also crucial.
Selection of the bypass side IIA
When only the unilateral IIA is involved, there is no need for a reverse endovascular bypass because of a rich pelvic collateral blood supply. If both IIAs are involved, the decision needs to be made about the side on which to perform the bypass. We believe the factors that need to be considered include the following: (1) The bypass side IIA should ideally be in good condition, and there should be no significant atherosclerosis or the degree of atherosclerosis should be less severe compared to the contralateral side. (2) The opening of the bypass side IIA should be closer to the iliac bifurcation compared to the contralateral side; this can minimize the length of the bypass stent graft and thus help maintain long-term stent patency. (3) The course of the bypass side IIA should be more conducive to operation. As inserting the ultra-election catheter in the opening of the bypass side IIA is rather difficult, the IIA that is closer to the sagittal plane of the body and has no obvious proximal curvatures will be a better choice.
Selection of the abdominal aortic main branch stent and the bypass stent graft
Regarding the selection of stent material, a VIABAHN stent was used as the bypass stent graft. We believe that it is better to use the same brand for the main branch stent and side branch stent in the abdominal aorta. Stents of the same or similar material can fit each other better in terms of support, hardness, and mutual shear stress, and this helps prevent endoleaks or migration.
Selection of the iliac artery side branch stent diameter
In our surgeries, as an additional bypass stent graft is inserted into the blood vessel, it will occupy certain space. If the iliac artery side branch stent is too large, it will compress the bypass stent graft. In contrast, the properly sized iliac artery side branch stent can fit with the bypass stent graft and to a certain degree secure it. Therefore, we believe selection of an appropriately sized iliac artery side branch stent is very important.
In the present report, the following formula was used for selecting the iliac artery side branch stent diameter: R’≥√(1.44*R2-r2) [11] (R’: main aortic endograft diameter, R: iliac artery diameter, r: chimney VIABAHN diameter).
Conclusion
Endovascular heparin-coated stent bypass in the IIA provides another option for patients with AAA in China who are of a relatively young age. However, currently, only few patients have received this operation, and the available data are not yet sufficient to identify factors that influence the patency of the bypass stent graft in AAA endovascular repair and the endovascular heparin-coated stent bypass in the IIA. In addition, long-term follow-up studies are still needed to determine whether the blood flow channels established in this operation can provide long-term blood supply to the IIA on the bypass side.
Disclosure of conflict of interest
None.
References
- 1.Cowie AG, Ashleigh RJ, England RE, McCollum CN. Endovascular aneurysm repair with the Talent stent-graft. J Vasc Interv Radiol. 2003;14:1011–1016. doi: 10.1097/01.rvi.0000082862.05622.63. [DOI] [PubMed] [Google Scholar]
- 2.Teufelsbauer H, Prusa AM, Wolff K, Polterauer P, Nanobashvili J, Prager M, Hölzenbein T, Thurnher S, Lammer J, Schemper M, Kretschmer G, Huk I. Endovascular stent grafting versus open surgical operation in patients with infrarenal aortic aneurysms: a propensity score-adjusted analysis. Circulation. 2002;106:782–787. doi: 10.1161/01.cir.0000028603.73287.7d. [DOI] [PubMed] [Google Scholar]
- 3.Howell MH, Strickman N, Mortazavi A, Hallman CH, Krajcer Z. Preliminary results of endovascular abdominal aortic aneurysm exclusion with the AneuRx stent-graft. J Am Coll Cardiol. 2001;38:1040–1046. doi: 10.1016/s0735-1097(01)01495-4. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford RB. Seminars in Vascular Surgery. Introduction. Semin Vasc Surg. 2012;25:1. doi: 10.1053/j.semvascsurg.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Peterson BG, Matsumura JS, Brewster DC, Makaroun MS. Five-year report of a multicenter controlled clinical trial of open versus endovascular treatment of abdominal aortic aneurysms. J Vasc Surg. 2007;45:885–890. doi: 10.1016/j.jvs.