Abstract
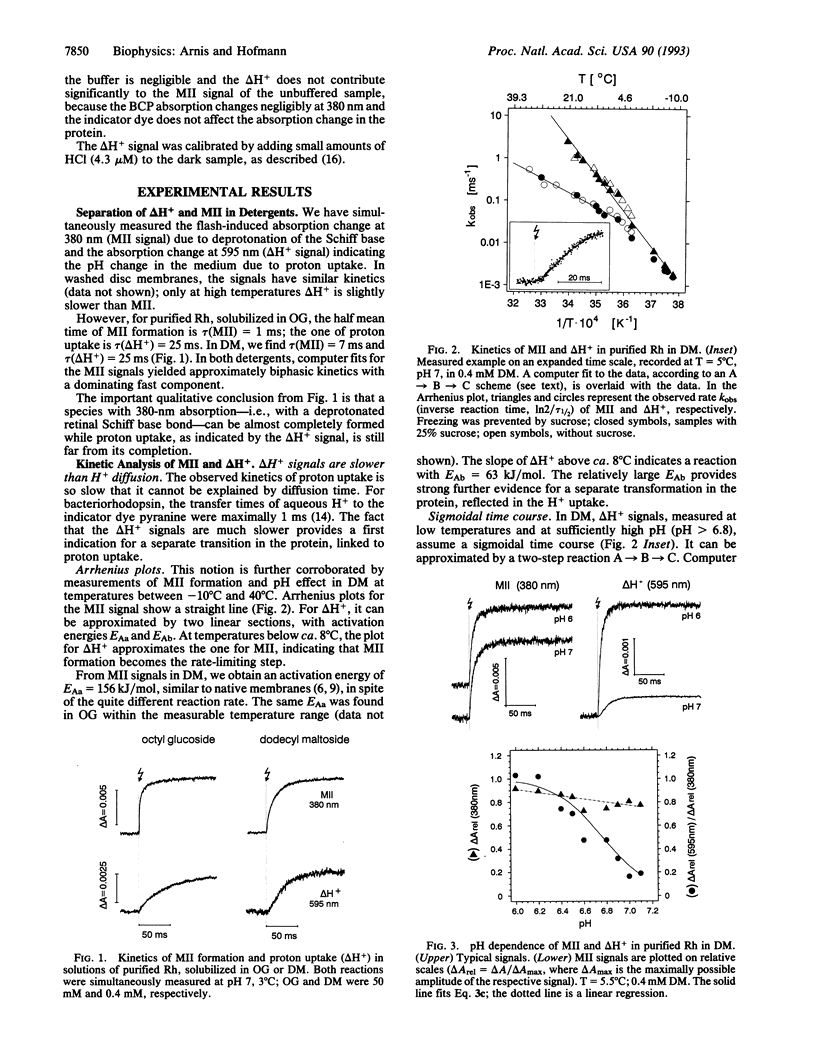
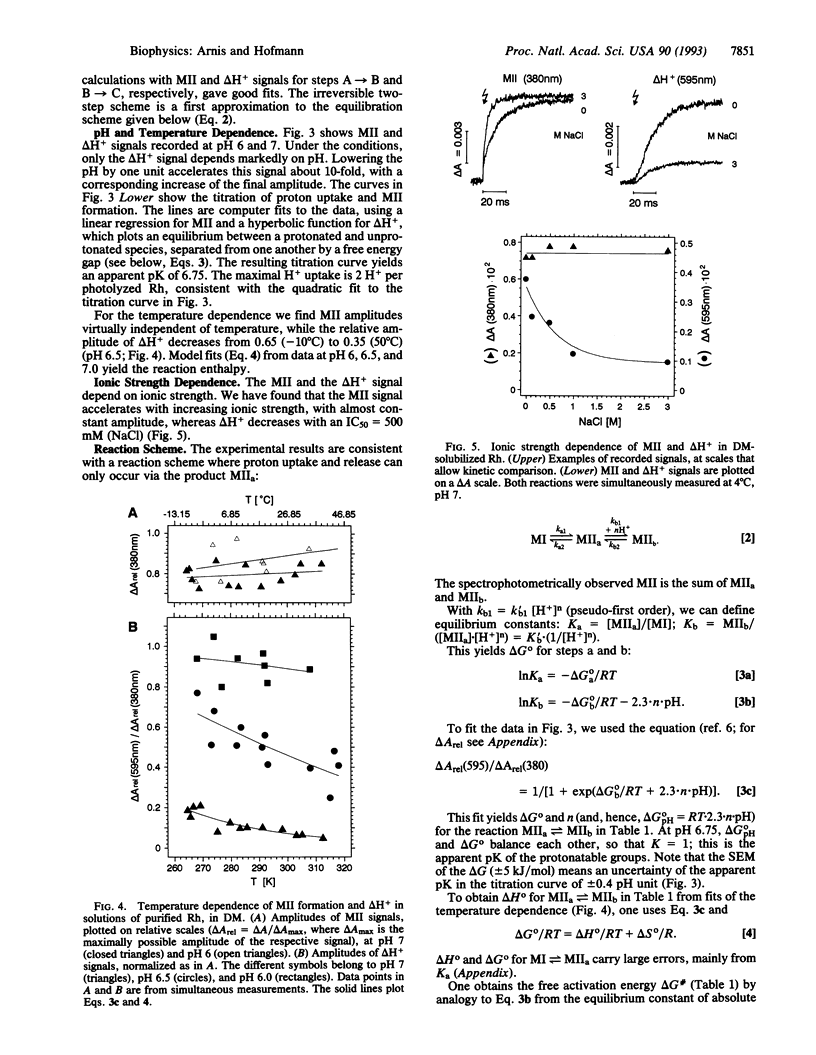
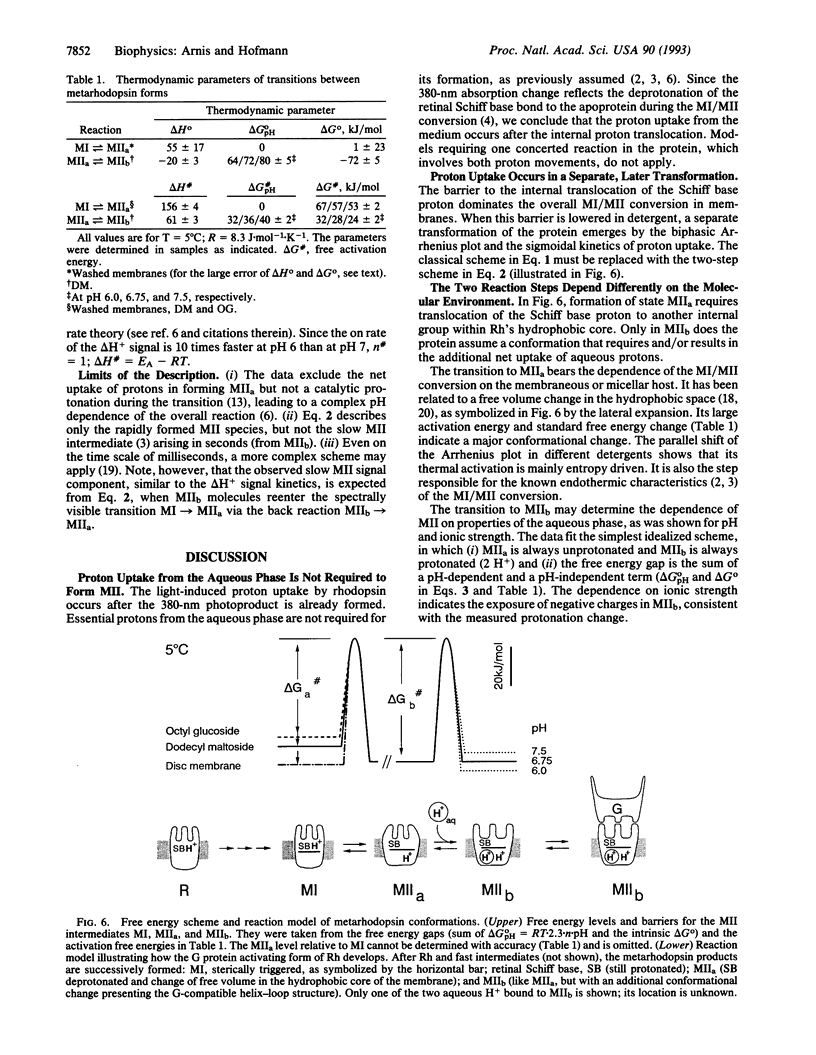
Rhodopsin is a retinal protein and a G-protein-coupled receptor; it shares with both of these families the seven helix structure. To generate the G-interacting helix-loop conformation, generally identified with the 380-nm absorbing metarhodopsin II (MII) photoproduct, the retinal Schiff base bond to the apoprotein must be deprotonated. This occurs as a key event also in the related retinal proteins, sensory rhodopsins, and the proton pump bacteriorhodopsin. In MII, proton uptake from the aqueous phase must be involved as well, since its formation increases the pH of the aqueous medium and is accelerated under acidic conditions. In the native membrane, the pH effect matches MII formation kinetically, suggesting that intramolecular and aqueous protonation changes contribute in concert to the protein transformation. We show here, however, that proton uptake, as indicated by bromocresol purple, and Schiff base deprotonation (380-nm absorption change) show different kinetics when the protein is solubilized in suitable detergents. Our data are consistent with a two-step reaction:
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett N. Optical study of the light-induced protonation changes associated with the metarhodopson II intermediate in rod-outer-segment membranes. Eur J Biochem. 1980 Oct;111(1):99–103. doi: 10.1111/j.1432-1033.1980.tb06079.x. [DOI] [PubMed] [Google Scholar]
- Doukas A. G., Aton B., Callender R. H., Ebrey T. G. Resonance Raman studies of bovine metarhodopsin I and metarhodopsin II. Biochemistry. 1978 Jun 13;17(12):2430–2435. doi: 10.1021/bi00605a028. [DOI] [PubMed] [Google Scholar]
