Abstract
The aim of the present study was to perform a meta-analysis to assess the diagnostic value of fluorine-18 fluorodeoxyglucose (18F-FDG) PET-CT/PET in the pre-operative evaluation of TNM staging in patients with primary colorectal cancer (CRC). The Medline, Embase and Web of Knowledge were searched for studies assessing the diagnostic value of 18F-FDG PET-CT/PET in the pre-operative evaluation of TNM staging in CRC patients. We pooled the sensitivity, specificity, positive and negative Likelihood ratio (LR+ and LR-) and Diagnostic Odds Ratio (DOR) and constructed summary receiver operating characteristic curves. A total of 28 studies including 2283 CRC patients were analyzed. The pre-operative tumor detecting rate of PET-CT was 95.35%, which was superior to CT (P < 0.05). The pooled sensitivity and specificity of pre-operative T staging by PET-CT/PET was 0.73 (95% CI: 0.65-0.81) and 0.99 (95% CI: 0.98-0.99), which the AUC and Q* were 0.96 and 0.91, respectively. Concerning pre-operative N staging, the pooled sensitivity and specificity of PET-CT/PET were 0.62 and 0.70, which the AUC and Q* were 0.76 and 0.70, respectively. As for M staging, the pooled sensitivity and specificity of PET-CT/PET were 0.91 (95% CI: 0.80-0.96) and 0.95 (95% CI: 0.91-0.98), which the AUC and Q* were 0.96 and 0.91, respectively. 18F-FDG PET-CT/PET had good performance in the pre-operative tumor detecting rate, T staging and M staging in patients with primary CRC, which might alter the therapeutic strategy. However, the diagnostic value of 18F-FDG PET-CT/PET in pre-operative N staging in CRC patients was not ideal.
Keywords: Colorectal cancer, 18F-FDG-PET, PET-CT, TNM staging, meta-analysis
Introduction
Colorectal cancer (CRC) is the third most common malignant tumor worldwide and one of the most frequent cause of cancer-related death [1]. Accurate preoperative staging of CRC is crucial for providing the optimal therapeutic strategy and evaluating the prognosis [2]. The depth of tumor invasion, lymph node involvement and distant metastasis are the main factors that influence the prognosis of CRC patients [3]. Conventional staging options include endo-rectal ultrasound scanning, computed tomography (CT) and magnetic resonance imaging (MRI). Some researches revealed that CT and MRI were the reliable technique for preoperative evaluation of T staging in CRC patients [4]. However, it was inadequate for N staging involving in differentiating metastasis from benign lymph nodes (LNs). A recent research presented that the overall accuracy of MDCT was 86% in T staging and 84% in N staging [5]. Though technical refinements, such as multi-detector CT, have supplied better quality for conventional imaging, an ideal pre-therapeutic staging has not been achieved.
Positron emission tomography (PET) using the radio-labeled glucose analog 18F-fluorodeoxyglucose (18F-FDG) was widely being applied in the diagnosis, staging, evaluation of treatment response and predicting prognosis in a lot of malignant tumors [6]. Some researches considered that 18F-FDG PET might be more accurate than CT in diagnosing gastrointestinal malignancies [7]. Many investigators suggested that 18F-FDG PET or PET-CT should be used in the pre-therapeutic diagnosis of CRC patients with potentially resectable live metastasis and in the detection of tumor recurrence [8]. Some researches consider that PET-CT is going to be the standard preoperative assessment of primary CRC in the near future [9]. Currently, there are increasing studies involving in pre-operative TNM staging of primary CRC patients using 18F-FDG PET or PET-CT. However, single studies are inconclusive owing to the application of all kinds of methods for assessing the value of 18F-FDG PET or PET-CT and limited sample sizes. So some results of recent studies concerning pre-operative TNM staging of primary CRC patients using 18F-FDG PET-CT/PET are controversial. The purpose of the present meta-analysis was to undertake a systematic review of all available studies to address the diagnostic performance of 18F-FDG PET-CT/PET in determining the pre-operative TNM staging of primary CRC patients.
Materials and methods
Literature search
A comprehensive computerized systematic literature search was performed to identify abstracts of English-language publications from studies that evaluated 18F-FDG PET or PET/CT, which are diagnostic tools for initial staging before surgery or any treatment in patients with colorectal cancer. We searched for relevant articles with PubMed/Medline, Embase databases and ISI Web of Knowledge (Last updated on 22 July 2014). We utilized a search algorithm that was according to a combination of the following text words: (a) positron emission tomography or 18F-FDG PET or PET or PET/CT, (b) colorectal cancer or colon cancer or rectal cancer or colorectal carcinoma or colon carcinoma or rectal carcinoma or colorectal neoplasm or colon neoplasm or rectal neoplasm, (c) staging. The searches were restricted to studies done in humans. Two investigators, who were blinded to the journal, institution, author and date of publication, independently checked the retrieved articles. Potentially relevant articles were evaluated through reviewing their titles and abstracts and all the studies matching the eligible criteria were retrieved. To identify additional relevant references, the reference lists of the publications retrieved were conducted manually. For studies using the same sample in different publications, only the most complete information was included. Patients’ files were retrieved to obtain clinical data (Table 1) with approval of the hospital’s ethics committee.
Table 1.
