Abstract
Chronic obstructive pulmonary disease (COPD) is a common respiratory disease characterized by airflow obstruction that is usually progressive and not fully reversible. It is accompanied by the abnormal inflammatory response of lung to toxic particles or gas. Studies indicate that chronic inflammatory injuries of airway, pulmonary parenchyma and pulmonary vessels are the characteristic changes of COPD. Adhesion of inflammatory cells is the important link of pulmonary infection. Intercellular adhesion molecule-1 (ICAM-1) is a glycoprotein involved in binding with mediated cells or with the extracellular matrix in the process called cell adhesion. IL-1β is an important inflammatory mediator as well as the promoter and critical inducer of cytokine cascade reaction. In this study, the rat model of COPD was established by smoking + intratracheal instillation of LPS (the experimental group). PaO2 and PaCO2 were measured. ICAM-1mRNA and IL-1βmRNA level in lung homogenate were detected by immunohistochemistry and RT-PCR and were compared with those of the rats treated by smoke exposure (the control group) and the healthy rats (the blank group) in order to investigate the effect of ICAM-1 and IL-1β in lung injury of COPD. This study showed that the respiratory function of rats with COPD was decreased. PaO2 of rats in the experimental group, the control group and the blank group decreased successively, and the comparison between any two groups had significant difference. PaCO2 increased successively, and the comparison between any two groups had significant difference. Immunohistochemistry results showed that protein expression of ICAM-1 and IL-1β in lung tissues of rats in the experimental group was higher than that in the control group and the blank group, and the comparison between any two groups had significant difference. RT-PCR results showed that ICAM-1mRNA and IL-1βmRNA level of rats in the experimental group was higher than that in the control group and the blank group, and the comparison between any two groups had significant difference. This study indicated that the decreased respiratory function of rats with COPD was associated with the imbalance of inflammatory cascade and the up-regulation of ICAM-1mRNA and IL-1βmRNA in lung tissues and cells caused inflammatory injury and decreased respiratory function.
Keywords: Chronic obstructive pulmonary disease, intercellular adhesion molecule-1mRNA, interleukin-1βmRNA
Introduction
Chronic obstructive pulmonary disease (COPD) has become the global public health problem due to its high incidence, high mortality and heavy medical burden [1,2]. COPD now is the fourth leading cause of death worldwide [3,4]. Around the world, smoking and recurrent respiratory tract infection are the most common risk factors of COPD [5-7]. In many countries, air pollution, occupational dust and indoor air pollution are also the major risk factors of COPD. However, the pathogenesis of COPD is now not quite clear, which affects the clinical treatment to a great extent. Studies indicate that chronic inflammatory injuries of airway, pulmonary parenchyma and pulmonary vessels are the characteristic changes of COPD [8].
Intercellular adhesion molecule-1 (ICAM-1) is a single strand glycoprotein in immunoglobulin superfamily, which activates the adhesive action between leukocytes and stromal cells as well as between leukocytes and vascular endothelial cells, activates the adhesion, aggregation and release of leukocytes, regulates the expression of multiple cytokines [9,10] and promotes the occurrence and development of inflammation [11,12]. The expression level of ICAM-1 may reflect the degree of inflammatory injuries. In normal physiological conditions, there is little expression or no expression of ICAM-1 in most of tissues, including lung tissues [13-17]. When risk factors lead to the increase of ICAM-1 in endothelial cells, ICAM-I may interact with the integrin on the surface of neutrophils, causing leukocyte chemotaxis. The activated leukocytes adhere, aggregate and release. The release of proinflammatory factors and the imbalance of inflammatory response lead to the inflammatory injury effect [18-21]. IL-1 includes three proteins highly homologous in amino acid sequence, IL-1α, IL-1β and IL-1γ. IL-1α is a membrane binding protein that takes effect in local part through paracrine and autocrine, while, IL-1β can pass into blood. Studies show that IL-1β is an important proinflammatory cytokine that may activate the cascade effect of inflammatory cascade [22]. Under the condition that the peripheral mononuclear cells are induced by most of the stimulants, IL-1βmRNA level is 20-25 times of IL-1α mRNA [23].