- Emrich H. M., Reich R. Uber Primärreaktionen beim Schvorgang. Thermodynamischer und kinetischer Einfluss des pH-Wertes auf die Metarhodopsin-I-II-Umwandlung. Protonenverbrauch als Auswirkung einer Konformationsänderung. Z Naturforsch C. 1974 Sep-Oct;29C(9-10):577–591. [PubMed] [Google Scholar]
- Fahmy K., Sakmar T. P. Regulation of the rhodopsin-transducin interaction by a highly conserved carboxylic acid group. Biochemistry. 1993 Jul 20;32(28):7229–7236. doi: 10.1021/bi00079a020. [DOI] [PubMed] [Google Scholar]
- Franke R. R., König B., Sakmar T. P., Khorana H. G., Hofmann K. P. Rhodopsin mutants that bind but fail to activate transducin. Science. 1990 Oct 5;250(4977):123–125. doi: 10.1126/science.2218504. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A., Hamm H. E., Hofmann K. P. Interaction of rhodopsin with the G-protein, transducin. Bioessays. 1993 Jan;15(1):43–50. doi: 10.1002/bies.950150107. [DOI] [PubMed] [Google Scholar]
- Heberle J., Dencher N. A. Surface-bound optical probes monitor protein translocation and surface potential changes during the bacteriorhodopsin photocycle. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5996–6000. doi: 10.1073/pnas.89.13.5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann W., Siebert F., Hofmann K. P., Kreutz W. Two distinct rhodopsin molecules within the disc membrane of vertebrate rod outer segments. Biochim Biophys Acta. 1978 Sep 7;503(3):450–461. doi: 10.1016/0005-2728(78)90144-5. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P., Junge W. Rapid calcium release and proton uptake at the disk membrane of isolated cattle rod outer segments. 2. Kinetics of light-stimulated calcium release and proton uptake. Biochemistry. 1981 Sep 15;20(19):5511–5516. doi: 10.1021/bi00522a025. [DOI] [PubMed] [Google Scholar]
