Abstract
The association between transforming growth factor-β1 (TGF-β1) polymorphisms with the risk of diabetes mellitus (DM) remains elusive. We aimed to evaluate the relationship between TGF-β1 polymorphisms and DM risk. We searched the association studies according to a predefined criteria using electronic databases. The strength of association between TGF-β1 codon 10/25 polymorphisms and the risk of DM was evaluated by odds ratio (OR) with the corresponding 95% confidence interval (CI). Six case-control studies were identified for the analysis of the association between TGF-β1 codon 10/25 polymorphism and the risk of DM. CC genotype at the codon 10 polymorphism was associated with the risk of type 2 DM (T2DM) (P = 0.026, OR = 1.397, 95% CI = 1.041-1.874). No marked association was observed between codon 25 polymorphism and the risk of DM. No evidence of marked publication bias was observed. CC genotype at the TGF-β1 codon 10 site may be an indicator for the risk of T2DM. However, further larger studies should be performed in the future.
Keywords: Diabetes mellitus, gene polymorphisms, transforming growth factor-β1, meta-analysis
Introduction
Diabetes mellitus (DM), a common, frequently-occurring disease, is accounting for 6% of the world’s population [1]. Its incidence is increasing with the aging of the population, economic growth, and changes of lifestyle [2]. The chronic and non-communicable features of DM greatly aggravate people’s health. On the other hand, DM is associated with a variety of complications which led to increased morbidity and mortality [3]. Diabetic nephropathy (DN) is one of the most important complications of DM and the main cause of end-stage renal disease (ESRD) in both developed and developing countries, nearly 20-40% of ESRD patients receiving initial therapy were primarily diagnosed as DM [4,5]. Due to the underlying harms of DM, strenuous efforts have been made to prevent the onset of DM. However, no favorable prevention effects have been obtained. Search for the potential biomarkers for DM susceptibility appears imperative.
Transforming growth factor-β1 (TGF-β1), a pleiotropic cytokine, is a key player in immunoregulation [6]. TGF-β1 plays an important role in the activation of inflammation and the resolution of inflammatory responses in a variety of autoimmune diseases [7]. High glucose induces the increase of TGF-β1 [8]. TGF-β1 also stimulates glucose uptake by enhancing the expression of glucose transporter 1 (GLUT1) in mesangial cells that leads to intracellular metabolic abnormalities in DM [9]. TGF-β1 regulates the production of almost every molecule of the extracellular matrix (ECM) [10]. Glucose intolerance is the hallmark of DM. The central feature of DN is an alteration in the composition of the ECM, including thickening of the glomerular basement membrane (GBM) and expansion of the mesangial matrix [11]. In terms of above-mentioned evidence, TGF-β1 expression may be associated with the risk of DM. Genetic polymorphisms were proven to affect the overall expression and secretion of cytokines [12]. For TGF-β1, the polymorphism at codon 10/25 has been reported to be associated with higher or lower TGF-β1 synthesis [13]. In this sense, TGF-β1 polymorphisms may be associated with the susceptibility of DM.
Currently, a number of studies have been performed to test the association between TGF-β1 codon 10/25 polymorphism and DM risk. However, the results remain in conflict among the reported studies [14-19]. A previous meta-analysis by Jia et al. [20] showed that TGF-β1 codon 10 polymorphism conferred an elevated risk of nephropathy in T2DM. A meta-analysis of the relationship between TGF-β1 polymorphism and DM risk was rare. With the accumulating evidence, we, therefore, summarized the available publications to perform a meta-analysis with the aim of clarifying the association between TGF-β1 polymorphisms and DM risk.
Materials and methods
Search strategy
According to the recommendations of the PRISMA statement [21] (Supplemental Table 1). The published papers were searched through April 2014 for relevant studies that tested the association between TGF-β1 gene polymorphisms and the risk of DM in humans using PubMed, Embase, Cochrane and China National Knowledge Infrastructure (CNKI) databases. No restriction was imposed on search language. The used search terms were as follows: (1) diabetes mellitus, DM, type 1 diabetes mellitus, type 2 diabetes mellitus, T1DM, T2DM; and (2) gene polymorphisms, transforming growth factor-β1, TGF-β1, codon 10, codon 25, 869T/C and 915G/C. We also scrutinized the reference lists of extracted articles and reviews. If the same data was included in more than one study we chose the study with the most complete analysis.