Clinical characteristics of included studies
| References and Study ID | Year | Number of patients | Design | Gender (% male) | Mean age (Year) | Primary tumor | Equipment | Blind | Total QUADAS Score |
|---|---|---|---|---|---|---|---|---|---|
| Engelmann BE et al. [16] | 2014 | 62 | P | 52.0 | 70.0 | CRC | PET-CT; CT | Yes | 12 |
| Lee JH et al. [17] | 2014 | 266 | R | 57.9 | 63.7 | CC | PET-CT; CT | NR | 10 |
| Ozis SE et al. [18] | 2014 | 97 | P | 60.8 | 59.6 | RC | PET-CT; CT | Yes | 12 |
| Makis W et al. [19] | 2013 | 189 | R | NR | NR | CRC | PET-CT | NR | 9 |
| Cipe G et al. [20] | 2013 | 64 | P | 69.0 | 59.0 | CRC | PET-CT; CT | NR | 11 |
| Huang SW et al. [21] | 2013 | 1109a | R | 59.6 | 53.2 | CRC | PET-CT | Yes | 12 |
| Zafar HM et al. [22] | 2012 | 24 | R | 50.0 | NR | CRC | PET-CT | NR | 9 |
| Yu LJ et al. [23] | 2012 | 68 | P | NR | NR | CRC | PET-CT | NR | 9 |
| Kwak JY et al. [24] | 2012 | 473 | R | NR | NR | CRC | PET-CT; CT | NR | 9 |
| Mainenti PP et al. [25] | 2011 | 34 | P | 58.8 | 63.0 | CRC | PET-CT | Yes | 12 |
| Kim DJ et al. [26] | 2011 | 30 | R | 70.0 | 62.0 | CRC | PET-CT; MRI | NR | 10 |
| Eglinton T et al. [27] | 2010 | 20 | P | 70.0 | 63.0 | RC | PET-CT; MRI | Yes | 12 |
| Kam MH et al. [28] | 2010 | 23 | R | 65.2 | 60.0 | RC | PET-CT; MRI | NR | 10 |
| Ono K et al. [29] | 2009 | 25 | R | 64.0 | 67.3 | CRC | PET; MRI | Yes | 12 |
| Akiyoshi T et al. [30] | 2009 | 65 | R | 55.4 | 62.0 | CRC | PET; CT | NR | 11 |
| Davey K et al. [31] | 2008 | 83 | P | 62.6 | 64.0 | RC | PET-CT; CT | NR | 10 |
| Nahas CS et al. [32] | 2008 | 93 | P | 66.7 | 59.0 | RC | PET | Yes | 12 |
| Tsunoda Y et al. [33] | 2008 | 88 | R | 59.1 | 60.6 | CRC | PET-CT | Yes | 13 |
| Llamas-Elvira et al. [34] | 2007 | 104 | P | 51.0 | 66.7 | CRC | PET; CT | NR | 9 |
| Tateishi U et al. [35] | 2007 | 53 | R | 60.4 | 61.0 | RC | PET-CT | NR | 9 |
| Veit-Haibach P et al. [36] | 2006 | 47 | P | 47.4 | 72.0 | CRC | PET-CT; CT | Yes | 13 |
| Furukawa H et al. [37] | 2006 | 44 | P | 75.0 | 61.4 | CRC | PET; CT | NR | 11 |
| Park IJ et al. [38] | 2006 | 100 | P | 60.0 | 57.0 | CRC | PET-CT; CT | NR | 11 |
| Gearhart SL et al. [39] | 2006 | 37 | P | 70.3 | 58.0 | RC | PET-CT; CT | NR | 9 |
| Heriot AG et al. [40] | 2004 | 46 | P | NR | NR | RC | PET | NR | 9 |
| Kantorova I et al. [41] | 2003 | 38 | P | 71.1 | 66.0 | CRC | PET; CT | NR | 10 |
| Mukai M et al. [42] | 2000 | 24 | R | NR | NR | CRC | PET | NR | 9 |
| Abdel-Nabi H et al. [43] | 1998 | 48 | P | 100 | 67.8 | CRC | PET; CT | NR | 9 |
P, Prospective; R, Retrospective; NR, Not Reported; RC, Rectal Cancer; CC, Colon Cancer; CRC, Colorectal Cancer; PET, Positron Emission Tomography; CT, Computed Tomography; MRI, Magnatic Resonance Imaging; QUADAS, Quality Assessment of Diagnostic Accuracy Studies.
1109 peoples performed PET-CT examination and 38 patients with colorectal cancer were confirmed.
Study selection criteria
Studies, prospective and retrospective, were selected if all of the following inclusion criteria were fulfilled: (a) 18F-FDG PET or PET/CT was utilized to evaluate patients with primary colorectal cancer without surgery or any other treatment; (b) pre-operative staging of colorectal cancer, including T stage or N stage, was involved in the articles; (c) articles were published in English and Chinese; (d) for per-patient level statistics, sufficient data were presented to calculate the true-positive (TP), false-positive (FP), true negative (TN) and false-negative (FN) values; (e) histological evaluation was utilized as a reference standard; (f) as for the quality of the study design, only the article in which the number of the answer “yes” for the 14 items in the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist [10] was more than nine was included; (g) 20 or more patients were included; (h) when data or subsets of data were published in more than one article, the article with the most recent article or the most details was selected. The authors of the abstracts and studies not showing sufficient data were contacted to request additional information. Review articles, case reports, letters, comments, conference records as well as articles that did not provide raw data, were excluded.
Quality assessment and data extraction
The methodological quality of the selected studies was assessed by two investigators independently, who discussed discrepancies and achieved a consensus. The QUADAS checklists were utilized to evaluate the methodological quality of included studies. To undergo accuracy analysis, we extracted data on characteristics of patients and studies, measurements executed and results. For each study, we extracted the following details: first author, year of publication, country of origin, sample size, characteristics of study population (gender and age), study design (retrospective, prospective or unknown); included criteria as well as the reasons for exclusions from the analysis; reference standard; whether 18F-FDG PET measurements were blinded to the pathological diagnosis, clinical results or other diagnostic methods used (CT and MRI).
For each study, we extracted the number of TP, FP, TN and FN findings for 18F-FDG PET or PET/CT in diagnosing the staging of primary colorectal cancer. The information was also recorded for CT and MRI, which was used for comparison with PET in the eligible studies.
Statistical analysis
Data on the diagnostic performance of 18F-FDG PET or PET/CT were combined quantitatively across eligible publications. Data were utilized to construct 2×2 contingency tables (including TP, FP, TN and FN results) in order to calculate sensitivity and specificity with confidence intervals (CIs). Dates were plotted graphically in forest plots. A value of 0.5 was added to all cells of studies which included a count of zero to avoid following problems in odds calculations for studies with sensitivities or specificities of 100%. The pooled sensitivity, specificity and diagnostic odds ratio estimators were weighted averages in which the weight of each study was the single sample size.
We used the derived estimates of sensitivity, specificity and respective variances to construct summary receiver operating characteristic (ROC) curves. The area under the summary ROC curves was utilized as an alternative general measure of test performance [11,12]. The SROC curve shows the trade-off between sensitivity and specificity across the included studies [13]. A summary ROC curve located near the upper left corner indicates the better diagnostic modality. Testing of the diagnostic threshold was performed by Spearman’s correlation test.
Likelihood ratios (LR) are also metrics that combine sensitivity and specificity in the calculations. The discrimination ability is deemed to be better with a higher positive LR and a lower negative LR. In previous papers, a test was considered clinically useful when positive LR was greater than 5.0 and negative LR was less than 0.2 [14]. Heterogeneity was evaluated by utilizing likelihood X 2-test. Concerning the likelihood ratio X 2-test, P < 0.05 was thought owning obvious heterogeneity. If heterogeneity existed, a random-effect model was used for the primary meta-analysis to get a summary estimate for sensitivity with 95% CI. To evaluate whether the diagnostic values were significantly influenced by heterogeneity between individual studies, we performed a subgroup analysis. Furthermore, we also tested the difference of tumor detecting rate assessed by Pearson X 2 test.
All the statistical computations were performed utilizing Meta-DiSc (version 1.4, http://www.hrc.es/investigacion/metadisc_en.htm) [15]. Meta-DiSc is freeware software to perform meta-analysis of studies of evaluation of screening and diagnostic tests. P < 0.05 was considered to be statistically significant.
Results
Literature search and selection of studies
After the computerized search was carried out and reference lists were comprehensively cross-checked, 720 articles were yielded, of which 210 were excluded on the basis of their titles and abstracts. After further retrieval, 466 publications were excluded because they did not meet the inclusion criteria. Then we screen the remaining 44 potentially appropriated articles in full text. Among them, 16 articles were excluded owing to following reasons: excluded reports (n=1), essential data missing to construction 2×2 contingency tables (n=14) and possible overlapping study population (n=1). Therefore, 28 eligible studies, fulfilled all of the inclusion criteria, were considered for the analysis (16-43). Among the 28 included articles, tumor detecting rate (n=12), T staging (n=4), N staging (n=20), M staging (n=5) and stage change (n=8) were studied respectively. The detailed procedure of study selection in the meta-analysis was shown in Figure 1.
Figure 1.