Our preliminary studies have confirmed the involvement of ICAM-1 [24,25] and IL-1β [26] in the occurrence of acute lung injury, while there is no relevant report on the involvement of ICAM-1 and IL-1β in the occurrence of COPD at home and abroad. In this study, the rat model of COPD was established by smoke exposure + intratracheal instillation of LPS (the experimental group) to imitate the risk factors of COPD: smoking and infection. Pulmonary function, pathological changes, expression of ICAM-1 protein and IL-1β protein in lung tissues and expression level of ICAM-1mRNA and IL-1βmRNA of rats with COPD were detected and compared with those of the rats treated by smoke exposure (the control group) and the healthy rats (the blank group) in order to investigate the effect of ICAM-1 and IL-1β in COPD. This study aims to provide new methods and laboratory basis for the treatment of COPD.
Materials and methods
Materials and reagents
IL-1β ELISA kit and ICAM-1 ELISA kit (Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd., Beijing, China); PCR instrument (PerkinElmer, Shanghai, China); Sanhua filter-tipped cigarettes (China Tobacco Henan Industrial Co., Ltd., Zhengzhou, China), containing 13 mg tar, 1.0 mg smoking nicotine (nicotine) and 14 mg carbon monoxide per cigarette; LPS (Shanghai Haoran Biological Technology Co., Ltd., Shanghai, China); chloral hydrate (Jinan Xinyuanda Industry, Jinan, China); rabbit anti-rat ICAM-1/IL-1β antibody (Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China); IgG antibody (Abcam Shanghai Office, Shanghai, China); diaminobenzidine (Beijing CellChip Biotechnology Co., Ltd., Beijing, China). Other lab consumables were provided by the Department of Functional Experiment, Xinxiang Medical University (Xinxiang, Henan).
Animal groups
In total, 60 CV healthy SD rats at body weight of (150±10) g provided by Laboratory Animal Center of Zhengzhou University (Zhengzhou University, Zhengzhou, China) were randomized into three groups, the experimental group, the control group and the blank group, 20 in each group. All rats were placed in the cage at appropriate temperature and humidity and they may eat and drink freely before this study. The experimental procedure was approved by the Ethics Committee of Xinxiang Medical University (Xinxiang, China). This study conformed to the ethical principles of Helsinki Declaration.
Animal experiment
According to the reference [27,28], the rats in the experimental group were placed in a smoke exposure box of 60 cm×40 cm×30 cm for 30 min, twice daily for 28 d. Lipopolysaccharide at the dose of 1 mg.Kg-1 was given by intratracheal instillation on 1d and 14 d. The rats were anesthetized by intraperitoneal injection of 4% chloral hydrate at the dose of 10 ml.kg-1 on 29 d and underwent cervical arterial catheterization. 2 ml arterial blood was taken for PaO2 and PaCO2 determination. The rats were sacrificed and the entire lung was removed. The lung tissues were treated in order to determine ICAM-1mRNA and IL-1βmRNA level by immunohistochemistry and RT-PCR. The rats in the control group were given equal normal saline by intratracheal instillation on 1 d and 14 d and the other operations were the same with the experimental group. The rats in the blank group were not treated with smoke exposure, LPS and normal saline and the other operations were the same with the experimental group.
Immunohistochemistry
Lung tissues were formalin-fixed, paraffin-embedded, deparaffinized, incubated with 3% H2O2 and blocked with normal goat serum. Rabbit anti-rat ICAM-1/IL-1β antibody and biotinylated anti-rabbit IgG were added drop by drop, incubated with diaminobenzidine (compound avidin-biotin substrate) and stained with hematoxylin. These steps were repeated by three times.