Inclusion and exclusion criteria
Inclusion criteria
1. A case-control study. 2. The outcome of interest was DM. 3. A minimum of two comparison groups (DM group vs control group).
Exclusion criteria
1. Case reports, reviews and editorials. 2. Association of other genes with DM risk. 3. Multiple publications of the same data. 4. Investigation of the role of TGF-β1 gene to diseases.
Data extraction and synthesis
We extracted the following characteristics from each study: First author’s last name, publication year, ethnicity of study population, number of cases and controls for TGF-β1 gene codon 10/25 genotype. Frequencies of allele (C, G) were calculated for case and control groups, from the corresponding genotype distribution. Two authors independently performed the data extraction and synthesis with any disagreements resolved by discussion.
Statistical analysis
STATA version 12.0 (Stata Corp, College Station, TX) was used to calculate the available data from each study. The pooled statistic was odds ratio (OR) to measure the association between TGF-β1 gene codon 10/25 polymorphism and DM risk across studies. Heterogeneity of ORs among studies was tested by using the Q statistic (significance level at P < 0.10). The I2 statistic, a quantitative measure of inconsistency across studies, was also calculated. The pooled ORs were calculated using either fixed-effects model or, in the presence of heterogeneity, random-effects model.
Mantel-Haenszel or I-V heterogeneity model was used.
Furthermore, 95% confidence intervals (CIs) were also calculated. A chi-square test using a web-based program was used to determine whether genotype distribution of the control groups reported conformed to Hardy-Weinberg equilibrium (HWE) (HWE; P < 0.05 was considered significant). Chi-square and exact tests were used. Sensitivity analysis was conducted when studies with controls were not in HWE. Potential publication bias was assessed by Begg’s test and Egger’s test at the P < 0.05 level of significance when the number of enrolled studies was more than two. Begg’s test and Egger’s test were used. P < 0.05 was considered statistically significant, except where otherwise specified.
Results
Study characteristics
We firstly retrieved 236 citations from the PubMed, Embase, Cochrane and China National Knowledge Infrastructure (CNKI) databases. Of these, 221 papers were excluded according to the inclusion and exclusion criteria. Six studies [14-19] were enrolled in our analysis for the association between TGF-β1 gene codon 10/25 polymorphism and DM risk (Figure 1).
Figure 1.
Flow chart of study selection.
Study characteristics for TGF-β1 gene codon 10 polymorphism with DM risk
Six studies [14-19] were identified for the analysis of the association between TGF-β1 gene codon 10 polymorphism and DM risk (Table 1). All studies were performed in Caucasians. A total of 1418 cases and 1024 controls were included. The average frequency of the C allele was 43.5% in cases and 41.3% in controls.
Table 1.
Characteristics of studies evaluating the effects of TGF-β1 polymorphisms on DM risk
| First author, year | Ethnicity | DM | Controls | M allele (%) | HWE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| MM | MN | NN | Total | MM | MN | NN | Total | Case | Control | P | ||
| TGF-β1 codon 10 C/T1 | ||||||||||||
| Tsiavou, 20044 | Caucasian | 9 | 11 | 12 | 32 | 7 | 24 | 8 | 39 | 45.3 | 48.7 | 0.351 |
| Buraczynska, 20074 | Caucasian | 126 | 224 | 153 | 503 | 76 | 181 | 143 | 400 | 47.3 | 41.6 | 0.387 |
| Javor, 20103 | Caucasian | 17 | 56 | 75 | 148 | 27 | 71 | 41 | 139 | 30.4 | 44.9 | 0.931 |
| Jahromi, 20103 | Caucasian | 78 | 208 | 102 | 388 | 75 | 80 | 74 | 229 | 46.9 | 50.2 | < 10-4 |
| El-Sherbini, 20134 | Caucasian | 10 | 50 | 39 | 99 | 3 | 27 | 68 | 98 | 34.3 | 35.1 | 0.332 |
| Bazzaz, 20143 | Caucasian | 39 | 126 | 83 | 248 | 15 | 57 | 47 | 119 | 41.1 | 36.5 | 0.383 |
| TGF-β1 codon 25 G/C2 | ||||||||||||
| Tsiavou, 20044 | Caucasian | 26 | 6 | 0 | 32 | 35 | 4 | 0 | 39 | 90.6 | 94.9 | 0.945 |
| Javor, 20103 | Caucasian | 137 | 11 | 0 | 148 | 117 | 22 | 0 | 139 | 96.3 | 92.1 | 0.599 |
| El-Sherbini, 20134 | Caucasian | 79 | 20 | 0 | 99 | 81 | 9 | 8 | 98 | 89.9 | 87.2 | < 10-4 |
| Bazzaz, 20143 | Caucasian | 201 | 44 | 3 | 248 | 97 | 21 | 1 | 119 | 90 | 90.3 | 0.905 |
C/T = M/N;
G/C = M/N;
Type 1 diabetes;
Type 2 diabetes.