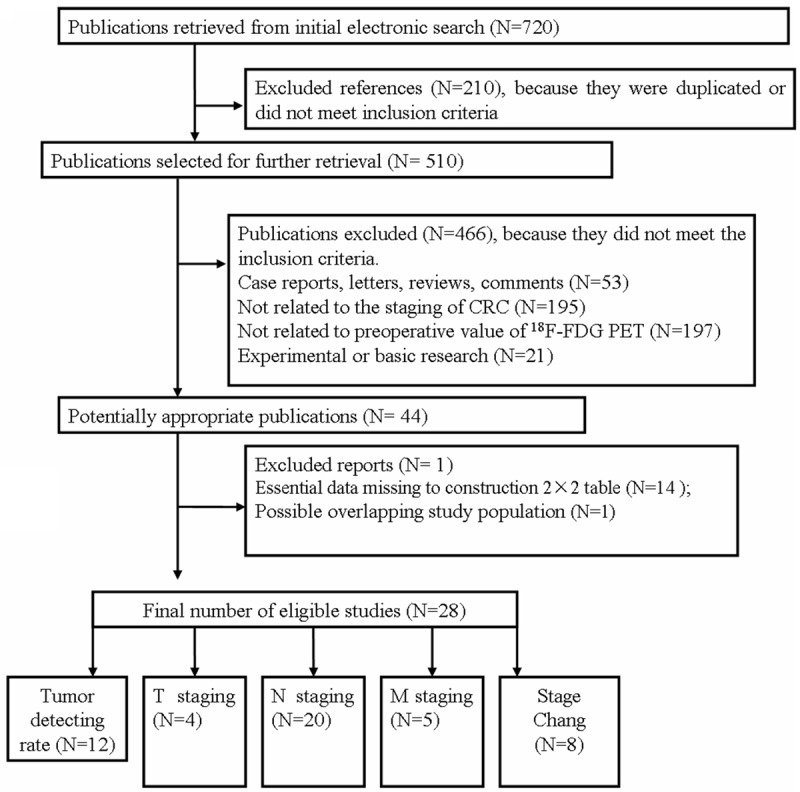
Flow chart of articles included in the meta-analysis. CRC, colorectal cancer; PET, Positron Emission Tomography; 18F-FDG, Fluorine-18 fluorodeoxyglucose.
Study characteristics, study quality and methodological quality assessment
The characteristics of the selected studies are shown in Table 1. We performed all analyses based on per-patients data analysis. There are a total of 2283 colorectal cancer patients in the 28 included articles. Among them, one study showed that 1109 persons performed PET-CT examination and 38 patients with colorectal cancer were confirmed. 16 studies enrolled patients prospectively while the other 12 studies were retrospective. The ratio of male and mean age of every study could be seen in Table 1. There are 8 studies enrolled only rectal cancer patients and 1 study involved in only colon cancer patients while the remained studies enrolled both colon cancer and rectal cancer patients. Furthermore, CRC patients performed PET-CT examination in 19 studies and the other CRC patients underwent PET scan in 9 studies. As the control tests, some conventional examinations were performed synchronously. Among them, there were 14 studies involving in CRC patients using CT scanning and 4 studies undergoing MRI examination. Moreover, there were 9 studies in which the PET or PET-CT reviewers were blinded to patients’ clinical data and other test results while the other 19 studies did not report whether they adopted the blinding.
We utilized the QUADAS tool to evaluate each included study. All selected studies in the meta-analysis met nine or more of the fourteen criteria in the QUADAS tool for methodological quality, which could be seen in Table 1. 19 studies were not blinded in the results of the index test results and the reference standard (67.9% for “No” response to questions 10 and 11). There were no uninterpretable and/or intermediate test results reported (100% for “No” response to question 14). Furthermore, the description of the gold standard was unclear in 3 studies.
Diagnostic accuracy of pre-operative N staging using PET-CT or PET
There are 20 studies, including 1530 CRC patients, involved in pre-operative N staging using PET-CT or PET [16,17,20,23-26,28-30,33-37,41-43]. The data of each study and the results of the statistical pooling are shown in Table 2. The pooled estimates of sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR-) and diagnostic odds ratio (DOR) of PET-CT/PET in the detection of pre-operative lymph node staging in CRC patients were 0.62 (95% CI: 0.59-0.66), 0.70 (95% CI: 0.67-0.73), 2.83 (95% CI: 1.96-4.06), 0.60 (95% CI: 0.50-0.71) and 6.14 (95% CI: 3.80-9.91), respectively.
Table 2.
Diagnositc value of PET-CT or PET in detection of involved lymph node in preoperative CRC patients
| Sensitivity | Specificity | Likelihood ratios | Diagnostic Odds Ratio | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| LR+ | LR- | ||||||||||||||
|
|
|||||||||||||||
| References | No. | TP | FP | FN | TN | V | 95% CI | V | 95% CI | V | 95% CI | V | 95% CI | V | 95% CI |
| Engelmann BE | 55 | 8 | 5 | 16 | 26 | 0.333 | 0.156-0.553 | 0.839 | 0.663-0.945 | 2.067 | 0.774-5.518 | 0.795 | 0.576-1.097 | 2.600 | 0.723-9.344 |
| Lee JH | 138 | 44 | 74 | 6 | 14 | 0.880 | 0.757-0.955 | 0.159 | 0.090-0.252 | 1.046 | 0.913-1.200 | 0.754 | 0.309-1.839 | 1.387 | 0.497-3.873 |
| Lee JH | 63 | 37 | 22 | 1 | 3 | 0.974 | 0.862-0.999 | 0.120 | 0.025-0.312 | 1.106 | 0.949-1.291 | 0.219 | 0.024-1.991 | 5.045 | 0.494-51.54 |
| Cipe G | 64 | 34 | 5 | 13 | 12 | 0.723 | 0.574-0.844 | 0.706 | 0.440-0.897 | 2.460 | 1.153-5.245 | 0.392 | 0.225-0.683 | 6.277 | 1.847-21.33 |
| Yu LJ | 68 | 19 | 6 | 1 | 42 | 0.950 | 0.751-0.999 | 0.875 | 0.748-0.953 | 7.600 | 3.571-16.17 | 0.057 | 0.008-0.387 | 133.0 | 14.95-1183 |
| Kwak JY | 473 | 161 | 91 | 84 | 137 | 0.657 | 0.594-0.716 | 0.601 | 0.534-0.665 | 1.646 | 1.371-1.977 | 0.571 | 0.466-0.699 | 2.886 | 1.985-4.194 |
| Mainenti PP | 34 | 12 | 3 | 4 | 15 | 0.750 | 0.476-0.927 | 0.833 | 0.586-0.964 | 4.500 | 1.542-13.13 | 0.300 | 0.125-0.719 | 15.00 | 2.800-80.35 |
| Kim DJ | 30 | 11 | 2 | 7 | 10 | 0.611 | 0.357-0.827 | 0.833 | 0.516-0.979 | 3.667 | 0.982-13.70 | 0.467 | 0.248-0.878 | 7.857 | 1.312-47.04 |
| Kam MH | 23 | 4 | 0 | 5 | 14 | 0.444 | 0.137-0.788 | 1.000 | 0.768-1.000 | 13.50 | 0.813-224.3 | 0.569 | 0.322-1.005 | 23.73 | 1.088-517.4 |
