Abstract
Four molecular subtypes have lately been established in endometrial cancer basing on estrogen receptor (ER), progesterone receptor (PR) and HER2 status: ER+/PR+/HER2+, ER+/PR+/HER2-, ER-/PR-/HER2+ and ER-/PR-/HER2-. The subtypes have shown diversity in terms of prognosis, clinicopathological and molecular characteristics, with ER+/PR+/HER2- and ER-/PR-/HER2+ group exhibiting exceptionally benign and aggressive behavior, respectively. We have further characterized the subtypes in the context of pathways known to drive endometrial carcinogenesis: phosphatidylinositol 3-kinase (PI3K)-AKT pathway (ERBB/PI3K pathway), TP53 system, and the mismatch repair (MMR) mechanism. Analysis of tumor heterogeneity was also included. ER+/PR+/HER2+ was characterized by active ERBB/PI3K pathway occurring in 58% of cases. Subtype ER-/PR-/HER2+ was characterized by the most frequent TP53 mutations (83% of cases). Triple negative phenotype utterly lacked active ERBB/PI3K pathway. Analyzed major pathways rarely correlated with clinicopathologial data but mutated TP53 and retained MMR did correlate with shorter overall survival (both P<0.01). The presence of tumor heterogeneity was most frequent in ER-/PR-/HER2+ subtype (53% of all cases). The presented results further emphasize that the molecular subtype distinction, along with MMR and TP53 status, could be a useful diagnostic tool in guiding individualized therapy.
Keywords: Molecular subtypes, ERBB/PI3K pathway, Mismatch Repair system, TP53, endometrial cancer
Introduction
Endometrial cancer (EC) is the most frequent malignancy of the female genital tract in the Western world, with approximately 90.000 new cases registered each year in the European Union [1]. We have recently established molecular subtype classification based on hormone receptor status (Estrogen Receptor-ER, Progesterone Receptor-PR, Human Epidermal growth factor Receptor 2-HER2) analyzed by immunohistochemistry (IHC). Four molecular subtypes have been distinguished: ER-positive and/or PR-positive, HER2-positive (ER+/PR+/HER2+); ER-positive and/or PR-positive, HER2-negative (ER+/PR+/HER2-); ER-negative and PR-negative, HER2-positive (ER-/PR-/HER2+); ER-negative and PR-negative, HER2-negative (ER-/PR-/HER2-). The subtypes have shown diversity in terms of prognosis, clinicopathological and molecular characteristics, with ER+/PR+/HER2- and ER-/PR-/HER2+ group exhibiting exceptionally benign and aggressive behaviour, respectively [2]. As the presented classification could find clinical application in terms of prognosis and treatment personalization, we have decided to further characterize the subtypes in the context of pathways known to drive endometrial carcinogenesis: phosphatidylinositol 3-kinase (PI3K)-AKT pathway (ERBB/PI3K pathway), TP53 system, and the mismatch repair (MMR) mechanism [3], hoping to present a clearer view on the different behavior of tumors belonging to the delineated subtypes. Protein tumor heterogeneity has been additionally analyzed in the context of the delineated subtypes as we have found out that also this parameter strongly correlates with patients’ clinicopathological characteristics and survival [4].
Patients and methods
Patients and tissues
The study included 400 formalin-fixed paraffin-embedded (FFPE) primary tumor samples retrospectively collected from a cohort of consecutive endometrial cancer patients who were operated in the Department of Gynaecology, Gynaecological Oncology and Gynaecological Endocrinology (Medical University of Gdansk) between 2000 and 2010. Samples included in the study were the total sum of eligible cases with available tissue material. Each patient was primarily treated by surgery, with the possible option of radiotherapy and/or chemotherapy administration. The inclusion criteria were operable endometrial cancer (IVB stage patients underwent cytoreductive surgery) confirmed by histological examination and a signed consent form. The study was accepted by the Independent Ethics Committee of the Medical University of Gdansk (NKEBN/269/2009). Procedures involving human subjects were in accordance with the Helsinki Declaration, as revised in 1983.
Immunohistochemistry (IHC) on tissue microarrays (TMA)
The following proteins were examined immunohistochemically: a) phosphatidylinositol 3-kinase (PI3K)-AKT pathway: ERBB1 (epidermal growth factor receptor), HER2 (v-erb-b2 erythroblastic leukaemia viral oncogene homolog 2, also known as HER2), ERBB3 (receptor tyrosine-protein kinase erbB-3), ERBB4 (v-erb-b2 avian erythroblastic leukemia viral oncogene homolog 4), PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase), phosphorylated AKT1-pAKT1 (v-akt murine thymoma viral oncogene homolog 1), stathmin 1 (STMN1), PTEN; b) TP53 system: TP53 (tumor protein p53); c) mismatch repair mechanism: MLH1 (mutL homolog 1), and MSH2 (mutS homolog 2); d) protein tumor heterogeneity: ER, PR, PIK3CA, pAKT1, MYC (v-myc avian myelocytomatosis viral oncogene homolog), TOP2A (DNA topoisomerase II alpha 170 kDa), CDKN2A (cyclin-dependent kinase inhibitor 2A, also known as p16), RAD21 (RAD21 homolog, S. pombe) and RUNX1 (runt-related transcription factor 1).
