Abstract
The aim of this study was to determine whether the cognitive impairment is associated with corpus callosum infarctions. Ten corpus callosum infarction patients were enrolled in this study. Their emotions, cognitive and language abilities, memory, comprehensive perception were assessed using the Chinese version of following measures: Mini Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), World Health Organization-University of California-Los Angeles Auditory Verbal Learning Test (WHO-UCLA AVLT), Wechsler Adult Intelligence Scale (WAIS) Digit Span subtest and so on. The same measurements were performed on healthy control participants as contrast for analysis. Infarction most frequently occurred in the body and/or splenium of the corpus callosum. The scores of the most cognitive tests in the corpus callosum infarction patients were significantly worse than those of the control participants (P<0.05). Except for the naming ability, the patients showed significantly poorer performance at the overall level of MMSE than the controls did (P<0.05). Consistently, the results of MoCA suggested a significant reduction in visuospatial abilities of execution, orientation, attention, calculation, delayed memory, language, and repetition capabilities in the patients with respect to the control (P<0.05). In addition, the scores in the case group were significantly worse than those in the control group in the auditory word learning test, digital span and Rey complex figure test (P<0.05). Corpus callosum infarction can cause cognitive dysfunction, which poses obstacles to memory in the acute phase, accompanied by different degrees of decline in visuospatial abilities, attention and calculating abilities.
Keywords: Corpus callosum, infarction, cognitive impairment, MMSE, MoCA
Introduction
The corpus callosum (CC) is the biggest commissural fibrous bundle of the central nervous system. The precise functions of the corpus callosum between the two hemispheres remain unclear. It is generally considered that the role of CC is transference, integration and coordination of information between homologous areas of the two cerebral hemispheres. Researchers have shown that it is involved in many advanced features of the brain, such as learning, memory, thinking, three-dimensional visual ability, executive functions, as well as visual reaction time [1,2]. Lesions of any part of the corpus callosum might lead to loss of contact between bilateral hemispheres that cause mental disorders, pseudobulbar palsy, speech and movement ataxia. It also might be associated with the disconnection syndrome of the corpus callosum like left hand apraxia, left side alien hand syndrome, etc. [3-5].
However, due to lack of specific in clinical manifestations, there are few reports on cognitive disorder caused by the corpus callosum infarction in the literature [6-9]. The most knowledge of the corpus callosum in cognitive functions is from research on hypoplasia, degeneration of the corpus callosum, and injury after surgery for epilepsy [4,10-13] performed a set of neuropsychological tests on few patients after operation of tumor resection in the 3rd or lateral ventricles via the corpus callosum. The results showed that partial infarction of the callosum may be related to verbal and visual memory impairment, dysfunction executive abilities, and, in particular, the significant impairment of procedural learning ability. We expected that there were similar dysfunctions related to the corpus callosum infarction. However, there are conditions thought to be distinct to corpus callosum infarction versus other damage to the corpus callosum. For example, the patients who undergo corpus callosotomy have clear epilepsy before surgery, which have affected their cognitive functions, while there is no such case in the patients with corpus callosum infarctions. After occurrence of corpus callosum infarction, the main changes show as demyelination but the nerve fibers are not completely interrupted. There are few papers illustrating the cognitive impairments associated with corpus callosum infarction in literature, while it is important to examine further to understand the damage on the patients.
Therefore, this study aims to analyze the characteristics of cognitive dysfunctions accompanied by corpus callosum infarction based on neuropsychological assessments.
Materials and methods
Subjects
Ten patients (all male; age range: 42-73 years, an average of 56.0±9.4; education level: primary school (5 patients) and secondary or higher education (5 patients); all right-handed) affected by corpus callosum infarction and 10 healthy controls (all male, 42-73 years old with an average of 56.9±7.8, education level matched) were included in this study. Except for the level of glycosylated hemoglobin (the case group was much higher with P<0.01), there was no significant difference in all of the other past blood disease risk factors and laboratory tests between the two groups (see Table 1).
Table 1.