Total RNA extraction and RT-PCR
Lung tissues preserved at -80°C were taken and total RNA was extracted by Trizol single-step method. RT-PCR was performed, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal reference gene. Primer sequence and target fragment: GAPDH upstream primer sequence: 5’-TCC CTC AAG ATT GTC AGC AA-3’; downstream primer sequence: 5’-AGA TCC ACA ACG GAT ACA TT-3’; amplified fragment length: 309 bp. ICAM-1 upstream primer sequence: 5’-CTTTGCCCTGGTCCTCCAAT-3’; downstream primer sequence: TGTCTTCCCCAATGTCGCTC-3’; amplified fragment length: 208 bp. IL-1β upstream primer sequence: 5’-GTCACTCATTGTGGCTGTGGA-3; downstream primer sequence: 5’-GTCGTTGCTTGTCTCTCCTTGT-3’, amplified fragment length: 219 bp. All primer sequence was synthesized by Invitrogen (Invitrogen Beijing Office, Beijing, China). The gray intensity ratio of PCR target band and internal reference band was analyzed by Bandleader 3.0.
Statistical method
All data were input into SPSS10.0 statistic software for statistical analysis. The measurement data were expressed by mean ± standard deviation (x̅±s) and tested by independent-samples T test. The enumeration data were analyzed by χ2 test, size of test α=0.05.
Results
PaO2 of the rats in the experimental group decreased while PaCO2 increased
PaO2 of the rats in the experimental group was lower than that in the control group and the blank group, while PaO2 of the rats in the control group was lower than that in the blank group; PaCO2 of the rats in the experimental group was higher than that in the control group and the blank group, while PaCO2 of the rats in the control group was higher than that in the blank group. The aforementioned results had statistic significance. See Figures 1, 2.
Figure 1.
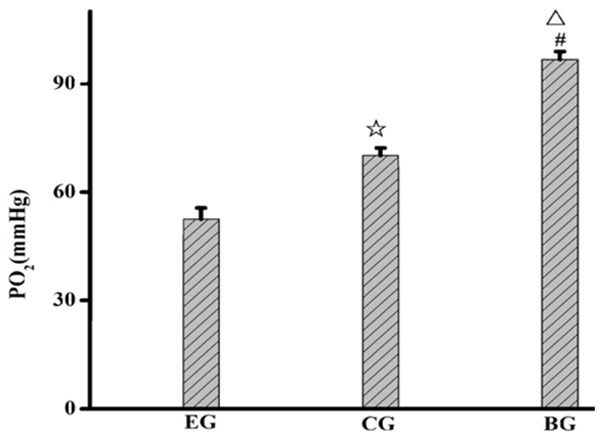
PaO2 in lung tissues of the rats in the three groups. EG, experiment group; CG, control group; BG, blank group; ☆, EG compared with CG, P=0.001; △, EG compared with BG, P=0.001; #, CG compared with BG, P=0.000.
Figure 2.
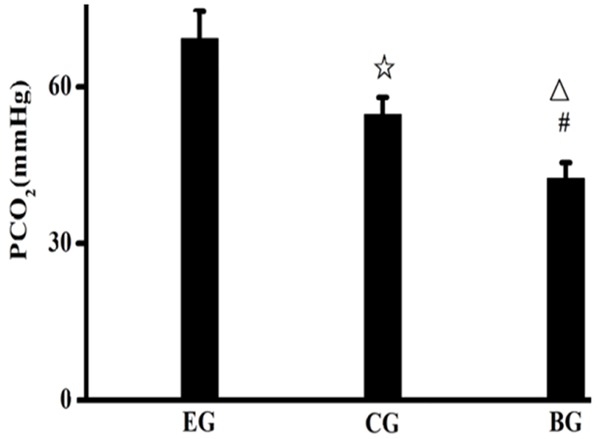
PaO2 of the rats of the three groups. EG, experiment group; CG, control group; BG, blank group; ☆, EG compared to CG, P=0.000; △, EG compared to BG, P=0.000; #, CG compared with BG, P<0.05.
ICAM-1 and IL-1β expression in lung tissues of the rats in the experimental group increased obviously
ICAM-1 content in lung homogenate of the rats in the experimental group was higher than that in the control group and the blank group, while ICAM-1 content in lung homogenate of the rats in the control group was higher than that in the blank group; IL-1β level in lung homogenate of the rats in the experimental group was higher than that in the control group and the blank group, while IL-1β level in lung homogenate of the rats in the control group was higher than that in the blank group. See Figures 3, 4.