Samples were collected by surgical excision prior to any systemic treatment and processed as reported before [4]. Protein expression was examined by immunohistochemistry on TMA blocks using the following antibodies: ER-clone SP1 (Roche, Switzerland), PR-clone 1E2 (Roche), ERBB1-clone EGFR113 (Novocastra, Germany), HER2-clone 4B5 (Roche), ERBB3-clone DAK-H3-IC (DAKO, Denmark), ERBB4-clone HFR1 (Abcam, United Kingdom), PIK3CA-clone C73F8 (Cell Signalling, Massachusetts, USA), pAKT1-clone D9E (Cell Signalling), STMN1-clone EPR1574 (Abcam), PTEN-clone 6H2.1 (DAKO, Denmark), TP53 (antibody directed against the mutant TP53 phosphoprotein)-clone BP-53-11 (Roche), MLH1-clone M1 (Roche), MSH2-clone G219-1129 (Roche), MYC-clone 9E11 (Novocastra), TOP2A-clone Ki-S1 (DAKO), CDKN2A-clone JC8 (Santa Cruz Biotechnology, Texas), RAD21-polyclonal antibody (Abcam) and RUNX1-clone DW71 (Santa Cruz Biotechnology). The staining has been performed in accordance with manufacturers’ guidelines, details are presented as supplementary material (Table S1; Figure S1).
Protein expression evaluation was performed by two pathologists (HM and JG) blinded to clinical data. ER and PR evaluation of the nuclear staining was performed based on Allred score [5]. HER2 receptor status was determined based on the criteria of HercepTestTM (DAKO) according to the manufacturer’s guidelines, as previously described [2,4]. The interpretation criteria for the remaining proteins were based on the intensity of the staining and/or the percentage of cells showing positive reaction (0-100%), as reported in the literature [6-10]. For proteins where both parameters could have been assessed the final staining score (histological score, H-score) was obtained as the result of multiplication [11]. Cut off point determination of expression positivity of all the proteins studied was performed with the use of Cutoff Finder Web Application [12], basing on the results’ distribution. The same cut off determination criteria were assumed for ERBB/PI3K pathway activation status (1 point for each protein classified as positive) where all the 8 proteins were scored together with a new cut off point determined (the only exception was made with regard to PTEN: as this protein is the suppressor, not the activator of ERBB/PI3K pathway, 1 point was scored when PTEN status was negative). MMR status was considered functional when the expression of at least one protein (MLH1 or MSH2) was assumed positive.
In addition to the studied pathways we have also included the analysis of tumor heterogeneity in the distinguished molecular subtypes. Protein tumor heterogeneity has been assessed as previously described [4]. In brief, cumulative heterogeneity was determined for each patient, based on 9 proteins which correlated with clinicopathological characteristics and/or survival (ER, PR, PIK3CA, pAKT1, MYC, TOP2A, CDKN2A, RAD21, RUNX1). For each patient a score between 0 and 9 was obtained (1 point for each protein classified as heterogeneous). Based on the results distribution, primary tumors with the score of at least 3 were classified as “globally” heterogenous. Cumulative tumor heterogeneity strongly correlated with the presence of metastases, higher stage, and higher grade of the disease (all p values below 0.05). It also carried negative prognostic value (P=0.0008).
Details of immunohistochemical evaluation of all the aforementioned proteins are presented as supplementary material (Table S2).
Statistical analysis
STATISTICA software (Statsoft Co, USA, version 10, Oklahoma) was used for all calculations. The tests that were performed and their applications were as follows: testing normality of the data set-Shapiro-Wilk test; comparison of pathway activation with molecular subtypes-crosstabs statistics with Pearson’s Chi-square test; correlations between pathway activation and clinicopathological data-crosstabs statistics with Pearson’s Chi-square test; comparison of global tumor heterogeneity with molecular subtypes-crosstabs statistics with Pearson’s Chi-square test. The Kaplan-Meier estimator was employed for survival analysis, and the generated curves were compared with the F Cox test. The endpoint for the study was overall survival (OS). OS was defined as the time from sample collection to death of any cause or censoring. Censoring was defined as loss of follow-up or alive at the end of follow-up. Statistical significance was assumed when P≤0.05.