| Ono K | 23 | 3 | 0 | 7 | 13 | 0.300 | 0.067-0.652 | 1.000 | 0.753-1.000 | 8.909 | 0.512-154.9 | 0.707 | 0.466-1.072 | 12.60 | 0.571-278.2 |
| Akiyoshi T | 56 | 15 | 1 | 20 | 20 | 0.429 | 0.263-0.606 | 0.952 | 0.762-0.999 | 9.000 | 1.280-63.30 | 0.600 | 0.443-0.812 | 15.00 | 1.806-124.6 |
| Tsunoda Y | 88 | 21 | 7 | 20 | 40 | 0.512 | 0.351-0.671 | 0.851 | 0.717-0.938 | 3.439 | 1.631-7.250 | 0.573 | 0.410-0.802 | 6.000 | 2.186-16.47 |
| Tsunoda Y | 88 | 5 | 6 | 3 | 74 | 0.625 | 0.245-0.915 | 0.925 | 0.844-0.972 | 8.333 | 3.261-21.30 | 0.405 | 0.165-0.994 | 20.56 | 3.926-107.6 |
| Liamas-Elvira | 53 | 10 | 2 | 38 | 40 | 0.208 | 0.105-0.350 | 0.952 | 0.838-0.994 | 4.375 | 1.015-18.85 | 0.831 | 0.708-0.976 | 5.263 | 1.082-25.60 |
| Tateishi U | 37 | 29 | 11 | 5 | 8 | 0.853 | 0.689-0.950 | 0.421 | 0.203-0.665 | 1.473 | 0.980-2.216 | 0.349 | 0.133-0.918 | 4.218 | 1.132-15.72 |
| Veit-Haibach P | 90 | 16 | 1 | 4 | 29 | 0.800 | 0.563-0.943 | 0.967 | 0.828-0.999 | 24.00 | 3.451-166.9 | 0.207 | 0.086-0.498 | 116.0 | 11.93-1128 |
| Furukawa H | 50 | 7 | 3 | 12 | 15 | 0.368 | 0.163-0.616 | 0.833 | 0.586-0.964 | 2.211 | 0.673-7.259 | 0.758 | 0.508-1.132 | 2.917 | 0.618-13.76 |
| Kantorova I | 32 | 2 | 3 | 5 | 22 | 0.286 | 0.037-0.710 | 0.880 | 0.688-0.975 | 2.381 | 0.490-11.57 | 0.812 | 0.497-1.325 | 2.933 | 0.383-22.46 |
| Mukai M | 24 | 2 | 2 | 7 | 13 | 0.222 | 0.028-0.600 | 0.867 | 0.595-0.983 | 1.667 | 0.282-9.856 | 0.897 | 0.601-1.341 | 1.857 | 0.213-16.18 |
| Abdel-Nabi H | 41 | 4 | 1 | 10 | 26 | 0.286 | 0.084-0.581 | 0.963 | 0.810-0.999 | 7.714 | 0.950-62.63 | 0.742 | 0.528-1.042 | 10.40 | 1.033-104.7 |
| Pooled data | 0.624 | 0.587-0.659 | 0.700 | 0.668-0.732 | 2.825 | 1.964-4.064 | 0.600 | 0.503-0.714 | 6.138 | 3.803-9.909 | |||||
CRC, Colorectal Cancer; PET, Positron Emission Tomography; CT, Computed Tomography; V, Value; CI, Confidence interval; LR, Likelihood ratio; TP, True positive; FP, False Positive; TN, True Negative; FN, False Negative.
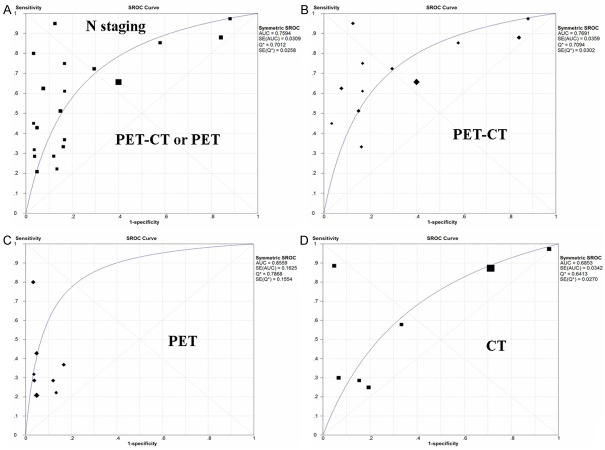
Heterogeneity were found in sensitivity, specificity, LR+, LR- and DOR between 20 included studies after evaluation by plotting the above parameters from each study on a forest plot and calculating the heterogeneity x2. The threshold effect was one important extra source of variation in the meta-analysis. To judge whether the threshold effect existed, the spearman correlation test was utilized to verify it. The spearman correlation coefficient was 0.529 and P value was 0.016, which suggested that the threshold effect existed in this meta-analysis. Then we fitted a summary ROC to evaluate the diagnostic accuracy of PET-CT/PET. An SROC curve for lymph node staging using PET-CT/PET has been illustrated in Figure 2A. The Q* index was calculated as a globe measure of diagnostic accuracy. The AUC and Q* were 0.76 and 0.70, respectively (Table 3 and Figure 2A).
Figure 2.
Summary ROC curve of diagnostic value of 18F-FDG PET-CT or PET and CT in the pre-operative N staging in patients with primary CRC. A. The sROC of 18F-FDG PET-CT or PET; B. The sROC of PET-CT; C. The sROC of PET; D. The sROC of CT. AUC, area under the curve; CRC, colorectal cancer; 18F-FDG, Fluorine-18 fluorodeoxyglucose; PET, Positron Emission Tomography; CT, computed tomography; SROC, summary receiver operating characteristic.
Table 3.
Comparison of the accuracy of pre-operative staging in CRC patients using PET-CT, PET and CT
| Staging | Diagnostic Method | Number of study | Diagnostic Threshold | Pooled Sensitivity | Pooled Specificity | Positive LR | Negative LR | Pooled DOR | AUC | Q* |
|---|---|---|---|---|---|---|---|---|---|---|
| T staging | PET-CT/PET | 4 | Yes | 0.73 (0.65-0.81) | 0.99 (0.98-0.99) | 9.26 (1.22-70.60) | 0.15 (0.02-1.02) | 75.02 (15.14-371.9) | 0.96 | 0.91 |
| N staging | PET-CT/PET | 20 | Yes | 0.62 (0.59-0.66) | 0.70 (0.67-0.73) | 2.83 (1.96-4.06) | 0.60 (0.50-0.71) | 6.14 (3.80-9.91) | 0.76 | 0.70 |
| CT | 7 | No | 0.79 (0.75-0.80) | 0.46 (0.41-0.51) | 1.42 (1.01-2.02) | 0.58 (0.37-0.90) | 3.71 (1.60-8.62) | 0.69 | 0.64 | |
| M staging | PET-CT/PET | 5 | No | 0.91 (0.80-0.96) | 0.95 (0.91-0.98) | 25.40 (4.90-131.6) | 0.14 (0.07-0.27) | 186.4 (52.8-657.6) | 0.97 | 0.92 |
| CT | 5 | No | 0.91 (0.87-0.94) | 0.16 (0.08-0.27) | 1.09 (0.85-1.38) | 0.29 (0.04-2.12) | 4.34 (0.42-45.42) | 0.87 | 0.80 |
CRC, Colorectal Cancer; PET, Positron Emission Tomography; CT, Computed Tomography; LR, Likelihood Ratio; DOR, Diagnostic Odds Ratio; AUC, area under the curve.