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 6.Jean-Baptiste E, Brizzi S, Bartoli MA, Sadaghianloo N, Baqué J, Magnan PE, Hassen-Khodja R. Pelvic ischemia and quality of life scores after interventional occlusion of the hypogastric artery in patients undergoing endovascular aortic aneurysm repair. J Vasc Surg. 2014;60:40–49. doi: 10.1016/j.jvs.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 7.Park KM, Yang SS, Kim YW, Park KB, Park HS, Do YS, Kim DI. Clinical outcomes after internal iliac artery embolization prior to endovascular aortic aneurysm repair. Surg Today. 2014;44:472–477. doi: 10.1007/s00595-013-0572-y. [DOI] [PubMed] [Google Scholar]
- 8.Unno N, Inuzuka K, Yamamoto N, Sagara D, Suzuki M, Konno H. Preservation of pelvic circulation with hypogastric artery bypass in endovascular repair of abdominal aortic aneurysm with bilateral iliac artery aneurysms. J Vasc Surg. 2006;44:1170–1175. doi: 10.1016/j.jvs.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Strobl FF, Sommer WH, Haack M, Nikolaou K, Meimarakis G, Koeppel TA, Weidenhagen R. Computed tomography angiography as the basis for optimized therapy planning before endovascular aneurysm repair (EVAR) Radiologe. 2013;53:495–502. doi: 10.1007/s00117-012-2450-9. [DOI] [PubMed] [Google Scholar]
- 10.Nakai M, Sato M, Sato H, Sakaguchi H, Tanaka F, Ikoma A, Sanda H, Nakata K, Minamiguchi H, Kawai N, Sonomura T, Nishimura Y, Okamura Y. Midterm results of endovascular abdominal aortic aneurysm repair: comparison of instruction-for-use (IFU) cases and non-IFU cases. Jpn J Radiol. 2013;31:585–592. doi: 10.1007/s11604-013-0223-7. [DOI] [PubMed] [Google Scholar]
- 11.Wu IH, Chan CY, Chen YS, Huang SC, Wang SS, Chi NH. Crossover chimney technique to preserve the internal iliac artery in abdominal aortic aneurysm with common iliac artery aneurysms. J Endovasc Ther. 2013;20:298–302. doi: 10.1583/13-4219R.1. [DOI] [PubMed] [Google Scholar]
- 12.Bobadilla JL, Suwanabol PA, Reeder SB, Pozniak MA, Bley TA, Tefera G. Clinical implications of non-contrast-enhanced computed tomography for follow-up after endovascular abdominal aortic aneurysm repair. Ann Vasc Surg. 2013;27:1042–1048. doi: 10.1016/j.avsg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 13.United Kingdom Small Aneurysm Trial Participants. Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346:1445–1452. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 14.Nevitt MP, Ballard DJ, Hallett JW Jr. Prognosis of abdominal aortic aneurysms. A population-based study. N Engl J Med. 1989;321:1009–1014. doi: 10.1056/NEJM198910123211504. [DOI] [PubMed] [Google Scholar]
- 15.Ke CC, Feng YP, Chang CC, Hung CJ. Extensive spinal cord ischemia following endovascular repair of an infrarenal abdominal aortic aneurysm: a rare complication. J Anesth. 2013;27:956–959. doi: 10.1007/s00540-013-1635-3. [DOI] [PubMed] [Google Scholar]
- 16.Freyrie A, Testi G, Gargiulo M, Faggioli G, Mauro R, Stella A. Spinal cord ischemia after endovascular treatment of infrarenal aortic aneurysm. Case report and literature review. J Cardiovasc Surg (Torino) 2011;52:731–734. [PubMed] [Google Scholar]
- 17.Altaf N, Abisi S, Yong Y, Saunders JH, Braithwaite BD, MacSweeney ST. Mid-term results of endovascular aortic aneurysm repair in the young. Eur J Vasc Endovasc Surg. 2013;46:315–319. doi: 10.1016/j.ejvs.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 18.Lobato AC. Sandwich technique for aortoiliac aneurysms extending to the internal iliac artery or isolated common/internal iliac artery aneurysms: a new endovascular approach to preserve pelvic circulation. J Endovasc Ther. 2011;18:106–111. doi: 10.1583/10-3320.1. [DOI] [PubMed] [Google Scholar]
- 19.Lobato AC, Camacho-Lobato L. A new technique to enhance endovascular thoracoabdominal aortic aneurysm therapy--the sandwich procedure. Semin Vasc Surg. 2012;25:153–160. doi: 10.1053/j.semvascsurg.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Karthikesalingam A, Hinchliffe RJ, Holt PJ, Boyle JR, Loftus IM, Thompson MM. Endovascular aneurysm repair with preservation of the internal iliac artery using the iliac branch graft device. Eur J Vasc Endovasc Surg. 2010;39:285–294. doi: 10.1016/j.ejvs.2009.11.018. [DOI] [PubMed] [Google Scholar]