Study characteristics for TGF-β1 gene codon 25 polymorphism with DM risk
Four studies [14,16,18,19] were enrolled for the analysis of the association between TGF-β1 gene codon 25 polymorphism and DM risk (Table 1). All studies were performed in Caucasians. A total of 527 cases and 395 controls were included. The average frequency of the G allele was 91.7% in cases and 90.6% in controls.
Association of TGF-β1 gene codon 10 polymorphism with DM risk
C allele and TT genotype were not associated with the risk of T1DM and T2DM (Table 2). No significant association between CC genotype and T1DM risk was observed (Table 2). CC genotype conferred a significantly increased risk of T2DM (Figure 2; Table 2). Sensitivity analysis showed similar results compared to those from non-sensitivity analysis.
Table 2.
Meta-analysis of the association of TGF-β1 polymorphisms with the risk of DM
| Genetic variant | DM | Studies | Q test | Model selected | OR (95% CI) | P-value |
|---|---|---|---|---|---|---|
|
|
||||||
| p-value | ||||||
| TGF-β1 codon 10 | ||||||
| C vs T | T1DM | 3 | 0.066 | Random | 0.901 (0.698-1.162) | 0.421 |
| T2DM | 3 | 0.033 | Random | 1.304 (0.858-1.984) | 0.214 | |
| CC vs (CT+TT) | T1DM | 3 | 0.137 | Fixed | 0.738 (0.478-1.140) | 0.171 |
| T2DM | 3 | 0.406 | Fixed | 1.397 (1.041-1.874) | 0.026 | |
| TT vs (CT+CC) | T1DM | 3 | 0.022 | Random | 1.043 (0.663-1.640) | 0.856 |
| T2DM | 3 | 0.095 | Fixed | 0.826 (0.528-1.292) | 0.402 | |
| Sensitivity analysis | ||||||
| C vs T | T1DM | 2 | 0.021 | Random | 0.875 (0.531-1.441) | 0.601 |
| CC vs (CT+TT) | T1DM | 2 | 0.107 | Fixed | 0.862 (0.415-1.791) | 0.690 |
| TT vs (CT+CC) | T1DM | 2 | 0.024 | Random | 1.202 (0.601-2.402) | 0.603 |
| TGF-β1 codon 25 | ||||||
| G vs C | T1DM | 2 | 0.767 | Fixed | 1.019 (0.866-1.199) | 0.820 |
| T2DM | 2 | 0.790 | Fixed | 1.010 (0.789-1.292) | 0.939 | |
| GG vs (GC+CC) | T1DM | 2 | 0.674 | Fixed | 1.044 (0.825-1.320) | 0.721 |
| T2DM | 2 | 0.876 | Fixed | 0.949 (0.664-1.356) | 0.774 | |
| CC vs (GC+GG) | T1DM | 2 | 0.738 | Fixed | 1.802 (0.282-11.520) | 0.534 |
| T2DM | 2 | 0.085 | Fixed | 0.532 (0.020-14.194) | 0.706 | |
| Sensitivity analysis | ||||||
| G vs C | T2DM | 1 | - | Fixed | 0.955 (0.593-1.539) | 0.851 |
| GG vs (GC+CC) | T2DM | 1 | - | Fixed | 0.905 (0.454-1.805) | 0.778 |
| CC vs (GC+GG) | T2DM | 1 | - | Fixed | 3.646 (0.144-92.551) | 0.433 |
OR: odds ratio, CI = confidence interval.
Figure 2.