Figure 3.

Expression of ICAM-1 in lung tissues of the rats in the three groups. EG, experiment group; CG, control group; BG, blank group; ☆, EG compared to CG, P<0.001; △, EG compared to BG P<0.001; #, CG compared with BG, P<0.05.
Figure 4.
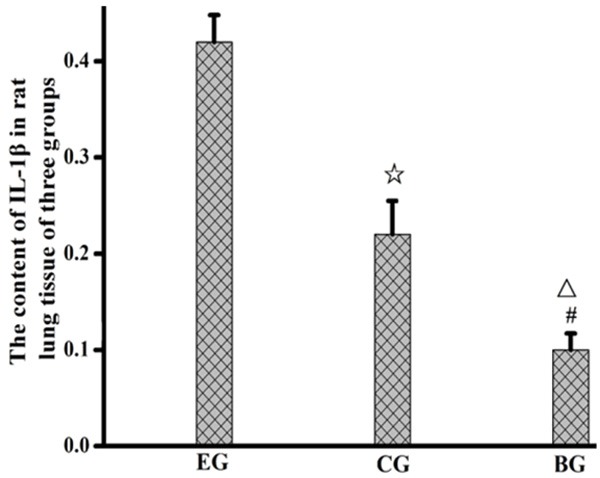
Expression of IL-1β in lung tissues of the rats in the three groups. EG, experiment group; CG, control group; BG, blank group; ☆, EG compared to CG, P<0.001; △, EG compared to BG, P<0.05; #, CG compared with BG, P<0.05.
ICAM-1mRNA and IL-1βmRNA level in lung tissues of the rats in the experimental group increased obviously
ICAM-1mRNA and IL-1βmRNA level of the three groups was detected by RT-PCR, with GAPDH as the internal reference. The results showed that ICAM-1 expression of the experimental group up-regulated in comparison with the control group and blank group, while ICAM-1 expression of the control group up-regulated in comparison with the blank group. IL-1β expression of the experimental group up-regulated in comparison with the control group and blank group, while IL-1β expression of the control group up-regulated in comparison with the blank group. The aforementioned results had statistic significance, P<0.05. See Figures 5, 6.
Figure 5.
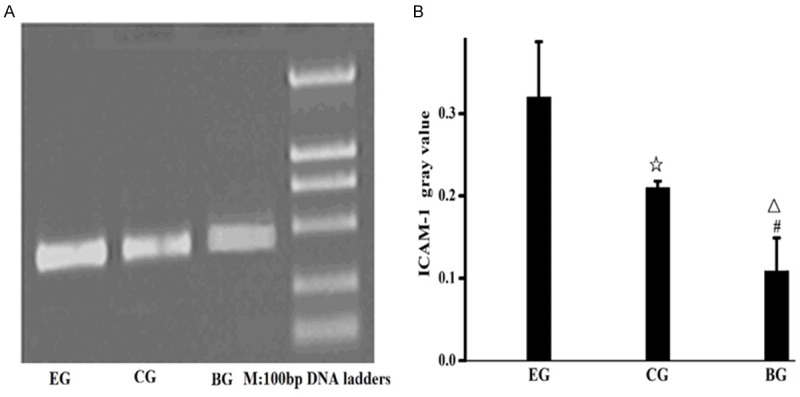
Expression level of ICAM-1mRNA in lung tissues of the rats in the three groups. A. Expression level of ICAM-1mRNA in lung tissues; B. Gray value of ICAM-1mRNA in lung tissues; EG, experiment group; CG, control group; BG, blank group; ☆, EG compared to CG, P=0.001; △, EG compared to BG, P=0.001; #, CG compared with BG, P<0.05.
Figure 6.