Results
Studied cohort and the flow of samples
The tumor samples included all stages of endometrial carcinoma, from stage IA to IVB, as distinguished by FIGO in 2009 (International Federation of Gynecology and Obstetrics) [13]. We analysed all primary carcinomas of the uterine corpus, separating them into type I (endometrioid) and type II tumors (non-endometrioid). The latter included serous, clear cell, mucinous, undifferentiated and mixed adenocarcinomas [14]. As the position of grade 3 endometrioid EC is not clear, and some authors regard them as intermediate group between low-grade endometrioid and serous EC in molecular terms [15], we analyzed it separately. Metastases included lymph node and distant metastases. The patients’ characteristics are summarised in Table 1. The median age was 64 (range 26-89 years). Patients with a body mass index higher than 30 were classified as obese [16]. A survival analysis was performed for 397 (99.3%) patients, 3 patients were lost to the follow-up. After a median follow-up of 72 months (range: 0-158), 113 (28.5%) patients had died. The last follow-up data were collected in September 2013. The study was performed in accordance with the REMARK criteria [17].
Table 1.
Clinicopathological data (N=400)
| Variable | Number of cases (%) |
|---|---|
| Menopausal status | |
| Premenopausal | 28 (7.0%) |
| Perimenopausal | 26 (6.5%) |
| Postmenopausal | 345 (86.3%) |
| Missing data | 1 (0.3%) |
| Age | |
| ≤50 years | 42 (10.5%) |
| >50 years | 358 (89.5%) |
| Obesity | |
| Absent | 197 (49.3%) |
| Present | 202 (50.5%) |
| Missing data | 1 (0.3%) |
| Diabetes | |
| Absent | 300 (75.0%) |
| Present | 100 (25.0%) |
| Hypertension | |
| Absent | 140 (35.0%) |
| Present | 260 (65.0%) |
| Histology | |
| Type I | 307 (76.8%) |
| Type II | 40 (10.0%) |
| Grade 3 | 40 (10.0%) |
| Missing data | 13 (3.3%) |
| Stage (FIGO*) | |
| IA-IB | 277 (69.3%) |
| II | 55 (13.8%) |
| IIIA-IIIC | 47 (11.8%) |
| IVA-IVB | 16 (4.0%) |
| Missing data | 5 (1.3%) |
| Cervical invasion | |
| Absent | 300 (75.0%) |
| Present | 95 (23.8%) |
| Missing data | 5 (1.3%) |
| Myometrial infiltration | |
| ≤1/2 | 198 (49.5%) |
| >1/2 | 197 (49.3%) |
| Missing data | 5 (1.3%) |
| Metastases | |
| Absent | 334 (83.5%) |
| Present | 63 (15.8%) |
| Missing data | 3 (0.8%) |
Constructed TMA blocks collectively included 400 patients. Information concerning the expression level of all three receptors simultaneously (ER, PR, HER2) was available for 400 (100.0%) cases. Within the group of 400 samples, 396 (99.0%), 399 (99.8%) and 400 (100.0%) had ERBB/PI3K pathway status, Mismatch Repair system status and TP53 system status assessed, respectively, as presented in Table 2.
Table 2.
Flow of the samples within the cohort of 400 patients where molecular subtype was determined (N=400)
| Protein expression level assessed | Number of samples | Number of positive samples (%) | System activation assessed | Number of samples | Number of positive samples (%) |
|---|---|---|---|---|---|
| PTEN | 399 | 279 (69.9%) | ERBB/PI3K pathway | 396 | 106 (26.8%) |
| ERBB1 | 399 | 166 (41.6%) | |||
| HER2 | 400 | 147 (36.8%) | |||
| ERBB3 | 400 | 141 (35.3%) | |||
| ERBB4 | 400 | 395 (98.8%) | |||
| PI3K | 400 | 7 (1.8%) | |||
| pAKT1 | 397 | 325 (81.9%) | |||
| STMN1 | 400 | 156 (39.0%) | |||
| TP53 | 400 | 160 (40.0%) | TP53 system | 400 | 160 (40.0%) |
| MLH1 | 399 | 13 (3.3%) | Mismatch Repair system | 399 | 93 (23.3%) |
| MSH2 | 400 | 90 (22.5%) |
Pathway activation in the context of molecular subtypes
ER+/PR+/HER2+ was characterized by overexpression of ERBB/PI3K-dependent molecules that occurred in 58% of cases. The least frequent rate of TP53 mutations was identified in ER+/PR+/HER2- subtype (22%), while ER-/PR-/HER2+ subtype showed the highest rate of this alteration (83% of cases); in addition, it often had active MMR system (33% of cases). Triple negative phenotype lacked active ERBB/PI3K pathway utterly (Table 3).
Table 3.