Baseline clinical characteristics in the corpus callosum infarction group and control
| Case group (n = 10) | Control (n = 10) | t/x2 | P | |
|---|---|---|---|---|
| Age (yr) | 56.0±9.4 | 56.9±7.8 | t = 0.23 | 0.82 |
| Sex (M/F) | 10/0 | 10/0 | - | - |
| Education (yr) | 7.8±2.1 | 8.4±2.4 | t = 0.60 | 0.56 |
| Past history (%) | - | - | - | - |
| Cerebrovascular disease | 0 | 0 | - | - |
| Hypertension | 7 (70%) | 8 (80%) | x2 = 0.27 | 0.61 |
| Coronary heart disease | 2 (20%) | 3 (30%) | x2 = 0.27 | 0.61 |
| Atrial fibrillation | 0 | 0 | - | - |
| Diabetes | 7 (70%) | 5 (50%) | x2 = 0.83 | 0.36 |
| Hyperlipidemia | 6 (60%) | 7 (70%) | x2 = 0.23 | 0.69 |
| smoke | 7 (70%) | 5 (50%) | x2 = 0.83 | 0.36 |
| Alcohol | 6 (60%) | 4 (40%) | x2 = 0.80 | 0.37 |
| SBP (mmHg) | 143.50±13.34 | 140.00±9.72 | t = 0.67 | 0.51 |
| DBP (mmHg) | 84.50±8.64 | 84.00±6.15 | t = 0.15 | 0.88 |
| Heart rate (bpm) | 75.30±4.76 | 4.76±6.96 | t = 0.34 | 0.74 |
| Total cholesterol (mmol/L) | 4.19±1.17 | 3.53±0.83 | t = 1.46 | 0.16 |
| Triglycerides (mmol/L) | 2.07±1.10 | 2.07±0.95 | t = 0.02 | 0.98 |
| LDL-C (mmol/L) | 2.29±0.89 | 2.22±0.60 | t = 0.18 | 0.86 |
| HDL-C (mmol/L) | 1.15±0.15 | 1.09±0.16 | t = 1.05 | 0.31 |
| GLU (mmol/L) | 8.13±3.28 | 7.47±2.52 | t = 0.19 | 0.85 |
| HbA1c (%) | 8.37±2.04 | 6.02±0.79 | t = 3.39 | 0.003* |
| HCY | 18.14±9.35 | 15.18±3.67 | t = 0.93 | 0.36 |
The difference is significant in statistics (P<0.05) for comparison of the corpus callosum infarction and control group.
Abbreviations: SBP: Systolic blood pressure; DBP: Diastolic blood pressure; LDL-C: Low-density lipoprotein cholesterol; HDL-C: High-density lipoprotein cholesterol; HbA1c: Hemoglobin A1c.
These 10 patients were inpatients at the Department of Neurology, Xuan Wu Hospital of Capital Medical University from January 2011 to August 2013. The criteria of selection were as follows: 1) compliance with WHO diagnostic criteria for cerebral infarction [14] (fulfillment of the definition of stroke by meeting WHO criteria; namely, sudden focal or comprehensive neurological deficit of cerebrovascular cause that persists beyond 24 hours, excluding cerebral dysfunction caused by other nonvascular factors); 2) head MRI confirming the infarction in the corpus callosum; 3) first cerebral apoplexy; 4) within seven days of onset; 5) clear consciousness; 6) before admission, mRS ≤ 2 and the patient is able to coordinate the examination; 7) informed consent. The exclusion criteria were any of the following: 1) non-first cerebral apoplexy; 2) NIHSS scores demonstrating: level of consciousness (1a) >0, or language related item (9,10) >1, or dual upper limb movement >0; excluded the interference of consciousness, language and movement disorders; 3) serious speech, vision, hearing disorders that affects communication; 4) explicit medical history of traumatic brain injury or mental, emotional, cognitive disorders before; 5) alcohol and/or drug abuse; 6) past medical history of diagnosed malignancy or suffering from severe heart, lung, liver, kidney or other vital organ diseases. There were 2968 cases of acute cerebral infarction during period of the study from 2011 to 2013, among of which, there were 28 cases of patients with corpus callosum infarction. Out of 18 cases of patients were excluded because of first stroke (3 cases), the duration of stroke more than 7days (4 cases), disturbance of consciousness (one case), language barriers (3 cases) and other reasons (7 cases).
The ten matched healthy controls were screened by following criteria: 1) Past medical history including no neurological or psychiatric disorders; 2) No systemic disease (e.g., liver and kidney dysfunction, hypothyroidism, vitamin deficiency) or alcohol or drug abuse, which may lead to cognitive disorders; 3) Normal intelligence examinations and clinical dementia rating (CDR) score of 0.