As a control test, CT scan has been simultaneously performed in 7 studies. The pooled estimates of sensitivity, specificity, LR+, LR- and DOR of CT in the detection of pre-operative lymph node staging in CRC patients were 0.79 (95% CI: 0.75-0.80), 0.46 (95% CI: 0.41-0.51), 1.42 (95% CI: 1.01-2.02), 0.58 (95% CI: 0.37-0.90) and 3.71 (95% CI: 1.60-8.62), respectively. Diagnostic threshold did not existed (spearman correlation coefficient was 0.321 and P =0.482). The AUC and Q* were 0.69 and 0.64, respectively (Table 3 and Figure 2D).
Diagnostic accuracy of pre-operative tumor detecting rate and T staging using PET-CT or PET
There are 12 studies [16-20,22,25,29,30,37,38,41,42], containing 773 CRC patients, involved in the tumor detecting rate using PET-CT/PET (Supplemental Table 1). Among them, 737 CRC patients were correctly detected while 36 CRC patients were omitted. So the tumor detecting rate was 95.35%. Furthermore, there are 7 studies, including 327 CRC patients, concerning the tumor detecting rate utilizing CT and the tumor detecting rate was 83.85%. Pearson X 2-Test revealed that PET-CT/PET was superior to CT in detecting tumor (X 2=43.99, P < 0.05).
There are 4 studies about the diagnostic accuracy of pre-operative T staging using PET-CT/PET [21,32,36,43]. The pooled estimates of sensitivity, specificity, LR+, LR- and DOR of PET-CT/PET in the detection of pre-operative T staging in CRC patients were 0.73 (95% CI: 0.65-0.81), 0.99 (95% CI: 0.98-0.99), 9.26 (95% CI: 1.22-70.60), 0.15 (95% CI: 0.02-1.02) and 75.02 (95% CI: 15.14-371.9), respectively. Diagnostic threshold existed in this meta-analysis. The AUC and Q* were 0.96 and 0.91, respectively (Table 3).
Diagnostic accuracy of pre-therapeutic M staging using PET-CT or PET
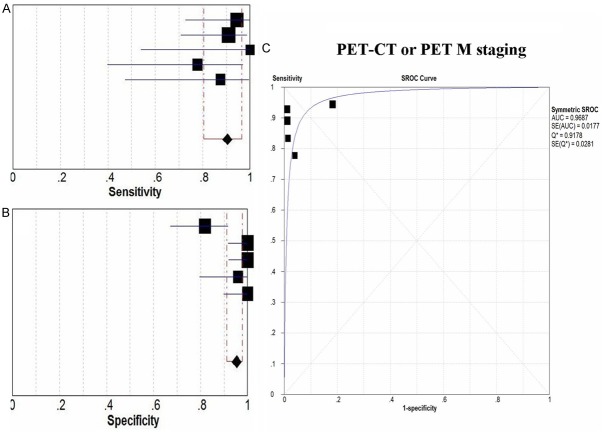
5 studies showed the pre-therapeutic M staging of CRC patients using PET-CT/PET and CT [16,30,36,41,43]. Spearman correlation test revealed that there were no threshold effect existed in the M staging utilizing PET-CT/PET or CT. The pooled estimates of sensitivity, specificity, LR+, LR- and DOR of PET-CT/PET in the detection of pre-therapeutic M staging in CRC patients were 0.91 (95% CI: 0.80-0.96), 0.95 (95% CI: 0.91-0.98), 25.40 (95% CI: 4.90-131.6), 0.14 (95% CI: 0.07-0.27) and 186.4 (95% CI: 52.8-657.6), respectively (Table 3). The forest plot of sensitivity and specificity of PET-CT/PET were presented in Figure 3. SROC illustrated that the AUC and Q* were 0.96 and 0.91, respectively (Figure 3C), which suggested that PET-CT/PET was a perfect option concerning the pre-therapeutic M staging in CRC patients. Meanwhile, the pooled estimates of sensitivity, specificity, LR+, LR- and DOR of CT in the pre-therapeutic M staging in CRC patients were 0.91 (95% CI: 0.87-0.94), 0.16 (95% CI: 0.08-0.27), 1.09 (95% CI: 0.85-1.38), 0.29 (95% CI: 0.04-2.21) and 4.34 (95% CI: 0.42-45.42), respectively (Table 3). And the AUC and Q* were 0.87 and 0.80, respectively.
Figure 3.
The diagnostic performance of PET-CT or PET in pre-operative M staging in CRC patients was illustrated. A, B. Revealed that the forest plot of pooled sensitivity and specificity of 18F-FDG PET-CT or PET in pre-operative M staging in CRC patients, respectively. C. Illustrated the SROC curve of 18F-FDG PET-CT or PET in pre-operative M staging in CRC patients. AUC, area under the curve; CRC, colorectal cancer; 18F-FDG, Fluorine-18 fluorodeoxyglucose; PET, Positron Emission Tomography; CT, computed tomography; SROC, summary receiver operating characteristic.
Subgroup analysis
Just as Table 3 shown, 20 studies investigated the pre-therapeutic N staging of CRC patients using PET-CT or PET. Among them, PET-CT was applied in the 12 studies and PET was utilized in the other 8 studies. The pooled estimates of sensitivity, specificity, LR+, LR- and DOR of PET-CT in the pre-therapeutic N staging in CRC patients were 0.70 (95% CI: 0.66-0.74), 0.63 (95% CI: 0.59-0.67), 2.38 (95% CI: 1.64-3.45), 0.52 (95% CI: 0.42-0.65) and 5.81 (95% CI: 3.26-10.34), respectively (Table 4). Because of existing of threshold effect, the SROC curve was constructed, which revealed that the AUC and Q* were 0.7891 and 0.7094, respectively. Furthermore, The pooled estimates of sensitivity, specificity, LR+, LR- and DOR of PET in the pre-therapeutic N staging in CRC patients were 0.36 (95% CI: 0.29-0.44), 0.93 (95% CI: 0.89-0.96), 4.22 (95% CI: 2.22-8.03), 0.72 (95% CI: 0.59-0.88) and 7.05 (95% CI: 2.93-17.01), respectively. There was no threshold effect and SROC showed that the AUC and Q* were 0.8559 and 0.7868, respectively.
Table 4.
Comparison of the accuracy of pre-operative N staging in colorectal cancer using PET-CT and/or PET
| Diagnostic Method | Number of study | Diagnostic Threshold | Pooled Sensitivity | Pooled Specificity | Positive LR | Negative LR | Pooled DOR | AUC | Q* |
|---|---|---|---|---|---|---|---|---|---|
| PET-CT/PET | 20 | Yes | 0.62 (0.59-0.66) | 0.70 (0.67-0.73) | 2.83 (1.96-4.06) | 0.60 (0.50-0.71) | 6.14 (3.80-9.91) | 0.7594 | 0.7012 |
| PET | 8 | No | 0.36 (0.29-0.44) | 0.93 (0.89-0.96) | 4.22 (2.22-8.03) | 0.72 (0.59-0.88) | 7.05 (2.93-17.01) | 0.8559 | 0.7868 |
| PET-CT | 12 | Yes | 0.70 (0.66-0.74) | 0.63 (0.59-0.67) | 2.38 (1.64-3.45) | 0.52 (0.42-0.65) | 5.81 (3.26-10.34) | 0.7691 | 0.7094 |
PET, Positron Emission Tomography; CT, Computed Tomography; LR, Likelihood Ratio; DOR, Diagnostic Odds Ratio; AUC, area under the curve.
