Abstract
This study aimed to investigate the expression levels of urothelial carcinoma associated 1 (UCA1) in cancer tissues and plasma of colon cancer patients, and evaluate its clinical significance. Quantitative real-time PCR was used to determine the expression levels of UCA1 in 80 pairs of colon cancer and adjacent normal tissues, plasma samples from 20 healthy controls, 20 colon cancer patients before and after tumor removal. The relationships between UCA1 expression and clinical features and overall survival were analyzed. Compared with adjacent normal tissues, UCA1 was significantly upregulated in colon cancer tissues, especially in cases with LNM and advanced TNM stages (P < 0.05). High UCA1 expression was associated with LMN, higher pT category, and advanced TNM stages (P < 0.05). Patients with high UCA1 expression had worse survival time than those with low UCA1 expression (adjusted HR = 2.002, 95% CI 1.007-3.981, P = 0.048). Furthermore, plasma levels of UCA1 in colon cancer patients were significantly higher than those of controls (P = 0.016). There was significant difference in plasma level of UCA1 between samples taken before and after surgery (P = 0.048). In conclusion, tissue expression of UCA1 is related to prognosis in colon cancer. Plasma UCA1 may serve as a potential biomarker for early diagnosis and disease monitoring of colon cancer patients.
Keywords: Long non-coding RNA, UCA1, colon cancer, metastasis, prognosis
Introduction
Colorectal cancer (CRC) is one of the most common malignant tumors worldwide, with 1.4 million new cases and 693,900 deaths occurring in 2012 [1]. With the continuous development in diagnostics and treatment, the prognosis of CRC has been significantly improved. However, the prognosis of advanced CRC is far from satisfactory. Therefore, identification of biomarkers for predicting CRC progression, metastasis, recurrence, and drug resistance helps improve its prognosis.
Besides large amounts of protein-coding messenger RNAs, there are numerous noncoding transcripts, including microRNA and long non-coding RNAs (lncRNAs), in the human transcriptome. LncRNAs are non-coding transcripts greater than 200 nt in length. Although lncRNAs were initially regarded as a transcription “noise” of the genome, recent studies have shown that lncRNAs regulate gene expression at epigenetic, transcriptional and post-transcriptional levels and are involved in various cell processes [2-5]. Abnormal expression of lncRNAs are associated with various diseases such as cancer [6-12]. For example, urothelial carcinoma associated 1 (UCA1) firstly identified in bladder cancer is an oncogenic lncRNA that participates in the progression, invasion, and drug resistance of bladder cancer [12-14]. Han et al. [15] found that UCA1 is upregulated in CRC and influences CRC cell proliferation, apoptosis, and cell cycle. Recent studies have also shown that circulating cancer-related lncRNAs can be stably detected. Circulating lncRNAs are potential noninvasive biomarkers for cancer [16,17]. Therefore, this study aimed to investigate the expression levels of UCA1 in CRC tissue and plasma samples and analyze the correlation between its expression and clinicopathologic features and prognosis of CRC.
Materials and methods
Patients
Patients undergoing curative-intent resection of colon cancer were recruited from Tongren Hospital between 2008 and 2011. A total of 80 patients with histologically confirmed colon cancer were enrolled. Of these, 48 cases were male and 32 cases female, with an average age of 65.1 years. All patients did not receive any antitumor treatment and have no other cancer history. Simultaneously, 40 plasma samples were collected from 20 colon cancer patients prior to surgery and after surgery for 14 days. In addition, 30 plasma samples were collected from 30 healthy subjects. Written informed consent was obtained from all subjects according to the research protocol approved by the Ethics Committee of Tongren Hospital.