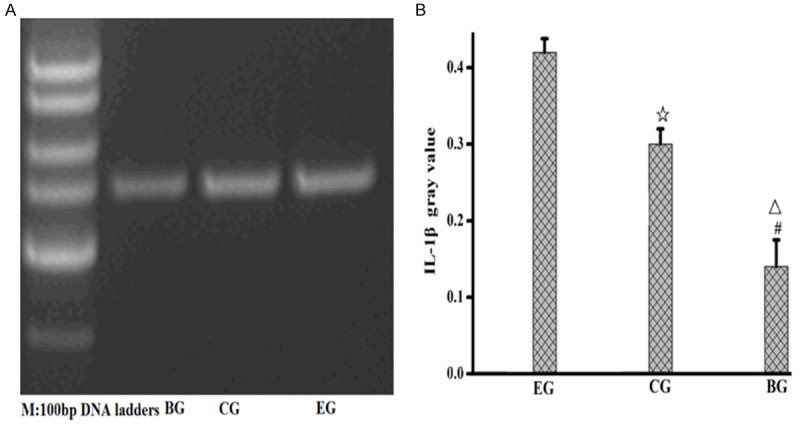
Expression level of IL-1βmRNA in lung tissues of the rats in the three groups. A. Expression level of IL-1βmRNA in lung tissues; B. Gray value of IL-1βmRNA in lung tissues; EG, experiment group; CG, control group; BG, blank group; ☆, EG compared to CG, P=0.001; △, EG compared to BG, P=0.001; #, CG compared with BG, P<0.05.
Discussion
It takes long time to induce COPD with pure smoking. In recent years, foreign studies are concentrated on smoking + LPS. LPS is an ingredient in the cell walls of Gram-negative bacilli and a kind of particulate matters. This study establishes the rat model of COPD by cigarette smoke exposure + intratracheal instillation of LPS (the experimental group). The lung injuries caused in this study are similar to the chronic lung injuries of COPD in clinic [29].
ICAM-1 is also known as CD54 and is mainly distributed in vascular endothelial cell and epithelial cell. It consists of 5 extracellular IgSF domains, a hydrophobic transmembrane domain, and a short cytoplasmic domain. The first and the third IgSF domains have the binding sites of integrins of αLβ2 and αMβ2. Thus, ICAM-1 may regulate the intercellular adhesion, activate the adhesive action between leukocytes and vascular endothelial cells, activate the adhesion, aggregation and release of leukocytes and promote the occurrence and development of inflammation. The expression level of ICAM-1 may reflect the degree of inflammatory injuries [30,31]. IL-1β is also called lymphocyte activating factor. It may increase vascular permeability, promote the trans-membrane migration of neutrophils and enhance the release of inflammatory mediators [32,33] as well as it plays the role of secondary action in the imbalance process of inflammatory cascade reaction.
This study is to investigate the effect of ICAM-1 and IL-1β in the rat models of COPD. Blood gas analysis indicated that the rat models of COPD (the experimental group) had respiratory disorder. Compared with the blank group, PaO2 and PaCO2 changes of the rat models of COPD (the experimental group) were obvious than those of the rats in the control group (Figures 1, 2), suggesting that smoking may lead to the inflammatory injury in the respiratory system. Harmful ingredients in cigarettes may directly impair the purification and defense function of the respiratory tract, which creates conditions for infection. Especially, when infected with Gram-negative (G-) bacteria, LPS in outer membrane of Gram-negative bacteria that is released by bacteria lysis binds with Toll-like receptor 4 (TLR-4) under the effect of the immunity system, which causes downstream inflammatory cascade, further aggravates pulmonary inflammation and leads to injuries to airway epithelium and alveolar epithelium [34,35]. Meanwhile, we find obvious inflammatory response in lung tissues of rat models of COPD. The expression of ICAM-1 and IL-1β (Figures 3, 4) as well as the level of ICAM-1mRNA and IL-1βmRNA of rats in the experimental group were obviously higher than those in the control group and the blank group (Figures 5 and 6), with significant difference. The control group (rats treated with pure smoking) had similar inflammatory response, but the severity is mild. It may be because the control group was not treated with LPS.