Moreover, 8 studies showed the change of pre-operative TNM staging in CRC patients using PET-CT/PET [17,20,27,31,34,36,39,40]. In the total 670 CRC patients, the stage of 114 cases changed. Among them, 6 studies showed that the stage of 82 cases changed in the total 520 patient, including 37 cases up-regulation and 45 cases down-regulation. Then the change rate of TNM staging was 15.8% through PET-CT/PET test. And the ratios of up-regulation and down-regulation were 45.1% and 54.9%, respectively.
Discussion
Though numerous articles involved in 18F-FDG PET-CT or PET in CRC patients have been presented by now, researches concerning pre-operative TNM staging of primary CRC utilizing 18F-FDG PET-CT/PET are relatively little. Currently, PET/CT is recommended only for the evaluation of the suspicious recurrence of CRC and in pre-operative assessment before metastasectomy. Recently, studies involving in pre-operative TNM staging of primary CRC utilizing 18F-FDG PET-CT/PET are increasingly reported. It is extraordinary necessary to evaluate the value of 18F-FDG PET-CT/PET in determining the pre-operative TNM stage of primary CRC. Therefore, in this study, 28 articles concerning the pre-operative TNM stage of primary CRC using 18F-FDG PET-CT/PET are selected to perform a meta-analysis.
In this study, the primary tumor detecting rate of PET-CT/PET and CT were 95.35% and 83.85%, respectively, which revealed that PET-CT/PET was superior to CT in detecting primary CRC and the difference was statistically significant. As for pre-operative T staging of primary CRC, the globe measure of diagnostic accuracy was 0.91. The performance of PET-CT/PET in determining the pre-operative N staging of primary CRC was better than that of CT scan, which the globe measure of diagnostic accuracy was 0.70 and 0.64, respectively. To our exciting, the value of PET-CT/PET in establishing the pre-operative M staging of primary CRC was perfect, which demonstrated that the pooled sensitivity, pooled specificity and Q* were 0.91, 0.95 and 0.92, respectively. As a control, the pooled specificity of CT scanning in determining the pre-operative of primary CRC was only 0.16 though the pooled sensitivity and Q* were 0.91 and 0.80, respectively.
In this meta-analysis, the primary tumor detecting rate using PET-CT/PET was assessed by 12 studies and the accuracy of pre-operative T staging utilizing PET-CT/PET was evaluated by 4 studies. The results revealed that PET-CT/PET was a satisfactory optional in detecting primary CRC and determining pre-operative T staging with a high specificity (0.99) and diagnostic accuracy (0.91) though the sensitivity was only 0.73, maybe because some small tumors (diameter less than 1 cm) were neglected by PET-CT or PET owing to relatively low spatial resolution. This result was consistent with those of other well-designed studies [16,25,44]. Engelmann BE et al. demonstrated that PET-CT readers identified 97-98% of primary tumors, especially T4 tumor [16]. Ozis SE et al. report that the tumor detecting was 98% and only two CRC cases were ignored, which histopathologies of two FDG negative tumors were mucinous and well-differentiated adenocarcinoma [18]. Cipe G et al. showed that PET-CT had the accuracy rate of 90.4% for T staging [20]. And Veit-Haibach reported that the accuracy rate of T staging using PET-CT was 86% [36], which was similar with our results.
In this study, 20 researches containing 1530 CRC patients determined the pre-operative N staging using PET-CT or PET. The pooled estimates of sensitivity, specificity, LR+, LR- and DOR were 0.62, 0.70, 2.83, 0.60 and 6.14, respectively. Owing to existing of threshold effect, we fit a SROC curve to evaluate the value of PET-CT/PET. The AUC and Q* were 0.76 and 0.70, respectively. Though the global measure of diagnostic accuracy of PET-CT/PET was higher than that of CT (Q*=0.64), the performance of PET-CT/PET in judging pre-operative N staging of CRC was not satisfactory. In order to distinguish the difference of diagnostic method, we performed a subgroup analysis (Table 4), including PET-CT group (12 studies), PET group (8 studies) and PET-CT/PET group (20 studies). The pooled estimates of sensitivity, specificity, LR+ and LR- of PET-CT in the pre-therapeutic N staging in CRC patients were 0.70, 0.63, 2.38 and 0.51, respectively while those of PET were 0.36, 0.93, 4.22 and 0.72, respectively. Because of the existing of threshold effect in PET-CT group, the SROC curve showed that the global measure of diagnostic accuracy of PET-CT and PET was 0.71 and 0.79, respectively. Though the pooled sensitivity of PET-CT (0.70) was superior to those of PET (0.36) and PET-CT/PET (0.62), the pooled specificity of PET was highest in the three groups. Interestingly, the overall diagnostic accuracy of PET was higher than that of PET-CT, maybe because PET was a specific examination in pre-operative N staging of primary CRC. In a word, the overall accuracy of PET-CT or PET for the detection of the pre-operative N staging of primary CRC is not ideal. So it is difficult to recommend the routine use of PET-CT or PET to determine the lymph node involvement in CRC patients. Some possible reasons for the unsatisfactory results of PET-CT/PET to establish lymph node involvement in CRC patients have been explained by some studies as following: firstly, metastatic deposits in lymph nodes are often microscopic and smaller than the detection limit for even the newest PET systems [16]; secondly, metastatic lymph nodes were the close proximity to the primary tumor or urinary bladder [20].
The most important finding of this study is that PET-CT/PET may be an ideal option in terms of pre-therapeutic M staging in patients with primary CRC. The pooled estimates of sensitivity, specificity, LR+, LR- and DOR of PET-CT/PET in the detection of pre-therapeutic M staging in CRC patients were 0.91, 0.95, 25.4, 0.14 and 186.5, respectively. And the AUC and Q* were 0.96 and 0.91, respectively. The performance of PET-CT/PET in detection of pre-therapeutic M staging in primary CRC was superior to that of CT because the overall diagnostic accuracy of CT was 0.80. This result was consistent with those of other well-designed studies [16,30,36]. Engelmann et al. demonstrated that the accuracy of PET-CT in pre-therapeutic M staging in primary CRC was 89% [16]. And Akiyoshi T et al. reported that the accuracy of PET-CT in pre-therapeutic M staging in primary CRC was 97% [30]. Furthermore, our results showed that the change rate of pre-operative TNM staging in patients with primary CRC was 15.8% through PET-CT/PET test, which might alter the therapeutic strategy.