Quantitative real-time PCR (qPCR)
RNA was isolated from tissue and plasma samples using Trizol Reagent (Invitrogen, CA, USA) and mirVana PARIS Kit (Ambion, TX, USA), respectively, according to manufacturer’s protocols. Meanwhile, the synthetic caenorhabditis elegans cel-miR-39 was added after the denaturation of plasma. cDNA PrimeScript TM Reagent Kit with gDNA Eraser Kit (Takara, Dalian, China) was used to synthesize the cDNA, whereas SYBR Premix Ex TaqII Kit was used for amplification reaction (Takara, Dalian, China). The amplification reaction was performed using ABI 7900HT Real-Time PCR System (Applied Biosystems, CA, USA). All reactions were performed in triplicate, and the average Ct values were determined for further analyses. RUN6 and cel-miR-39 were used for internal controls for tissue and plasma samples, respectively, to normalize UCA1 expression. The relative expression level of UCA1 was calculated using formula 2-ΔΔCt.
Statistical analyses
All statistical analyses were performed using the IBM SPSS Statistics 20.0 (SPSS Inc., IL, USA). Paired t-test was used to compare the difference in UCA1 expression between colon cancer and adjacent normal tissues. Other groups were compared with one another in terms of the expression level of UCA1 via Mann-Whitney U test analysis. Chi-square and Fisher exact probability tests were used to analyze the relationship between UCA1 expression and clinicopathologic features of colon cancer patients. Kaplan-Meier method and log-rank test were performed for survival analyses. Cox proportional hazard model was used to analyze the effect of UCA1 and clinicopathologic features on the prognosis of colon cancer patients. All P values were two-sided and a P value < 0.05 was considered statistically significant.
Results
UCA1 is upregulated in colon cancer
UCA1 was overexpressed in colon cancer tissues. The expression levels of UCA1 in colon cancer tissues were significantly higher than those in paracancerous tissues (P < 0.001, Figure 1). Furthermore, the expression levels of UCA1 in cases with TNM stages III and IV significantly upregulated compared with those with TNM stages I and II (P = 0.032), whereas patients with lymph node metastasis (LNM) showed higher levels of UCA1 than those without LNM (P = 0.008).
Figure 1.
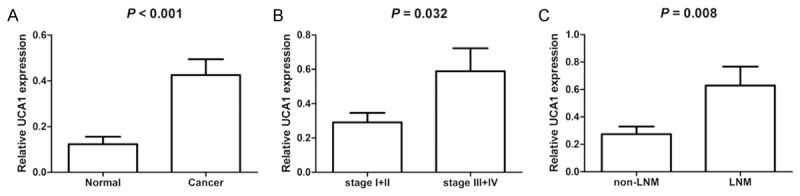
UCA1 is upregulated in colon cancer tissues. A. UCA1 expression in colon and adjacent noncancerous tissues. B. UCA1 expression at different TNM stages. C. UCA1 expression in cases with non-LNM and LNM.
Association between UCA1 expression and clinicopathologic features of colon cancer patients
To examine the association between UCA1 expression and clinicopathologic features, all patients were divided into low (n = 60) and high UCA1 expression groups (n = 20) according to the fourth quartile of the expression level of UCA1. As shown in Table 1, high UCA1 expression was closely related to LNM (P = 0.035), pT category (P = 0.013), and advanced TNM stage (P = 0.018). No significant correlation existed between UCA1 expression and age, sex, differentiation, tumor size (P > 0.05).
Table 1.