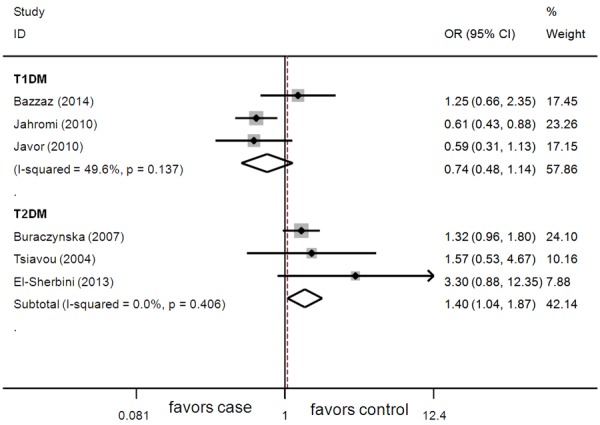
Association between CC genotype and DM risk.
Association of TGF-β1 gene codon 25 polymorphism with DM risk
TGF-β1 gene codon 25 polymorphism was not associated with the risk of DM (Table 2). Sensitivity analysis did not changed the overall results significantly.
Evaluation of publication bias
No significant publication bias was observed (codon 10 C vs T for T1DM/T2DM: Begg P = 0.602/0.603, Egger P = 0.382/0.325; codon 10 CC vs. (CT+TT) for T1DM/T2DM: Begg P = 0.602/0.117, Egger P = 0.793/0.286; codon 10 TT vs (CT+CC) for T1DM/T2DM: Begg P = 0.117/0.602, Egger P = 0.663/0.444).
Discussion
Increasing attention has been focused on the etiology of DM. The confirmation of possible genetic origin of DM would give an insight to early prevention of DM.
A number of investigations reported that gene polymorphism was associated with the susceptibility of DM and could be used as a marker to predict the onset of DM, such as ACE I/D gene polymorphism [22,23]. In our meta-analysis, we found that CC genotype at the TGF-β1 codon 10 site was a risk factor for the onset of T2DM. Several facts may account for the association of TGF-β1 gene polymorphism with DM risk. First, TGF-β1 plays a role of both pro-inflammation and anti-inflammation in many pathophysiological conditions. TGF-β1 inhibits and reverses the activation of macrophages and downregulates central effector mechanisms of the innate immunity [24]. The innate immune system modulates the effects of many factors, such as genes, fetal programming, nutrition, and age on the later development of metabolic sequela associated with insulin resistance [25]. On the other hand, TGF-β1 can also positively regulate immune responses. For example, TGF-β1 supports the differentiation of T-helper 17 (Th17) cells that are activated in many proinflammatory conditions in the presence of Interleukin-6 (IL-6). Of note, IL-6 levels were increased before the onset of T2DM [26]. TGF-β1 could possibly prevent or slow down the autoimmune-mediated destruction of pancreatic Langerhans islets, leading to an absolute lack of insulin production [27]. In this sense, the activation of the innate immune system and the development of a systemic low-grade chronic inflammation are closely involved in the development of T2DM. In terms of above-mentioned evidence, TGF-β1 is closely associated with the susceptibility of DM. Second, the ability of an individual to produce high or low levels of TGF-β1 may be genetically predetermined. For example, the inflammatory and anti-inflammatory activities of TGF-β1 and its signaling pathway is often inactivated by mutation or altered expression of its components [28]. Gene polymorphisms can influence cytokine production or function, they may contribute to genetic predisposition to the disease. Polymorphism at codon 10/25 may be associated with higher or lower TGF-β1 synthesis [13]. SNPs in codon 10/25 of TGF-β1 alter the amino acid sequence (Leu10Pro/Arg25Pro) and also affect TGF-β1 level. TGF-β1 Pro10 (C) allele secrets almost twice as much as Leu10 (T) allele [29]. The C allele was repeatedly associated with increased TGF-β1 production, resulting from a leucine-to proline substitution in the signal amino-acid sequence of the protein, which indicated that certain allele/genotype may affect the risk of DM.
Our findings agreed partially with the above-mentioned evidence. We found that codon 10 CC genotype increased the risk of T2DM, which was consistent with the notion that C allele was linked to an increased production of TGF-β1. However, we observed that codon 10/25 polymorphism was not associated with the susceptibility of T1DM, which might be due to the following facts: first, T1DM develops as a result of progressive T cell-mediated autoimmune destruction of only pancreatic β cells [30]. TGF-β1 is not directly associated with the damage of pancreatic β cells, which indicated that TGF-β1 gene polymorphism may not affect the T1DM risk obviously.