Pathway activation in the context of molecular subtypes
| Molecular subtype | Number of positive samples (%) | ||
|---|---|---|---|
|
| |||
| Activation of ERBB/PI3K pathway | Mismatch Repair system functioning | TP53 mutated | |
| PR+/ER+/HER2+ | 73/127 (57.5%) | 43/128 (33.6%) | 78/129 (60.5%) |
| PR+/ER+/HER2- | 29/222 (13.1%) | 37/224 (16.5%) | 50/224 (22.3%) |
| PR-/ER-/HER2+ | 4/18 (22.2%) | 6/18 (33.3%) | 15/18 (83.3%) |
| PR-/ER-/HER2- | 0/29 (0%) | 7/29 (24.1%) | 17/29 (58.6%) |
| P value (chi square) | <0.00001 | 0.002 | <0.000001 |
Pathway activation in the context of clinicopathological data
The major pathways analysed rarely correlated with clinicopathologial data. Associations were observed for TP53 and MMR. Mutated TP53 correlated with more aggressive tumor characteristics: histology type II (P<0.00001), stage III and IV (P=0.01), grade 3 (P<0.000001). MMR system was mostly active in non-obese patients (P=0.009). It correlated with the presence of metastases (P=0.02), type II EC (P=0.0006) and grade 3 (P=0.002) (Table 4).
Table 4.
Pathway activation in the context of clinicopathological data
| Variable | Activation of ERBB/PI3K pathway | Mismatch Repair system functioning | TP53 mutated | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Number of positive samples (%) | p value | Number of positive samples (%) | p value | Number of positive samples (%) | p value | |
| Menopausal status | ||||||
| Premenopausal | 8/27 (29.6%) | 0.73 | 6/28 (21.4%) | 0.80 | 10/28 (35.7%) | 0.64 |
| Peri- and postmenopausal | 98/368 (26.6%) | 87/370 (23.5%) | 149/371 (40.2%) | |||
| Age | ||||||
| ≤50 years | 11/41 (26.8%) | 0.99 | 7/42 (16.7%) | 0.28 | 12/42 (28.6%) | 0.11 |
| >50 years | 95/355 (26.8%) | 86/357 (24.1%) | 148/358 (41.3%) | |||
| Obesity | ||||||
| Absent | 57/195 (29.2%) | 0.29 | 57/197 (28.9%) | 0.009 | 83/197 (42.1%) | 0.41 |
| Present | 49/200 (24.5%) | 36/ 201 (17.9%) | 77/202 (38.1%) | |||
| Diabetes | ||||||
| Absent | 81/297 (27.3%) | 0.69 | 65/299 (21.7%) | 0.20 | 116/300 (38.7%) | 0.35 |
| Present | 25/99 (25.3%) | 28/100 (28.0%) | 44/100 (44.0%) | |||
| Hypertension | ||||||
| Absent | 41/138 (28.7%) | 0.33 | 33/140 (23.6%) | 0.93 | 64/140 (45.7%) | 0.09 |
| Present | 65/258 (25.2%) | 60/259 (23.2%) | 96/260 (36.9%) | |||
| Histology | ||||||
| Type I | 81/301 (26.9%) | 0.60 | 62/303 (20.5%) | 0.002 | 100/304 (32.9%) | <0.000001 |
| Type II | 10/40 (25.0%) | 18/40 (45.0%) | 29/40 (72.5%) | |||
| Grade 3 | 13/38 (34.2%) | 8/38 (21.1%) | 23/38 (60.5%) | |||
| Stage (FIGO*) | ||||||
| IA, IB, II | 86/328 (26.2%) | 0.70 | 73/331 (22.1%) | 0.10 | 123/332 (37.1%) | 0.01 |
| IIIA, IIB, IIIC, IVA, IVB | 18/63 (28.6%) | 20/63 (31.8%) | 34/63 (54.0%) | |||
| Cervical invasion | ||||||
| Absent | 83/297 (28.0%) | 0.28 | 68/299 (22.7%) | 0.72 | 125/300 (41.7%) | 0.17 |
| Present | 21/94 (22.3%) | 25/95 (26.3%) | 32/95 (33.7%) | |||
| Myometrial infiltration | ||||||
| ≤1/2 | 49/194 (25.3%) | 0.55 | 45/197 (22.8%) | 0.47 | 71/198 (35.9%) | 0.11 |
| >1/2 | 55/197 (27.9%) | 48/197 (24.4%) | 86/197 (43.7%) | |||
| Metastases | ||||||
| Absent | 87/326 (26.7%) | 0.94 | 78/354 (22.0%) | 0.02 | 127/330 (38.5%) | 0.12 |
| Present | 16/61 (26.3%) | 14/36 (38.9%) | 30/61 (49.2%) | |||
FIGO-International Federation of Gynecology and Obstetrics.
Survival analysis
Mutated TP53 and retained MMR system correlated with shorter overall survival. ERBB/PI3K pathway had no prognostic significance (Figure 1).
Figure 1.