Association of UCA1 expression with clinicopathologic parameters
| Characteristics | UCA1 expression | P value | |
|---|---|---|---|
|
| |||
| Low | High | ||
| Age (years) | |||
| ≥ 65 | 32 (53.3) | 10 (50.0) | 0.803 |
| < 65 | 28 (46.7) | 10 (50.0) | |
| Sex | |||
| Male | 39 (65.0) | 9 (45.0) | 0.125 |
| Female | 21 (35.0) | 11 (55.0) | |
| Histologic grade | |||
| 1 | 7 (11.7) | 1 (5.0) | 0.686 |
| 2 | 37 (61.7) | 13 (65.0) | |
| 3 | 16 (26.7) | 6 (30.0) | |
| Tumor size (cm) | |||
| ≥ 5 | 37 (61.7) | 10 (50.0) | 0.435 |
| < 5 | 23 (38.3) | 10 (50.0) | |
| LNM | |||
| Positive | 21 (35.0) | 13 (65.0) | 0.035 |
| Negative | 39 (65.0) | 7 (35.0) | |
| pT category | |||
| T1 | 0 (0.0%) | 3 (15.0) | 0.013 |
| T2 | 14 (23.3%) | 4 (20.0) | |
| T3 | 36 (60.0%) | 8 (40.0) | |
| T4 | 10 (25.0%) | 5 (25.0) | |
| TNM stage | |||
| I+II | 38 (63.3) | 6 (30.0) | 0.018 |
| III+IV | 22 (36.7) | 14 (70.0) | |
The effect of UCA1 expression on the prognosis of colon cancer patients
Survival analysis revealed that median survival time of patients with high UCA1 expression was dramatically lower than that of patients with low UCA1 expression (33 months vs 56 months, P = 0.006, log-rank test Figure 2). In univariate analysis, LNM [hazard ratio (HR) = 2.254, 95% CI 1.174-4.327, P = 0.015), TNM stage (HR = 2.645, 95% CI 1.358-5.151, P = 0.004), and high UCA1 expression (HR = 2.457, 95% CI 1.262-4.782, P = 0.008) were associated with poor survival of colon cancer patients (Table 2). In multivariable analysis, high UCA1 expression remained an independent predictor of poor survival (adjusted HR = 2.002, 95% CI 1.007-3.981, P = 0.048). The other variable associated with overall survival by multivariate analysis was TNM stage (adjusted HR = 5.202, 95% CI 1.148-23.569, P = 0.032).
Figure 2.
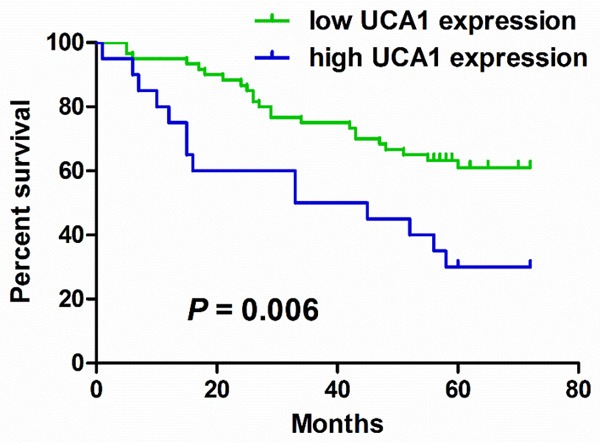
Kaplan-Meier estimates of overall survival for colon cancer patients according to UCA1 expression.
Table 2.
Univariate and multivariate analysis for overall survival
| Characteristics | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (years), ≥ 65 < 65 | 0.539 (0.279-1.040) | 0.065 | ||
| Sex, male vs female | 0.767 (0.400-1.472) | 0.425 | ||
| Histologic grade, 3 vs 1+2 | 1.307 (0.656-2.602) | 0.447 | ||
| Tumor size (cm), 2) ≥ 5 vs < 5 | 1.694 (0.888-3.230) | 0.11 | ||
| LNM, positive vs negative | 2.254 (1.174-4.327) | 0.015 | 2.395 (0.550-10.442) | 0.244 |
| pT category, T3+T4 vs T1+T2 | 1.229 (0.561-2.693) | 0.606 | ||
| TNM, III+IV vs I+II | 2.645 (1.358-5.151) | 0.004 | 5.202 (1.148-23.569) | 0.032 |
| UCA1 expression, high vs low | 2.457 (1.262-4.782) | 0.008 | 2.002 (1.007-3.981) | 0.048 |
Plasma UCA1 is upregulated in colon cancer patients
LncRNA can be secreted by cancer cells or released into the circulation from dead cancer cells. Thus, we further investigated whether colon cancer patients had aberrant circulating levels of UCA1. Colon cancer patients had higher plasma levels of UCA1 compared with healthy controls (P = 0.016, Figure 3). In addition, plasma levels of UCA1 significantly decreased 14 days after surgery (P = 0.042).