Second, the etiology, metabolic status and disease-specific genetic background vary quite a lot between T1DM and T2DM. Compared with T1DM, T2DM is a complex metabolic disease characterized by insulin resistance and/or pancreatic β-cell dysfunction, and is always associated with metabolic abnormalities, including obesity, hypertension and hyperlipidemia, which were associated with the inflammatory responses [31]. We speculated that TGF-β1 may interacted with these factors through its pro- and anti-inflammatory effects to increase the risk of T2DM. Interestingly, although TGF-β1 gene polymorphism at codon 25 is significantly associated with TGF-β1 production, we observed that TGF-β1 codon 25 polymorphism was not associated with DM, which might be due to the facts that only six studies were included in the investigation, and the studied populations were limited to Caucasians, which might not preclude the possibility that TGF-β1 gene polymorphism exerts effects in DM risk in a population-specific manner. In the past, the association between TGF-β1 gene polymorphism and metabolic diseases were investigated in a number of studies. Scaglione et al. [32] reported that TGF-β1 gene polymorphism was associated with left ventricular geometry and function in hypertensive subjects. Long et al. [33] reported that TGF-β1 genes were associated with obesity phenotypes. Argano et al. [34] reported that TGF-β1 T29C gene polymorphism was associated with the severity of hypertension. Rosmond et al. [35] reported that Pro10 allele in the TGF-β1 gene pathway contributed to obesity. Fuku et al. [36] reported that total, leg, and appendicular fat-free mass index were significantly lower in male subjects with CT/TT genotypes compared with those with CC genotypes. All these evidences strongly indicated the possibility that TGF-β1 gene polymorphism might be associated with the development of DM.
Several limitations should be considered in our study. First, the heterogeneities might affect the results of our meta-analysis, although a random-effects model had been conducted. Second, most participants were Caucasians, which might result in selection bias. More studies in other regions, such as Africa and Asia should be conducted in the future. Finally, although no evidence of significant publication bias was observed, the relative small number of participants decreases the statistical power. The stages of DM and interactions among different sites of TGF-β1 gene or other genes might affect the results. More in-depth analysis in terms of these factors should be performed in the future.
Taken together, the results of our study suggest that CC genotype at the TGF-β1 codon 10 site may be an indicator for the risk of T2DM. However, more studies are needed in the future.
Acknowledgements
This study was supported by Grants from the Research and innovation Project for College Graduates of Jiangsu Province, China (grant number CXLX13_556).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Meetoo D, McGovern P, Safadi R. An epidemiological overview of diabetes across the world. Br J Nurs. 2007;16:1002–1007. doi: 10.12968/bjon.2007.16.16.27079. [DOI] [PubMed] [Google Scholar]
- 2.Mou X, Liu WH, Zhou DY, Liu YH, Hu YB, Ma GL, Shou CM, Chen JW, Zhao JX. Association of Chinese medicine constitution susceptibility to diabetic nephropathy and transforming growth factor-β1 (T869C) gene polymorphism. Chin J Integr Med. 2011;17:680–684. doi: 10.1007/s11655-011-0845-5. [DOI] [PubMed] [Google Scholar]
- 3.Imam K. Management and treatment of diabetes mellitus. Adv Exp Med Biol. 2012;771:356–380. doi: 10.1007/978-1-4614-5441-0_26. [DOI] [PubMed] [Google Scholar]
- 4.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 5.McDonald S, Excell L, Livingston B. Appendix II in ANZDATA (Australia and New Zealand dialysis and transplant) registry report. Aust N Z J Public Health. 2008;27:499–518. [Google Scholar]