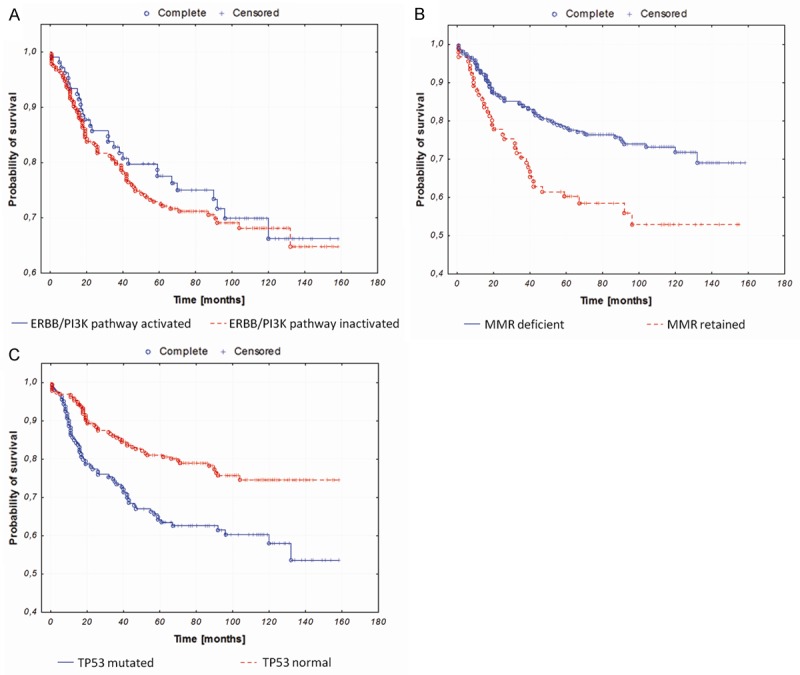
Kaplan-Meier curves illustrating overall survival of endometrial cancer patients stratified against: A. ERBB/PI3K pathway activation (P=0.31); B. MMR status (P=0.002); C. TP53 status (P=0.0002).
Molecular subtypes in the context of tumor heterogeneity
The presence of tumor heterogeneity correlated strongly with ER-/PR-/HER2+ subtype. The same phenomenon was rare in ER+/PR+/HER2- subtype, as presented in Table 5.
Table 5.
Global tumor heterogeneity in the context of molecular subtypes
| Molecular subtype | Number of samples classified as globally heterogenous (%) |
|---|---|
| ER+/PR+/HER2+ | 12/117 (10.3%) |
| ER+/PR+/HER2- | 11/193 (5.7%) |
| ER-/PR-/HER2+ | 9/17 (52.9%) |
| ER-/PR-/HER2- | 7/26 (27.0%) |
| P value (chi square) | <0.000001 |
Discussion
The distinction of molecular subtypes based on ER, PR and HER2 status has introduced valuable information about underlying tumor biology of endometrial cancer. Performed analysis revealed that the elucidated subgroups differed fundamentally in terms of prognosis, clinicopathological, and molecular characteristics. The greatest distinction was observed between ER-/PR-/HER2+ subtype exhibiting exceptionally aggressive tumor characteristics and ER+/PR+/HER2- subtype being the most benign. ER/PR-/HER2+ subtype was characterized by exceptionally short overall survival, often showing histological features of type II endometrial carcinomas, advanced stage or grade, with frequent signs of cervical invasion, myometrial infiltration, or metastases. The other subtypes have fallen in the middle of this distinction, showing intermediate clinicopathological, prognostic and molecular characteristics, with ER-/PR-/HER2- subtype classified as the second least favourable and ER+/PR+/HER2+ subtype-the second most favourable [2]. Encouraged by the described findings, we have decided to study the elucidated subtypes in the context of pathways known to drive endometrial carcinogenesis: ERBB/PI3K pathway, TP53 system and MMR. This study revealed underlying mechanisms of the tumor formation within different subtypes.
ER+/PR+/HER2+ exhibited the highest rate of ERBB/PI3K pathway activation, which was in compliance with the results obtained in breast cancer where ERBB/PI3K pathway activation signature was found common for luminal B subtype [18]. This subtype was also often characterized by the highest frequency of functional MMR system. Nevertheless, it is difficult to verify this data in the available literature as the data on this subject in EC are nonexistent and the frequency as well as the significance of DNA mismatch repair deficiency in breast cancer (the only surrogate with molecular subtypes distinguished) is unclear [19]. ER+/PR+/HER2- subtype exhibited TP53 mutations only in 22% which was the lowest value for all the studied subtypes. This might partially explain its benignity. Similar frequency of TP53 mutations was observed for luminal A breast cancer. Subtype ER-/PR-/HER2+ was characterized by the most frequent TP53 mutations, which is also common in HER2 amplified breast tumors [20]. Triple negative phenotype utterly lacked active ERBB/PI3K pathway.