Figure 3.
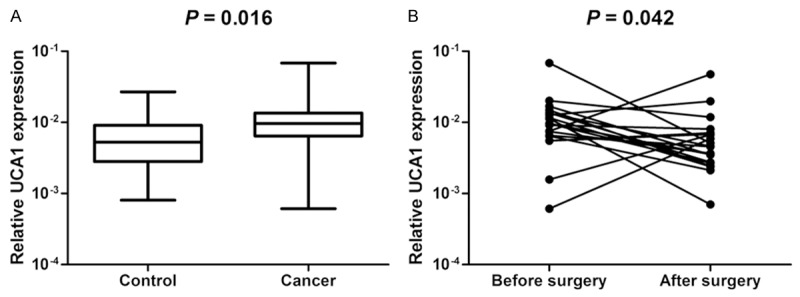
Plasma UCA1 is upregulated in colon cancer patients. A. Plasma levels of UCA1 in colon cancer patients and controls. B. Plasma levels of UCA1 in patients before and after surgical removal of the tumor.
Discussion
Many studies have shown that lncRNAs are closely related to cancer development, progression, and metastasis [6-10]. Therefore, investigating the role of lncRNA in cancer has gained increasing attention over the past few years. LncRNA is also an important molecule source in the diagnosis and treatment of specific diseases. However, the function of lncRNA in CRC remains poorly understood. For example, MALAT1 is overexpressed in CRC [18]. MALAT1 can combine with tumor-suppressors such as SFPQ, and thereby release oncogene PTBP2 from the SFPQ/PTB2 complex. Released PTBP2 promotes the proliferation and metastasis of CRC cells [6]. In the present study, the expression levels of UCA1 were increased in both tumor tissues and plasma from colon cancer patients, and its plasma levels were decreased after surgery. Additionally, high UCA1 expression in CRC tissues is closely related to lymph node metastasis, high invasion, high TNM stage, and poor prognosis. These results implied that UCA1 may be a prognostic biomarker for CRC.
UCA1 is overexpressed in many types of cancer, including bladder cancer, gastric cancer, hepatocellular carcinoma, CRC, melanoma, esophageal carcinoma, and ovarian cancer [12,15,19-24], especially in advanced-stage cases [20-23]. Han et al. [15] found that UCA1 overexpression can promote proliferation, invasion, and metastasis of CRC cells, as well as inhibit cell apoptosis, and was related to advanced TNM stage, poor differentiation, larger tumor, greater tumour depth, and poor survival. In this study, we also analyzed the relationship between UCA1 expression in colon cancer tissues and clinicopathologic features and prognosis. The results showed that differences were observed in UCA1 expression with regard to LNM, high pT stage, advanced TNM stage, and poor prognosis. These results indicate that UCA1 play an important role in CRC metastasis and invasion. There are several differences in associations of UCA1 expression with clinicopathologic features between the results of our study and Han et al. [15], which may be attributable to sample heterogeneity. The findings require further verification with large samples.