We should acknowledge that there are some limitations in this meta-analysis. Firstly, the interpretation of 18F-FDG PET-CT or PET scans was carried out qualitatively in the majority of studies. And only 9 studies in which the PET or PET-CT reviewers were blinded to patients’ clinical data and other test results while the other 19 studies did not report whether they adopted the blinding. Thus, there is a risk of subjective interpretation. Secondly, 16 studies enrolled patients prospectively while the other 12 studies were retrospective. And only 18 studies included conventional imaging (CT or MRI) as a control while the other 10 articles only had the data of PET-CT or PET, which impaired the performance and application of PET-CT/PET. Thirdly, the existence of clinical heterogeneity in patient population, study design and literature quality in these included studies influences the generalization of the results. In order to minimize bias in the selection of studies and in the data extraction, reviewers who blinded to the journal, authors, publication date and institution independently retrieved articles based on the inclusion criteria. Furthermore, we used the QUADAS tool to guarantee that all the selected articles were high quality articles. Fourth, we did not perform analysis according to the location of primary lesions because one studies involved in only patients with colon cancer and the other studies enrolled patients with rectal cancer or colorectal cancer. Finally, the current analysis did not allow invasion depth-by-invasion depth or node-by-node comparison, which might provide additional crucial information.
In conclusion, the meta-analysis indicated that 18F-FDG PET-CT/PET had good performance in the pre-operative tumor detecting rate, T staging and M staging in patients with primary colorectal cancer when compared with CT, which might alter the therapeutic strategy. However, the diagnostic value of 18F-FDG PET-CT/PET in pre-operative N staging in CRC patients was not ideal, which could be used combining with other conventional imaging in pre-therapeutic CRC patients with suspected lymph node involvement.
Acknowledgements
This study was supported by Department of Gastrointestinal Surgery and Institute of Clinical Medicine, The First Affiliated Hospital, Zhengzhou University and National Natural Science Foundation of China, Grant No. 81201955.
Disclosure of conflict of interest
None.
Abbreviations
- CRC
Colorectal Cancer
- RC
Rectal Cancer
- CC
Colon Cancer
- 18F-FDG
Fluorine-18 2-fluoro-2-deoxy-D-glucose
- PET
Positron Emission Tomography
- CT
Computed Tomography
- MRI
Magnatic Resonance Imaging
- QUADAS
Quality Assessment of Diagnostic Accuracy Studies
- CI
Confidence interval
- LR
Likelihood ratio
- TP
True positive
- FP
False Positive
- TN
True Negative
- FN
False Negative
- DOR
Diagnostic Odds Ratio
- AUC
area under the curve
Supporting Information
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Sun CH, Li ZP, Meng QF, Yu SP, Xu DS. Assessment of spiral CT pneumocolon in preoperative colorectal carcinoma. World J Gastroenterol. 2005;11:3866–70. doi: 10.3748/wjg.v11.i25.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L. Preoperative T and N staging of colorectal cancer: accuracy of contrastenhanced multi-detector row CT colonography-initial experience. Radiology. 2004;231:83–90. doi: 10.1148/radiol.2311021152. [DOI] [PubMed] [Google Scholar]
- 4.Torricelli P. Rectal cancer staging. Surg Oncol. 2007;16:S49–50. doi: 10.1016/j.suronc.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 5.Ahmetoglu A, Cansu A, Baki D, Kul S, Cobanoglu U, Alhan E, Ozdemir F. MDCT with multiplanar reconstruction in the preoperative local staging of rectal tumor. Abdom Imaging. 2011;36:31–7. doi: 10.1007/s00261-009-9591-y. [DOI] [PubMed] [Google Scholar]
- 6.Pakos EE, Fotopoulos AD, Ioannidis JP. 18F-FDG PET for evaluation of bone marrow infiltration in staging of lymphoma: a meta-analysis. J Nucl Med. 2005;46:958–63. [PubMed] [Google Scholar]
- 7.Guo H, Zhu H, Xi Y, Li L, Huang Y, Zhang J, Fu Z, Yang G, Yuan S, Yu J. Diagnostic and prognostic value of 18F-FDG PET/CT for patients with suspected recurrence fromsquamous cell carcinoma of the esophagus. J Nucl Med. 2007;48:1251–8. doi: 10.2967/jnumed.106.036509. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, Coleman RE, Wahl R, Paschold JC, Avril N, Einhorn LH, Suh WW, Samson D, Delbeke D, Gorman M, Shields AF. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 9.Kalff V, Hicks R, Ware R, Hogg A, Binns D, McKenzie A. The clinical impact of 18F-FDG PET in patients with suspected or confirmed recurrence of colorectal cancer: a prospective study. J Nucl Med. 2002;43:492–9. [PubMed] [Google Scholar]
- 10.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25–37. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for metaanalysis of diagnostic accuracy studies. Biostatistics. 2007;8:239–51. doi: 10.1093/biostatistics/kxl004. [DOI] [PubMed] [Google Scholar]
- 12.Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med. 2001;20:2865–84. doi: 10.1002/sim.942. [DOI] [PubMed] [Google Scholar]
- 13.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary ROC curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 14.Kang S, Kim SK, Chung DC, Seo SS, Kim JY, Nam BH, Park SY. Diagnostic value of 18F-FDG PET for evaluation of para-aortic nodal metastasis in patients with cervical carcinoma: a meta-analysis. J Nucl Med. 2010;51:360–7. doi: 10.2967/jnumed.109.066217. [DOI] [PubMed] [Google Scholar]
- 15.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelmann BE, Loft A, Kjær A, Nielsen HJ, Berthelsen AK, Binderup T. Positron emission tomography/computed tomography for optimized colon cancer staging and follow up. Scand J Gastroenterol. 2014;49:191–201. doi: 10.3109/00365521.2013.863967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Lee MR. Positron emission tomography/computed tomography in the staging of colon cancer. Ann Coloproctol. 2014;30:23–7. doi: 10.3393/ac.2014.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozis SE, Soydal C, Akyol C, Can N, Kucuk ON, Yagcı C, Erkek AB, Kuzu MA. The role of 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the primary staging of rectal cancer. World J Surg Oncol. 2014;12:26. doi: 10.1186/1477-7819-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Makis W, Kurzencwyg D, Hickeson M. 18F-FDG PET/CT superior to serum CEA in detection of colorectal cancer and its recurrence. Clinical Imaging. 2013;37:1094–7. doi: 10.1016/j.clinimag.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Cipe G, Ergul N, Hasbahceci M, Firat D, Bozkurt S, Memmi N, Karatepe O, Muslumanoglu M. Routine use of positron-emission tomography/computed tomography for staging of primary colorectal cancer: does it affect clinical management? World J Surg Oncol. 2013;11:49. doi: 10.1186/1477-7819-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang SW, Hsu CM, Jeng WJ, Yen TC, Su MY, Chiu CT. A comparison of positron emission tomography and colonoscopy for the detection of advanced colorectal neoplasms in subjects undergoing a health check-up. PLoS One. 2013;8:e69111. doi: 10.1371/journal.pone.0069111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zafar HM, Kramer S, Bonaccorsi D, Langlotz CP, Armstrong K. Predictors of initial 18F-fluorodeoxyglucose-positron emission tomography indication among patients with colorectal cancer. Nucl Med Commun. 2012;33:739–46. doi: 10.1097/MNM.0b013e328353b249. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Tian M, Gao X, Wang D, Qin Y, Geng J. The method and efficacy of 18F-fluorodeoxyglucose positron emission tomography/computed tomography for diagnosing the lymphatic metastasis of colorectal carcinoma. Acad Radiol. 2012;19:427–33. doi: 10.1016/j.acra.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Kwak JY, Kim JS, Kim HJ, Ha HK, Yu CS, Kim JC. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36:1898–905. doi: 10.1007/s00268-012-1575-3. [DOI] [PubMed] [Google Scholar]