- König B., Welte W., Hofmann K. P. Photoactivation of rhodopsin and interaction with transducin in detergent micelles. Effect of 'doping' with steroid molecules. FEBS Lett. 1989 Oct 23;257(1):163–166. doi: 10.1016/0014-5793(89)81811-3. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton transfer and energy coupling in the bacteriorhodopsin photocycle. J Bioenerg Biomembr. 1992 Apr;24(2):169–179. doi: 10.1007/BF00762675. [DOI] [PubMed] [Google Scholar]
- Lewis J. W., Kliger D. S. Photointermediates of visual pigments. J Bioenerg Biomembr. 1992 Apr;24(2):201–210. doi: 10.1007/BF00762678. [DOI] [PubMed] [Google Scholar]
- Longstaff C., Calhoon R. D., Rando R. R. Deprotonation of the Schiff base of rhodopsin is obligate in the activation of the G protein. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4209–4213. doi: 10.1073/pnas.83.12.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell D. C., Straume M., Miller J. L., Litman B. J. Modulation of metarhodopsin formation by cholesterol-induced ordering of bilayer lipids. Biochemistry. 1990 Oct 2;29(39):9143–9149. doi: 10.1021/bi00491a007. [DOI] [PubMed] [Google Scholar]
- Parkes J. H., Liebman P. A. Temperature and pH dependence of the metarhodopsin I-metarhodopsin II kinetics and equilibria in bovine rod disk membrane suspensions. Biochemistry. 1984 Oct 9;23(21):5054–5061. doi: 10.1021/bi00316a035. [DOI] [PubMed] [Google Scholar]
- RADDING C. M., WALD G. The stability of rhodopsin and opsin; effects of pH and aging. J Gen Physiol. 1956 Jul 20;39(6):923–933. doi: 10.1085/jgp.39.6.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleicher A., Hofmann K. P. Proton uptake by light induced interaction between rhodopsin and G-protein. Z Naturforsch C. 1985 May-Jun;40(5-6):400–405. doi: 10.1515/znc-1985-5-619. [DOI] [PubMed] [Google Scholar]