Previous studies have revealed that HIF-1α and C/EBPα that are commonly overexpressed in various types of cancer can regulate UCA1 expression [7,9]. Thus, this may partly explain why UCA1 is overexpressed in many types of cancer. UCA1 overexpression can prevent BRG1 from combining with the promoter of p21 and p27 and suppress their expression, thereby leading to the promotion of cancer growth, metastasis, invasion, and inhibition of apoptosis [7-10]. Simultaneously, UCA1 overexpression increases Wnt6 expression and activates the Wnt signaling pathway in a Wnt6-dependent manner, leading to drug-resistance in bladder cancer cells [14]. Drug resistance is a major cause of tumor recurrence and the failure of therapeutic treatments for cancer. A most recent study showed that UCA1 can function as an endogenous sponge for miR-26b, leading to restoring expression of fibroblast growth factor receptor 1 and the activation of ERK signaling pathway [22]. Wang et al. [24] found that UCA1 overexpression upregulated the expression of SPRK1 and promoted ovarian cancer cell proliferation and induce cisplatin resistance. A study by Li et al. [25] showed that UCA1 upregulated hexokinase 2 expression through the activation of STAT3 and inhibition of miR-143 expression to enhance glycolysis, which can further attenuate the inhibition of mTOR by rapamycin. Therefore, cancer patients with high UCA1 expression are more likely to have poor prognosis [15,19-23]. The above results indicate that UCA1 may exert an important function on the development, progression and metastasis of various types of cancer, including CRC. Thus, UCA1 expression may be used as a biomarker to predict the survival of cancer patients.
Given the abundant RNase in the circulation, lncRNAs were originally considered as highly unstable. However, recent studies have demonstrated that circulating lncRNAs can be stably detected even when degraded by RNase [16,17,26]. Moreover, circulating lncRNAs are derived mainly from cancer cells [16,17]. Thus, abnormal expression levels of circulating lncRNAs may reflect abnormal physiological state of patients. For example, linc-POUSFS is overexpressed in esophageal squamous cell carcinoma (ESCC) and stimulates the growth and cloning formation of ESCC cells by promoting POU3F3 gene methylation [2]. Moreover, elevated plasma linc-POUSFS is derived primarily from ESCC cells significantly increase the plasma level, implying that plasma linc-POUSFS is a potential biomarker for the diagnosis of ESCC [16]. Dong et al. [26] also found that a serum three-lncRNA signature exhibited high sensitivity and specificity for the diagnosis of gastric cancer. However, the underlying mechanism why circulating lncRNAs are not degraded by endogenous RNase remains unclear. This phenomenon can be partly explained by the following: 1) circulating lncRNAs may be encapsulated in the particles such as exosomes, microvesicles, and apoptotic bodies; 2) circulating lncRNAs may tend to be more resistant to degradation after modified by methylation, adenylation, and uridylation [27]; 3) lncRNAs exhibit a secondary structure that is relatively more stable [28]. In the present study, we found that plasma UCA1 was significantly upregulated in colon cancer patients, and its level was decreased after surgery. In clinical practice, peripheral blood-based detection for cancer demonstrates advantages of low trauma and high repeatability. Given high stability of lncRNAs in the circulation, plasma UCA1 may be a promising marker for CRC diagnosis.
In conclusion, our results showed that increased UCA1 expression may be involved in the development and progression of colon cancer. UCA1 may serve as a potential diagnostic and prognostic biomarker for colon cancer. However, due to small plasma sample size, whether plasma UCA1 can be used for early diagnosis and monitoring for colon cancer requires further verification in large-scale samples.