- 25.Mainenti PP, Iodice D, Segreto S, Storto G, Magliulo M, De Palma GD, Salvatore M, Pace L. Colorectal cancer and 18FDG-PET/CT: what about adding the T to the N parameter in locoregional staging? World J Gastroenterol. 2011;17:1427–33. doi: 10.3748/wjg.v17.i11.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim DJ, Kim JH, Ryu YH, Jeon TJ, Yu JS, Chung JJ. Nodal staging of rectal cancer: high-resolution pelvic MRI versus 18F-FDGPET/C. J Comput Assist Tomogr. 2011;35:531–4. doi: 10.1097/RCT.0b013e318225720f. [DOI] [PubMed] [Google Scholar]
- 27.Eglinton T, Luck A, Bartholomeusz D, Varghese R, Lawrence M. Positron-emission tomography/computed tomography (PET/CT) in the initial staging of primary rectal cancer. Colorectal Dis. 2010;12:667–73. doi: 10.1111/j.1463-1318.2009.01873.x. [DOI] [PubMed] [Google Scholar]
- 28.Kam MH, Wong DC, Siu S, Stevenson AR, Lai J, Phillips GE. Comparison of magnetic resonance imaging-fluorodeoxy- glucose positron emission tomography fusion with pathological staging in rectal cancer. Br J Surg. 2010;97:266–8. doi: 10.1002/bjs.6866. [DOI] [PubMed] [Google Scholar]
- 29.Ono K, Ochiai R, Yoshida T, Kitagawa M, Omagari J, Kobayashi H, Yamashita Y. Comparison of diffusion-weighted MRI and 2-[fluorine-18] -fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) for detecting primary colorectal cancer and regional lymph node metastases. J Magn Reson Imaging. 2009;29:336–40. doi: 10.1002/jmri.21638. [DOI] [PubMed] [Google Scholar]
- 30.Eglinton T, Luck A, Bartholomeusz D, Varghese R, Lawrence M. Positron-emission tomography/computed tomography (PET/CT) in the initial staging of primary rectal cancer. Colorectal Dis. 2010;12:667–73. doi: 10.1111/j.1463-1318.2009.01873.x. [DOI] [PubMed] [Google Scholar]
- 31.Davey K, Heriot AG, Mackay J, Drummond E, Hogg A, Ngan S, Milner AD, Hicks RJ. The impact of 18-fluorodeoxyglucose positron emission tomography-computed tomography on the staging and management of primary rectal cancer. Dis Colon Rectum. 2008;51:997–1003. doi: 10.1007/s10350-008-9244-1. [DOI] [PubMed] [Google Scholar]
- 32.Nahas CS, Akhurst T, Yeung H, Leibold T, Riedel E, Markowitz AJ, Minsky BD, Paty PB, Weiser MR, Temple LK, Wong WD, Larson SM, Guillem JG. Positron emission tomography detection of distant metastatic or synchronous disease in patients with locally advanced rectal cancer receiving preoperative chemoradiation. Ann Surg Oncol. 2008;15:704–11. doi: 10.1245/s10434-007-9626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tsunoda Y, Ito M, Fujii H, Kuwano H, Saito N. Preoperative diagnosis of lymph node metastases of colorectal cancer by FDG-PET/CT. Jpn J Clin Oncol. 2008;38:347–53. doi: 10.1093/jjco/hyn032. [DOI] [PubMed] [Google Scholar]
- 34.Llamas-Elvira JM, Rodríguez-Fernández A, Gutiérrez-Sáinz J, Gomez-Rio M, Bellon-Guardia M, Ramos-Font C, Rebollo-Aguirre AC, Cabello-García D, Ferrón-Orihuela A. Fluorine-18 fluorodeoxyglucose PET in the preoperative staging of colorectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:859–67. doi: 10.1007/s00259-006-0274-4. [DOI] [PubMed] [Google Scholar]
- 35.Tateishi U, Maeda T, Morimoto T, Miyake M, Arai Y, Kim EE. Non-enhanced CT versus contrast-enhanced CT in integrated PET/CT studies for nodal staging of rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:1627–34. doi: 10.1007/s00259-007-0455-9. [DOI] [PubMed] [Google Scholar]
- 36.Veit-Haibach P, Kuehle CA, Beyer T, Stergar H, Kuehl H, Schmidt J, Börsch G, Dahmen G, Barkhausen J, Bockisch A, Antoch G. Diagnostic accuracy of colorectal cancer staging with whole-body PET/CT colonography. JAMA. 2006;296:2590–600. doi: 10.1001/jama.296.21.2590. [DOI] [PubMed] [Google Scholar]
- 37.Furukawa H, Ikuma H, Seki A, Yokoe K, Yuen S, Aramaki T, Yamagushi S. Positron emission tomography scanning is not superior to whole body multidetector helical computed tomography in the preoperative staging of colorectal cancer. Gut. 2006;55:1007–11. doi: 10.1136/gut.2005.076273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park IJ, Kim HC, Yu CS, Ryu MH, Chang HM, Kim JH, Ryu JS, Yeo JS, Kim JC. Efficacy of PET/CT in the accurate evaluation of primary colorectal carcinoma. Eur J Surg Oncol. 2006;32:941–7. doi: 10.1016/j.ejso.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 39.Gearhart SL, Frassica D, Rosen R, Choti M, Schulick R, Wahl R. Improved staging with pretreatment positron emission tomography/computed tomography in low rectal cancer. Ann Surg Oncol. 2006;13:397–404. doi: 10.1245/ASO.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 40.Heriot AG, Hicks RJ, Drummond EG, Keck J, Mackay J, Chen F, Kalff V. Does positron emission tomography change management in primary rectal cancer? A prospective assessment. Dis Colon Rectum. 2004;47:451–8. doi: 10.1007/s10350-003-0089-3. [DOI] [PubMed] [Google Scholar]
- 41.Kantorová I, Lipská L, Bêlohlávek O, Visokai V, Trubaĉ M, Schneiderová M. Routine 18F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44:1784–8. [PubMed] [Google Scholar]
- 42.Mukai M, Sadahiro S, Yasuda S, Ishida H, Tokunaga N, Tajima T, Makuuchi H. Preoperative evaluation by whole-body 18F-fluorodeoxyglucose positron emission tomography in patients with primary colorectal cancer. Oncol Rep. 2000;7:85–7. [PubMed] [Google Scholar]
- 43.Abdel-Nabi H, Doerr RJ, Lamonica DM, Cronin VR, Galantowicz PJ, Carbone GM, Spaulding MB. Staging of primary colorectal carcinomas with fluorine-18 fluorodeoxyglucose wholebody PET: correlation with histopathologic and CT findings. Radiology. 1998;206:755–60. doi: 10.1148/radiology.206.3.9494497. [DOI] [PubMed] [Google Scholar]
- 44.Luboldt W, Volker T, Wiedemann B, Zöphel K, Wehrmann U, Koch A, Toussaint T, Abolmaali N, Middendorp M, Aust D, Kotzerke J, Grünwald F, Vogl TJ, Luboldt HJ. Detection of relevant colonic neoplasms with PET/CT: promising accuracy with minimal CT dose and a standardised PET cut-off. Eur Radiol. 2010;20:2274–85. doi: 10.1007/s00330-010-1772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.