Even though ERBB/PI3K activation in EC showed multiple correlations with aggressive tumor behaviour at the gene copy number variation level, as we have reported previously [21], no such association could have been observed in hereby study. Interesting results, however, were obtained in case of MMR system. The DNA mismatch repair is critical for the maintenance of genomic stability and its deficiency is common in many cancers. It might seem that high expression of MMR proteins will be present mostly in low-grade, early stage tumors but in fact retained MMR system correlated with type II histology and grade 3 (P=0.002) and the presence of metastases (P=0.02). Additionally, a trend was observed towards association of MMR system and higher stages of the disease (P=0.10). Similar results, also in endometrial cancer, were reported lately [22], contrary to many reports where deficiency of MMR system showed no associations with clinicopathological features or prognosis [23-25]. Furthermore, retained MMR system was less common in obese patients (P=0.009). Joehlin-Price et al. reported that obesity itself might be less crucial driver of the EC initiation. In their study, obese women had a lower overall MMR deficiency, however statistically significant BMI differences were not observed in all the studied subgroups and were highly dependent on the particular MMR protein analyzed [25]. In our study the accumulation of mutated TP53 correlated with type II EC (P<0.00001) and higher stage of the disease (P=0.01). Other authors also reported similar findings, with TP53 accumulation associated with adverse tumor characteristics [26,27].
In our study ERBB/PI3K activation showed no prognostic significance. We were also unable to find any prognostic significance of that pathway at the DNA level [21]. Nout et al. managed to present a trend regarding the prognostic significance of high PI3K-AKT pathway activation in EC [28]. Much more informative with regard to OS was MMR status. We have shown that MMR-deficient cases had significantly longer OS, similarly to the data presented by Kato et al. [22]. High expression of mutated TP53 strongly correlated with unfavourable prognosis. The data are in consistence with the results found in the literature [29,30].
Intratumoral heterogeneity analyzed in the studied specimen was the most frequently observed event in PR-/ER-/HER2+ subtype (P<0.000001). As PR-/ER-/HER2+ subtype carries by far the worst prognosis [2] and tumor heterogeneity has also shown to be of strong adverse prognostic significance [4], it is possible that overexpression of HER2 drives uncontrollable divisions characterized by an extremely high mutation rate, generating genetically diverse subclones within the tumor. To the best of our knowledge, we are the first to present the data concerning intratumoral heterogeneity in the context of molecular subtypes based on ER, PR and HER2 receptor status.
Methodology utilized in hereby study, exploited on a large sample size, showed satisfying reaction performance, specificity, high specimen quality, and thus low sample loss. The protocols used allowed for highly coherent protein expression measurements. Crucial limitation of the study was the lack of treatment response data as both the subtypes and the studied molecular systems carry implications for therapy selection.
EC molecular subtypes based on ER, PR and HER2 status have proven to differ not only in terms of prognosis and clinicopathological data but also in molecular characteristics. The proposed classification, along with molecular characterization, might serve as a clinically valid molecular marker. Continued investigation of the presented data, especially in the aspect of targeted therapies, is necessary, as it has been proven that MMR deficiency can be associated with resistance to standard therapeutic agents (eg. platinum compounds, methylating agents, fluoropyrimidine agents) [31]. Furthermore, loss of MMR could provide predisposition to adjuvant radiotherapy sensitivity [22]. There are also reports suggesting it might be worthwhile to include effective PI3K/Akt inhibitors in the current treatment regime of endometrial cancer [32]. Moreover, testing of TP53 expression level was suggested to help in the selection of patients eligible for pelvic lymphadenectomy in clinical stage I endometrial carcinoma [33] and tumors overexpressing TP53 have been shown more likely to benefit from anti-IGF-IR therapies [34].
Even with some molecular differences not being striking among the elucidated subtypes and with the lack of correlations between the studied systems and/or clinicopathological data, the presented results further emphasize that the molecular subtype distinction, along with MMR and TP53 status, could become a useful diagnostic tool in guiding individualized therapy, as suggested before [2,35,36]. The presented results should be subject to further investigation which could be therewith verified in a clinical setting. Additionally, studies of MMR system in a larger cohort of patients are necessary to explain observed discrepancies in reports concerning endometrial cancer.