In conclusion, we think cigarette smoke contacts respiratory membrane through airway, which leads to injuries to alveolar epithelium, causes inflammatory response in airway, releases pro-inflammatory cytokines TNF-α and IL-1β and expands inflammatory cascade injuries. It is the important pathophysiological basis of respiratory function injury of COPD.
Acknowledgements
This study was supported by Henan Province technology department project (No. 132300410160); Henan Province education department project (No. 2011GGJS-127) and Henan Province education department project (No. 2010B180024).
Disclosure of conflict of interest
None.
References
- 1.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 2.Menezes AM, Perez-Padilla R, Jardim JR, Muino A, Lopez MV, Valdivia G, Montes de Oca M, Talamo C, Hallal PC, Victora CG PLATINO Team. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): A prevalence study. Lancet. 2005;366:1875–81. doi: 10.1016/S0140-6736(05)67632-5. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rabe KF, Hurd S, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Man SF. Why are patients with chronic obstructive pulmonary disease at increased risk of cardiovascular diseases The otential role of systemic inflammation in chronic obstructive pulmonary disease. Circulation. 2003;107:1514–1519. doi: 10.1161/01.cir.0000056767.69054.b3. [DOI] [PubMed] [Google Scholar]
- 7.Chinese Thoracic Society COPD Study Group. Diagnosis and Management of Chronic Obstructive Pulmonary Disease (Revised Edition of 2013) [J] Chinese Journal of Tuberculosis and Respiratory Diseases. 2013;36:255–265. [Google Scholar]
- 8.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 10.Porter JC, Hall A. Epithelial ICAM-1 and ICAM-2 regulate the egression ofhumanT cells across the bronchialepithelium. FASEB J. 2009;23:492–502. doi: 10.1096/fj.08-115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gahmberg CG, Tolvanen M, Kotovuori P. Leukocyte adhesion-structure and function of human leukocyte beta2-integrins and their cellular ligands. Eur J Biochem. 1997;245:215–232. doi: 10.1111/j.1432-1033.1997.00215.x. [DOI] [PubMed] [Google Scholar]
- 12.Vogel SM, Orrington-Myers J, Broman M, Malik AB. De novo ICAM-1 synthesis in the mouse lung: model of assessment of protein expression in lungs. Am J Physiol Lung Cell Mol Physiol. 2006;291:L496–501. doi: 10.1152/ajplung.00353.2005. [DOI] [PubMed] [Google Scholar]
- 13.Rahman A, Fazal F. Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration. Antioxid Redox Signal. 2009;11:823–839. doi: 10.1089/ars.2008.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frey RS, Rahman A, Kefer JC, Minshall RD, Malik AB. PKC zeta regulates TNF-alpha induced activation of NADPH oxidase in endothelial cells. Circ Res. 2002;90:1012–1019. doi: 10.1161/01.res.0000017631.28815.8e. [DOI] [PubMed] [Google Scholar]
- 15.Rahman A, Anwar KN, Malik AB. Protein kinase C-zeta mediates TNF-alpha-induced ICAM-1 gene transcription in endothelial cells. Am J Physiol Cell Physiol. 2000;279:C906–914. doi: 10.1152/ajpcell.2000.279.4.C906. [DOI] [PubMed] [Google Scholar]
- 16.Adams DH, Nash GB. Disturbance of leucocyte circulation and adhesion to the endothelium as factors in circulatory pathology. Br J Anaesth. 1996;77:17–31. doi: 10.1093/bja/77.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Roebuck KA, Finnegan A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J Leukoc Biol. 1999;66:876–888. doi: 10.1002/jlb.66.6.876. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, Liu ZS, Yao H, Kang SX, He CY, Hu JS, Wu GF, Wang FL. Effects of TNF-alpha on ICAM-1 and LFA-1 expression in peripheral blood mononuclear cells of children with febrile seizures. Zhongguo Dangdai Erke Zazhi. 2011;13:285–287. [PubMed] [Google Scholar]