Acknowledgements
The project was supported by Shanghai Municipal Health and Family Planning Commission (grant No. XBR2013124), and the National Natural Science Foundation of China (grant No. 81471605).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Li W, Zheng J, Deng J, You Y, Wu H, Li N, Lu J, Zhou Y. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology. 2014;146:1714–1726. e1715. doi: 10.1053/j.gastro.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Ling H, Spizzo R, Atlasi Y, Nicoloso M, Shimizu M, Redis RS, Nishida N, Gafa R, Song J, Guo Z, Ivan C, Barbarotto E, De Vries I, Zhang X, Ferracin M, Churchman M, van Galen JF, Beverloo BH, Shariati M, Haderk F, Estecio MR, Garcia-Manero G, Patijn GA, Gotley DC, Bhardwaj V, Shureiqi I, Sen S, Multani AS, Welsh J, Yamamoto K, Taniguchi I, Song MA, Gallinger S, Casey G, Thibodeau SN, Le Marchand L, Tiirikainen M, Mani SA, Zhang W, Davuluri RV, Mimori K, Mori M, Sieuwerts AM, Martens JW, Tomlinson I, Negrini M, Berindan-Neagoe I, Foekens JA, Hamilton SR, Lanza G, Kopetz S, Fodde R, Calin GA. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–1461. doi: 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guttman M, Donaghey J, Carey BW, Garber M, Grenier JK, Munson G, Young G, Lucas AB, Ach R, Bruhn L, Yang X, Amit I, Meissner A, Regev A, Rinn JL, Root DE, Lander ES. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature. 2011;477:295–300. doi: 10.1038/nature10398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang JF, Yin QF, Chen T, Zhang Y, Zhang XO, Wu Z, Zhang S, Wang HB, Ge J, Lu X, Yang L, Chen LL. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014;24:513–531. doi: 10.1038/cr.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han Z, Sui H, Tang Y, Wang Y, Liu N, Ren J, Hou F, Li Q. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br J Cancer. 2014;111:736–748. doi: 10.1038/bjc.2014.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Gong Y, Jin B, Wu C, Yang J, Wang L, Zhang Z, Mao Z. Long non-coding RNA urothelial carcinoma associated 1 induces cell replication by inhibiting BRG1 in 5637 cells. Oncol Rep. 2014;32:1281–1290. doi: 10.3892/or.2014.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xue M, Li X, Wu W, Zhang S, Wu S, Li Z, Chen W. Upregulation of long non-coding RNA urothelial carcinoma associated 1 by CCAAT/enhancer binding protein alpha contributes to bladder cancer cell growth and reduced apoptosis. Oncol Rep. 2014;31:1993–2000. doi: 10.3892/or.2014.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xue M, Li X, Li Z, Chen W. Urothelial carcinoma associated 1 is a hypoxia-inducible factor-1alpha-targeted long noncoding RNA that enhances hypoxic bladder cancer cell proliferation, migration, and invasion. Tumour Biol. 2014;35:6901–6912. doi: 10.1007/s13277-014-1925-x. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Zhou N, Watabe K, Lu Z, Wu F, Xu M, Mo YY. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1) Cell Death Dis. 2014;5:e1008. doi: 10.1038/cddis.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thum T, Condorelli G. Long Noncoding RNAs and MicroRNAs in Cardiovascular Pathophysiology. Circ Res. 2015;116:751–762. doi: 10.1161/CIRCRESAHA.116.303549. [DOI] [PubMed] [Google Scholar]
- 12.Wang F, Li X, Xie X, Zhao L, Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. FEBS Lett. 2008;582:1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Yang C, Li X, Wang Y, Zhao L, Chen W. Long non-coding RNA UCA1 regulated cell cycle distribution via CREB through PI3-K dependent pathway in bladder carcinoma cells. Gene. 2012;496:8–16. doi: 10.1016/j.gene.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Fan Y, Shen B, Tan M, Mu X, Qin Y, Zhang F, Liu Y. Long non-coding RNA UCA1 increases chemoresistance of bladder cancer cells by regulating Wnt signaling. FEBS J. 2014;281:1750–1758. doi: 10.1111/febs.12737. [DOI] [PubMed] [Google Scholar]