Acknowledgements
The research has been financed by the Ministry of Science and Higher Education grant N407571538.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Colombo N, Preti E, Landoni F, Carinelli S, Colombo A, Marini C, Sessa C ESMO Guidelines Working Group. Endometrial cancer: Esmo clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi33–8. doi: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 2.Łapińska-Szumczyk S, Supernat A, Majewska H, Gulczyński J, Luczak A, Biernat W, Wydra D, Żaczek AJ. Her2-positive endometrial cancer subtype carries poor prognosis. Clin Transl Sci. 2014;7:482–488. doi: 10.1111/cts.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samarnthai N, Hall K, Yeh IT. Molecular profiling of endometrial malignancies. Obstet Gynecol Int. 2010;2010:162363. doi: 10.1155/2010/162363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Supernat A, Lapińska-Szumczyk S, Majewska H, Gulczyński J, Biernat W, Wydra D, Zaczek AJ. Tumor heterogeneity at protein level as an independent prognostic factor in endometrial cancer. Transl Oncol. 2014;7:613–619. doi: 10.1016/j.tranon.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 6.Ljuslinder I, Malmer B, Isaksson-Mettävainio M, Oberg A, Henriksson R, Stenling R, Palmqvist R. Erbb 1-4 expression alterations in primary colorectal cancers and their corresponding metastases. Anticancer Res. 2009;29:1489–1494. [PubMed] [Google Scholar]
- 7.de Jong RA, Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, Hollema H. Molecular markers and clinical behavior of uterine carcinosarcomas: Focus on the epithelial tumor component. Mod Pathol. 2011;24:1368–1379. doi: 10.1038/modpathol.2011.88. [DOI] [PubMed] [Google Scholar]
- 8.Altavilla G, Fassan M, Busatto G, Orsolan M, Giacomelli L. Microsatellite instability and hmlh1 and hmsh2 expression in renal tumors. Oncol Rep. 2010;24:927–932. doi: 10.3892/or.2010.927. [DOI] [PubMed] [Google Scholar]
- 9.Wik E, Birkeland E, Trovik J, Werner HM, Hoivik EA, Mjos S, Krakstad C, Kusonmano K, Mauland K, Stefansson IM, Holst F, Petersen K, Oyan AM, Simon R, Kalland KH, Ricketts W, Akslen LA, Salvesen HB. High phosphostathmin(serine38) expression identifies aggressive endometrial cancer and suggests an association with pi3k inhibition. Clin Cancer Res. 2013;19:2331–2341. doi: 10.1158/1078-0432.CCR-12-3413. [DOI] [PubMed] [Google Scholar]
- 10.Cui W, Cai Y, Wang W, Liu Z, Wei P, Bi R, Chen W, Sun M, Zhou X. Frequent copy number variations of pi3k/akt pathway and aberrant protein expressions of pi3k subunits are associated with inferior survival in diffuse large b cell lymphoma. J Transl Med. 2014;12:10. doi: 10.1186/1479-5876-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.da Costa AA, D›Almeida Costa F, Ribeiro AR, Guimarães AP, Chinen LT, Lopes CA, de Lima VC. Low pten expression is associated with worse overall survival in head and neck squamous cell carcinoma patients treated with chemotherapy and cetuximab. Int J Clin Oncol. 2015;20:282–289. doi: 10.1007/s10147-014-0707-1. [DOI] [PubMed] [Google Scholar]
- 12.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff finder: A comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One. 2012;7:e51862. doi: 10.1371/journal.pone.0051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pecorelli S. Revised figo staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 14.Tavassoli FA, Devilee P. Tumor of the breast and female genital organs. Lyon: 2003. Who classification of tumors. Pathology and genetics. [Google Scholar]
- 15.Lee CH, Mariño-Enriquez A, Ou W, Zhu M, Ali RH, Chiang S, Amant F, Gilks CB, van de Rijn M, Oliva E, Debiec-Rychter M, Dal Cin P, Fletcher JA, Nucci MR. The clinicopathologic features of ywhae-fam22 endometrial stromal sarcomas: A histologically high-grade and clinically aggressive tumor. Am J Surg Pathol. 2012;36:641–653. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 16.WHO Expert Consultation. Appropriate bodymass index for asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 17.Altman DG, McShane LM, Sauerbrei W, Taube SE. Reporting recommendations for tumor marker prognostic studies (remark): Explanation and elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fu X, Osborne CK, Schiff R. Biology and therapeutic potential of pi3k signaling in er+/her2-negative breast cancer. Breast. 2013;22(Suppl 2):S12–18. doi: 10.1016/j.breast.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wen YH, Brogi E, Zeng Z, Akram M, Catalano J, Paty PB, Norton L, Shia J. Dna mismatch repair deficiency in breast carcinoma: a pilot study of triple-negative and non-triple-negative tumors. Am J Surg Pathol. 2012;36:1700–1708. doi: 10.1097/PAS.0b013e3182627787. [DOI] [PubMed] [Google Scholar]