- 19.Eniola AO, Krasik EF, Smith LA, Song G, Hammer DA. I-domain of lymphocyte function associated antigen-1 mediates rolling of polystyrene particles on ICAM-1 under flow. Biophys J. 2005;89:3577–3588. doi: 10.1529/biophysj.104.057729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forlow SB, Ley K. Selectin-independent leukocyte rolling and adhesion in mice deficient in E-, P- and L-selectin and ICAM-1. Am J Physiol Heart Circ Physiol. 2001;280:H634–H641. doi: 10.1152/ajpheart.2001.280.2.H634. [DOI] [PubMed] [Google Scholar]
- 21.Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li WF, Li W, Wan D, et al. In-vitro study on effect of cigarette smoke on secretion of IL-1, IL-6 and IL-8 in lung tissues. Journal of Environment and Health. 2011;28:287–289. [Google Scholar]
- 23.Gu Y, Kuida K, Tsutsui H, Kupper TS, Asahi K, Okamura H, Nakanishi K, Suzuki M, Kayagaki N, Black RA, Miller DK, Nakashima K, Shimizu M, Mizutani H. Aetivation of interferon-gamma inducing factor Mediated by interleukin-lbeta converting enzyme. Scienee. 1997;275:206. doi: 10.1126/science.275.5297.206. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Ji M, Chen L, Wu X, Wang L. Breviscapine reduces acute lung injury induced by left heart ischemic reperfusion in rats by inhibiting the expression of ICAM-1 and IL-18. Exp Ther Med. 2013;6:1322–1326. doi: 10.3892/etm.2013.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang YX, Song XR, Ji ML, Wang JG, Guo Y, Liu ZG. Expressions of ICAM-1 and TNF-oL in rats with acute lung injury induced by left heart ischemic reperfusion. Journal of Zhengzhou University (Medical Science) 2012;47:475–478. [Google Scholar]
- 26.Ji ML, Wang YX, Guo H, Yang Y, Gao W, Wang JG, Fu SZ. Effects of breviscapine on the expression of interleukin-1β in lung tissues with left heart ischemic reperfusion of rabbits. Journal of Xinxiang Medical College. 2011;28:428–430. [Google Scholar]
- 27.Zheng HA. Research progress in establishment method of animal model of COPD. Acta Laboratorium Animalis Scientia Sinica. 2003;11:249–252. [Google Scholar]
- 28.Zhang L, Li JT, Liu YQ, Li J, Zhang Y. Study on pathologic features of rat model of COPD. Sichuan Journal of Zoology. 2010;29:285–287. [Google Scholar]
- 29.Jan S, Arjan R, Philip D. Induction of emphysema and bronchial mucus cell hyperplasia by intracheal instillation of lipopolysacharide in the hamster. J Pathol. 1992;167:349–356. doi: 10.1002/path.1711670314. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Zhu Y, Xu H, Sun Y, Li Q. Activation of hypoxia-inducible factor-1alpha vianuclear factor-kappa Bin rats with chronic obstructive ulmonary diseases. Acta Biochim Biophys Sin (Shanghai) 2010;42:483–488. doi: 10.1093/abbs/gmq041. [DOI] [PubMed] [Google Scholar]
- 31.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Li WF, Wan D, Liu FR, et al. Experimental study on effect of cigarette smoke on expression of IL-1, IL-6 and IL-8 in lung and A549 cells of rats. Modern Preventive Medicine. 39:3336–3338. [Google Scholar]
- 33.Chen QK, Jiang GN, Ding JA, et al. Analysis on expression of proinflammatory cytokine IL-1βmRNA after pulmonary ischemia-reperfusion injury. Fudan University Journal of Medical Sciences. 2008;35:560–563. [Google Scholar]
- 34.Sabroe I, Whyte MK, Wilson AG, Dower SK, Hubbard R, Hall I. Toll-like receptor (TLR) 4 polymorphisms and COPD. Thorax. 2004;59:81–84. [PMC free article] [PubMed] [Google Scholar]
- 35.Comer DM, Kidney JC, Ennis M, Elborn JS. Airway epithelial cell apoptosis and inflammation in COPD, smokers and nonsmokers. Eur Respir J. 2013;41:1058–1067. doi: 10.1183/09031936.00063112. [DOI] [PubMed] [Google Scholar]