- 6.Lawrence DA. Transforming growth factor-beta: An overview. Kidney Int Suppl. 1995;49:S19–S23. [PubMed] [Google Scholar]
- 7.Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang M, Zhou F, Zhang W, Guo Z, Shang Y, Lu H, Lu R, Zhang Y, Chen Y, Zhong M. The role of thrombospondin-1-mediated TGF-β1 on collagen type III synthesis induced by high glucose. Mol Cell Biochem. 2011;46:49–56. doi: 10.1007/s11010-010-0590-7. [DOI] [PubMed] [Google Scholar]
- 9.Inoki K, Haneda M, Maeda S, Koya D, Kikkawa R. TGF-beta 1 stimulates glucose uptake by enhancing GLUT1 expression in mesangial cells. Kidney Int. 1999;55:1704–1712. doi: 10.1046/j.1523-1755.1999.00438.x. [DOI] [PubMed] [Google Scholar]
- 10.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, Rowley DR. Reactive stroma in human prostate cancer: Induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–2923. [PubMed] [Google Scholar]
- 11.Yamagishi S, Fukami K, Ueda S, Okuda S. Molecular mechanisms of diabetic nephropathy and its therapeutic intervention. Curr Drug Targets. 2007;8:952–959. doi: 10.2174/138945007781386884. [DOI] [PubMed] [Google Scholar]
- 12.Gu X, Ji X, Shi LH, Yi CH, Zhao YP, Wang AH, Lu LG, Yu WB, Gao CF. Transforming growth factor beta1 gene variation Leu10Pro affects secretion and function in hepatic cells. Dig Dis Sci. 2012;57:2901–2909. doi: 10.1007/s10620-012-2238-9. [DOI] [PubMed] [Google Scholar]
- 13.Grainger DJ, Heathcote K, Chiano M, Snieder H, Kemp PR, Metcalfe JC, Carter ND, Spector TD. Genetic control of the circulating concentration of transforming growth factor type beta 1. Hum Mol Genet. 1999;8:93–97. doi: 10.1093/hmg/8.1.93. [DOI] [PubMed] [Google Scholar]
- 14.Tsiavou A, Hatziagelaki E, Chaidaroglou A, Manginas A, Koniavitou K, Degiannis D, Raptis SA. TNF-alpha, TGF-beta1, IL-10, IL-6 gene polymorphisms in latent autoimmune diabetes of adults (LADA) and type 2 diabetes mellitus. J Clin Immunol. 2004;24:591–599. doi: 10.1007/s10875-004-6239-0. [DOI] [PubMed] [Google Scholar]
- 15.Buraczynska M, Baranowicz-Gaszczyk I, Borowicz E, Ksiazek A. TGF-beta1 and TSC-22 gene polymorphisms and susceptibility to microvascular complications in type 2 diabetes. Nephron Physiol. 2007;106:p69–75. doi: 10.1159/000104874. [DOI] [PubMed] [Google Scholar]
- 16.Javor J, Ferencik S, Bucova M, Stuchlikova M, Martinka E, Barak L, Strbova L, Grosse-Wilde H, Buc M. Polymorphisms in the genes encoding TGF-beta1, TNF-alpha, and IL-6 show association with type 1 diabetes mellitus in the Slovak population. Arch Immunol Ther Exp (Warsz) 2010;58:385–393. doi: 10.1007/s00005-010-0092-z. [DOI] [PubMed] [Google Scholar]
- 17.Jahromi MM, Millward BA, Demaine AG. Significant correlation between association of polymorphism in codon 10 of transforming growth factor-beta1 T (29) C with type 1 diabetes and patients with nephropathy disorder. J Interferon Cytokine Res. 2010;30:59–66. doi: 10.1089/jir.2009.0026. [DOI] [PubMed] [Google Scholar]
- 18.El-Sherbini SM, Shahen SM, Mosaad YM, Abdelgawad MS, Talaat RM. Gene polymorphism of transforming growth factor-β1 in Egyptian patients with type 2 diabetes and diabetic nephropathy. Acta Biochim Biophys Sin (Shanghai) 2013;45:330–338. doi: 10.1093/abbs/gmt003. [DOI] [PubMed] [Google Scholar]
- 19.Bazzaz JT, Amoli MM, Taheri Z, Larijani B, Pravica V, Hutchinson IV. TGF-β1 and IGF-I gene variations in type 1 diabetes microangiopathic complications. J Diabetes Metab Disord. 2014;13:45–49. doi: 10.1186/2251-6581-13-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jia H, Yu L, Gao B, Ji Q. Association between the T869C polymorphism of transforming growth factor-beta 1 and diabetic nephropathy: a meta-analysis. Endocrine. 2011;40:372–378. doi: 10.1007/s12020-011-9503-0. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. doi: 10.1016/j.ijsu.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Bai H, Jing D, Guo A, Yin S. Association between interleukin 10 gene polymorphisms and risk of type 2 diabetes mellitus in a Chinese population. J Int Med Res. 2014;42:702–10. doi: 10.1177/0300060513505813. [DOI] [PubMed] [Google Scholar]