- Straume M., Mitchell D. C., Miller J. L., Litman B. J. Interconversion of metarhodopsins I and II: a branched photointermediate decay model. Biochemistry. 1990 Oct 2;29(39):9135–9142. doi: 10.1021/bi00491a006. [DOI] [PubMed] [Google Scholar]
- Szundi I., Stoeckenius W. Surface pH controls purple-to-blue transition of bacteriorhodopsin. A theoretical model of purple membrane surface. Biophys J. 1989 Aug;56(2):369–383. doi: 10.1016/S0006-3495(89)82683-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Lewis J. W., Wallace-Williams S. E., Kliger D. S. Photolysis of rhodopsin results in deprotonation of its retinal Schiff's base prior to formation of metarhodopsin II. Photochem Photobiol. 1992 Dec;56(6):1135–1144. doi: 10.1111/j.1751-1097.1992.tb09738.x. [DOI] [PubMed] [Google Scholar]
- Wong J. K., Ostroy S. E. Hydrogen ion changes of rhodopsin I. Proton uptake during the metarhodopsin I 478 metarhodopsin II 308 reaction. Arch Biochem Biophys. 1973 Jan;154(1):1–7. doi: 10.1016/0003-9861(73)90028-3. [DOI] [PubMed] [Google Scholar]
- Yan B., Nakanishi K., Spudich J. L. Mechanism of activation of sensory rhodopsin I: evidence for a steric trigger. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9412–9416. doi: 10.1073/pnas.88.21.9412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao V. J., Spudich J. L. Primary structure of an archaebacterial transducer, a methyl-accepting protein associated with sensory rhodopsin I. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11915–11919. doi: 10.1073/pnas.89.24.11915. [DOI] [PMC free article] [PubMed] [Google Scholar]