- 15.Han Y, Yang YN, Yuan HH, Zhang TT, Sui H, Wei XL, Liu L, Huang P, Zhang WJ, Bai YX. UCA1, a long non-coding RNA up-regulated in colorectal cancer influences cell proliferation, apoptosis and cell cycle distribution. Pathology. 2014;46:396–401. doi: 10.1097/PAT.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 16.Tong YS, Wang XW, Zhou XL, Liu ZH, Yang TX, Shi WH, Xie HW, Lv J, Wu QQ, Cao XF. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol Cancer. 2015;14:3. doi: 10.1186/1476-4598-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Wang F, Shen J, Sun Y, Xu W, Lu J, Wei M, Xu C, Wu C, Zhang Z, Gao X, Liu Z, Hou J, Huang J, Sun Y. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 derived miniRNA as a novel plasma-based biomarker for diagnosing prostate cancer. Eur J Cancer. 2013;49:2949–2959. doi: 10.1016/j.ejca.2013.04.026. [DOI] [PubMed] [Google Scholar]
- 18.Xu C, Yang M, Tian J, Wang X, Li Z. MALAT-1: a long non-coding RNA and its important 3’ end functional motif in colorectal cancer metastasis. Int J Oncol. 2011;39:169–175. doi: 10.3892/ijo.2011.1007. [DOI] [PubMed] [Google Scholar]
- 19.Tian Y, Zhang X, Hao Y, Fang Z, He Y. Potential roles of abnormally expressed long noncoding RNA UCA1 and Malat-1 in metastasis of melanoma. Melanoma Res. 2014;24:335–341. doi: 10.1097/CMR.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 20.Li JY, Ma X, Zhang CB. Overexpression of long non-coding RNA UCA1 predicts a poor prognosis in patients with esophageal squamous cell carcinoma. Int J Clin Exp Pathol. 2014;7:7938–7944. [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava AK, Singh PK, Rath SK, Dalela D, Goel MM, Bhatt ML. Appraisal of diagnostic ability of UCA1 as a biomarker of carcinoma of the urinary bladder. Tumour Biol. 2014;35:11435–11442. doi: 10.1007/s13277-014-2474-z. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Ying HQ, He BS, Pan YQ, Deng QW, Sun HL, Chen J, Liu X, Wang SK. Upregulated lncRNA-UCA1 contributes to progression of hepatocellular carcinoma through inhibition of miR-216b and activation of FGFR1/ERK signaling pathway. Oncotarget. 2015;6:7899–7917. doi: 10.18632/oncotarget.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Q, Wu F, Dai WY, Zheng DC, Zheng C, Ye H, Zhou B, Chen JJ, Chen P. Aberrant expression of UCA1 in gastric cancer and its clinical significance. Clin Transl Oncol. 2015;17:640–6. doi: 10.1007/s12094-015-1290-2. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Zhou J, Xie X, Hu J, Chen L, Hu Q, Guo H, Yu C. Involvement of SRPK1 in cisplatin resistance related to long non-coding RNA UCA1 in human ovarian cancer cells. Neoplasma. 2015;62:432–438. [PubMed] [Google Scholar]
- 25.Li Z, Li X, Wu S, Xue M, Chen W. Long noncoding RNA UCA1 promotes glycolysis by upregulating hexokinase 2 through the mTORSTAT3/microRNA143 pathway. Cancer Sci. 2014;105:951–955. doi: 10.1111/cas.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong L, Qi P, Xu MD, Ni SJ, Huang D, Xu QH, Weng WW, Tan C, Sheng WQ, Zhou XY, Du X. Circulating CUDR, LSINCT-5 and PTENP1 long noncoding RNAs in sera distinguish patients with gastric cancer from healthy controls. Int J Cancer. 2015;137:1128–35. doi: 10.1002/ijc.29484. [DOI] [PubMed] [Google Scholar]
- 27.Redova M, Sana J, Slaby O. Circulating miRNAs as new blood-based biomarkers for solid cancers. Future Oncol. 2013;9:387–402. doi: 10.2217/fon.12.192. [DOI] [PubMed] [Google Scholar]
- 28.Reis EM, Verjovski-Almeida S. Perspectives of Long Non-Coding RNAs in Cancer Diagnostics. Front Genet. 2012;3:32. doi: 10.3389/fgene.2012.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]