- 23.Shaikh R, Shahid SM, Mansoor Q, Ismail M, Azhar A. Genetic variants of ACE (Insertion/Deletion) and AGT (M268T) genes in patients with diabetes and nephropathy. J Renin Angiotensin Aldosterone Syst. 2014;15:124–30. doi: 10.1177/1470320313512390. [DOI] [PubMed] [Google Scholar]
- 24.Li MO, Wan YY, Flavell RA. T cell-produced transforming growth factor-beta1 controls T cell tolerance and regulates Th1- and Th17-celldifferentiation. Immunity. 2007;26:579–591. doi: 10.1016/j.immuni.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Lazenby MG, Crook MA. The innate immune system and diabetes mellitus: the relevance of periodontitis? A hypothesis. Clin Sci (Lond) 2010;119:423–429. doi: 10.1042/CS20100098. [DOI] [PubMed] [Google Scholar]
- 26.Herder C, Carstensen M, Ouwens DM. Antiinflammatory cytokines and risk of type 2 diabetes. Diabetes Obes Metab. 2013;3:39–50. doi: 10.1111/dom.12155. [DOI] [PubMed] [Google Scholar]
- 27.Patel A, Scott WR, Lympany PA, Rippin JD, Gill GV, Barnett AH, Bain SC Warren 3/UK GoKind Study Group. The TGF-beta 1 gene codon 10 polymorphism contributes to the genetic predisposition to nephropathy in Type 1 diabetes. Diabet Med. 2005;22:69–73. doi: 10.1111/j.1464-5491.2005.01376.x. [DOI] [PubMed] [Google Scholar]
- 28.Elliott RL, Blobe GC. Role of transforming growth factor Beta in human cancer. J. Clin. Oncol. 2005;23:2078–2093. doi: 10.1200/JCO.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 29.Wang H, Mengsteab S, Tag CG, Gao CF, Hellerbrand C, Lammert F, Gressner AM, Weiskirchen R. Transforming growth factor-beta1 gene polymorphisms are associated with progression of liver fibrosis in Caucasians with chronic hepatitis C infection. World J Gastroenterol. 2005;11:1929–1936. doi: 10.3748/wjg.v11.i13.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Notkins AL, Lernmark A. Autoimmune type 1 diabetes: resolved and unresolved issues. J Clin Invest. 2001;108:1247–1252. doi: 10.1172/JCI14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandal A. Study of Prevalence of Type 2 Diabetes Mellitus and Hypertension in Overweight and Obese People. J Family Med Prim Care. 2014;3:25–28. doi: 10.4103/2249-4863.130265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scaglione R, Argano C, Duro G, Di Chiara T, Nuzzo D, Colomba D, Fiore MC, Corrao S, Licata G. The Relationship between the Transforming Growth Factor β1 T29C Gene Polymorphism and Left Ventricular Geometry and Function in Hypertensive Subjects. Int J Hypertens. 2010;2010:647147. doi: 10.4061/2010/647147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long JR, Liu PY, Liu YJ, Lu Y, Xiong DH, Elze L, Recker RR, Deng HW. APOE and TGF-beta1 genes are associated with obesity phenotypes. J Med Genet. 2003;40:918–924. doi: 10.1136/jmg.40.12.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Argano C, Duro G, Corrao S, Di Chiara T, Nuzzo D, Colomba D, Scaglione R, Licata G. Transforming growth factor beta1 T29C gene polymorphism and hypertension: relationship with cardiovascular and renal damage. Blood Press. 2008;17:220–226. doi: 10.1080/08037050802431416. [DOI] [PubMed] [Google Scholar]
- 35.Rosmond R, Chagnon M, Bouchard C, Björntorp P. Increased abdominal obesity, insulin and glucose levels in nondiabetic subjects with a T29C polymorphism of the transforming growth factor-beta1 gene. Horm Res. 2003;59:191–194. doi: 10.1159/000069323. [DOI] [PubMed] [Google Scholar]
- 36.Fuku N, Mori S, Murakami H, Gando Y, Zhou H, Tanaka M, Miyachi M. Association of 29C>T polymorphism in the transforming growth factor-β1 gene with lean body mass in community-dwelling Japanese population. Geriatr Gerontol Int. 2012;12:292–297. doi: 10.1111/j.1447-0594.2011.00768.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