- 20.Bertheau P, Lehmann-Che J, Varna M, Dumay A, Poirot B, Porcher R, Turpin E, Plassa LF, de Roquancourt A, Bourstyn E, de Cremoux P, Janin A, Giacchetti S, Espié M, de Thé H. P53 in breast cancer subtypes and new insights into response to chemotherapy. Breast. 2013;22(Suppl 2):S27–29. doi: 10.1016/j.breast.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Supernat A, Łapińska-Szumczyk S, Majewska H, Gulczyński J, Biernat W, Wydra D, Żaczek AJ. A multimarker qpcr platform for the characterisation of endometrial cancer. Oncol Rep. 2014;31:1003–1013. doi: 10.3892/or.2013.2924. [DOI] [PubMed] [Google Scholar]
- 22.Kato M, Takano M, Miyamoto M, Sasaki N, Goto T, Tsuda H, Furuya K. Dna mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 2015;26:40–45. doi: 10.3802/jgo.2015.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo YL, Cheah PL, Shahruddin SI, Omar SZ, Arends M. The immunohistochemistry signature of mismatch repair (mmr) proteins in a multiethnic asian cohort with endometrial carcinoma. Int J Gynecol Pathol. 2014;33:554–559. doi: 10.1097/PGP.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz I, Martín-Arruti M, Lopez-Lopez E, Garcia-Orad A. Lack of association between deficient mismatch repair expression and outcome in endometrial carcinomas of the endometrioid type. Gynecol Oncol. 2014;134:20–23. doi: 10.1016/j.ygyno.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 25.Schröer A, Köster F, Fischer D, Dubitscher RM, Woll-Hermann A, Diedrich K, Friedrich M, Salehin D. Immunohistochemistry of dna mismatch repair enzyme msh2 is not correlated with prognostic data from endometrial carcinomas. Anticancer Res. 2009;29:4833–4837. [PubMed] [Google Scholar]
- 26.Seeger A, Kölbl H, Petry IB, Gebhard S, Battista MJ, Böhm D, Steiner E. P53 is correlated with low bmi negative progesterone receptor status and recurring disease in patients with endometrial cancer. Gynecol Oncol. 2012;125:200–207. doi: 10.1016/j.ygyno.2011.12.443. [DOI] [PubMed] [Google Scholar]
- 27.Markova I, Duskova M, Lubusky M, Kudela M, Zapletalová J, Procházka M, Pilka R. Selected immunohistochemical prognostic factors in endometrial cancer. Int J Gynecol Cancer. 2010;20:576–582. doi: 10.1111/IGC.0b013e3181d80ac4. [DOI] [PubMed] [Google Scholar]
- 28.Nout RA, Bosse T, Creutzberg CL, Jürgenliemk-Schulz IM, Jobsen JJ, Lutgens LC, van der Steen-Banasik EM, van Eijk R, Ter Haar NT, Smit VT. Improved risk assessment of endometrial cancer by combined analysis of msi, pi3kakt, wnt/β-catenin and p53 pathway activation. Gynecol Oncol. 2012;126:466–473. doi: 10.1016/j.ygyno.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Kudela M, Pilka R, Lubuský M, Hejtmánek P, Dzubák P, Brychtová S. [Prognostic importance of selected molecular genetic immunohistochemical markers and dna ploidy in endometrial cancer] . Ceska Gynekol. 2011;76:194–199. [PubMed] [Google Scholar]
- 30.Lee EJ, Kim TJ, Kim DS, Choi CH, Lee JW, Lee JH, Bae DS, Kim BG. P53 alteration independently predicts poor outcomes in patients with endometrial cancer: A clinicopathologic study of 131 cases and literature review. Gynecol Oncol. 2010;116:533–538. doi: 10.1016/j.ygyno.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 31.Guillotin D, Martin SA. Exploiting dna mismatch repair deficiency as a therapeutic strategy. Exp Cell Res. 2014;329:110–115. doi: 10.1016/j.yexcr.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Zhao KN, Li R, Shao R, Chen C. Activation of pi3k/akt/mtor pathway and dual inhibitors of pi3k and mtor in endometrial cancer. Curr Med Chem. 2014;21:3070–3080. doi: 10.2174/0929867321666140414095605. [DOI] [PubMed] [Google Scholar]
- 33.Fayallah EA, Hemida RA, Gamal AM, Abd Elhady E, Anwar KI, Nada NA, Sherif LS, Sayed-Ahmed MT. Pretreatment study of p53 overexpression for selection of candidates for pelvic lymphadenectomy in clinical stage i endometrial carcinoma: A randomized-controlled study. Arch Gynecol Obstet. 2011;283:617–622. doi: 10.1007/s00404-010-1468-3. [DOI] [PubMed] [Google Scholar]
- 34.Attias-Geva Z, Bentov I, Kidron D, Amichay K, Sarfstein R, Fishman A, Bruchim I, Werner H. P53 regulates insulin-like growth factor-i receptor gene expression in uterine serous carcinoma and predicts responsiveness to an insulin-like growth factor-i receptor-directed targeted therapy. Eur J Cancer. 2012;48:1570–1580. doi: 10.1016/j.ejca.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 35.Sho T, Hachisuga T, Nguyen TT, Urabe R, Kurita T, Kagami S, Kawagoe T, Matsuura Y, Shimajiri S. Expression of estrogen receptor-α as a prognostic factor in patients with uterine serous carcinoma. Int J Gynecol Cancer. 2014;24:102–106. doi: 10.1097/IGC.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 36.Buza N, Roque DM, Santin AD. Her2/neu in endometrial cancer: A promising therapeutic target with diagnostic challenges. Arch Pathol Lab Med. 2014;138:343–350. doi: 10.5858/arpa.2012-0416-RA. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