Participation in this study was voluntary. All tests performed gained informed consent signed by subjects or guardians, and were given consent by Capital Medical University Xuan Wu Hospital Ethics Committee.
Neuropsychological tests
Semi-structured interviews were used to collect all subjects’ demographic data, vascular risk factors (hypertension, diabetes, high cholesterol, heart disease, smoking, etc.) as well as their current and past medical histories. All subjects were also given a detailed neurological examination and neuropsychological assessment. The main test questionnaire included:
The Chinese version of Mini Mental State Examination (MMSE-C). It includes six factors: orientation, immediate recall, attention, short-term memory, and language, with a maximum score of 30.
The Chinese version of Montreal Cognitive Assessment (MoCA-C). It includes attention and concentration, executive functions, memory, language, visuospatial abilities, abstract thinking, calculating abilities and orientation. The cutoff value of MoCA on Chinese population is ≥ 26 with education ≤ 12. The final score is the actual measured score plus one point.
The Chinese version of World Health Organization University of California-Los Angeles, Auditory Verbal Learning Test (WHO-UCLAAVLT): This test primarily for verbal memory, patients’ short-term memory and the ability to learn new things, including immediate recall, delayed recall, long delayed recognition.
The Chinese version of Wechsler Adult Intelligence Scale (WAIS) digit span subtest: It is an individual memory test to primarily detect patients’ attention/working memory functions.
The Rey-Osterrieth complex figure test (ROCF), to examine the ability of visual memory and planning.
The above neuropsychological tests were completed within two weeks after the onset of the stroke and were carried out by the same neuropsychological examination staff/researchers for each patient. They did not know the diagnosis of any of the patient. All tests were completed once with 10 minute breaks offered after each test.
MRI examination
All patients underwent cranial MRI to clearly show the corpus callosum infarction. Regular MRI series included axial T2 weighted image (T2W1), T1 weighted image (T1W1), fluid attenuated inversion recovery image (FLAIR), and diffusion weighted image (DWI). All the images were acquired using Siemens Trio Tim 3.0T scanner (12-channel coil, 45 T/m). Scan sequences included: T2W1: TR/TE = 3830/98 ms, FOV: 230 × 218 mm, matrix 179 × 218; TlWI: TR/TE = 155/2.81 ms, FOV 230 × 186 mm, matrix 156 × 320; FLAIR: TR/TE = 8500/87 ms, FOV: 230 × 201, matrix 224 × 256; DWI: TR/TE = 3000/9 Ims, FOV: 240 × 240, matrix = 160 × 160, b value (0,500, and 1000 s/mm2. All above sequences kept the same slice locations, thickness and space (z = 5.0 mm, space = 1 mm). The spatial range is the whole brain from bottom to top.
Statistics
SPSS 17.0 (www.ibm.com/software/analytics/spss/) was used for the analysis. Categorical variables were described using the number of cases and percentage. Measurement data were presented as average ± standard deviation (x ± s). An individual t-test or Rank test was used for the comparison between groups. Logistic regression analysis was used to evaluate the relationship between cognitive impairment with corpus callosum infarctions. Difference at P<0.05 was regarded as significant.
Results
Demographic characteristics and clinical features
NIHSS scores of the case group were 2.8±1.9. The main clinical manifestations included limb weakness (n = 6), dysarthria (n = 5), dizziness (n = 2), memory loss (n = 2), syncope (n = 1), and unsteady gait (n = 1). The MRI results showed the new corpus callosum infarction in the following regions (Figure 1): involvement of the left (n = 6), involvement of right (n = 4), body (of the corpus callosum, same as follows) (n = 3), genu (n = 2), splenium (n = 2), body and splenium (n = 2), body and genu (n = 1). The vascular assessment showed stenosis in the anterior cerebral artery (n = 2), moderate to severe stenosis in the carotid artery (n = 2), and occlusion in vertebral artery (n = 2). Most of the corpus callosum infarction patients had moderate to severe arterial stenosis or occlusion (n = 9), 7 of which cases showed moderate to severe arterial stenosis in the anterior cerebral artery. Corresponding MR image (T1) of each patient with callosum infarction were showed in the Figure 1, and the characteristics and features on each patient were showed in the Table 2.
Figure 1.
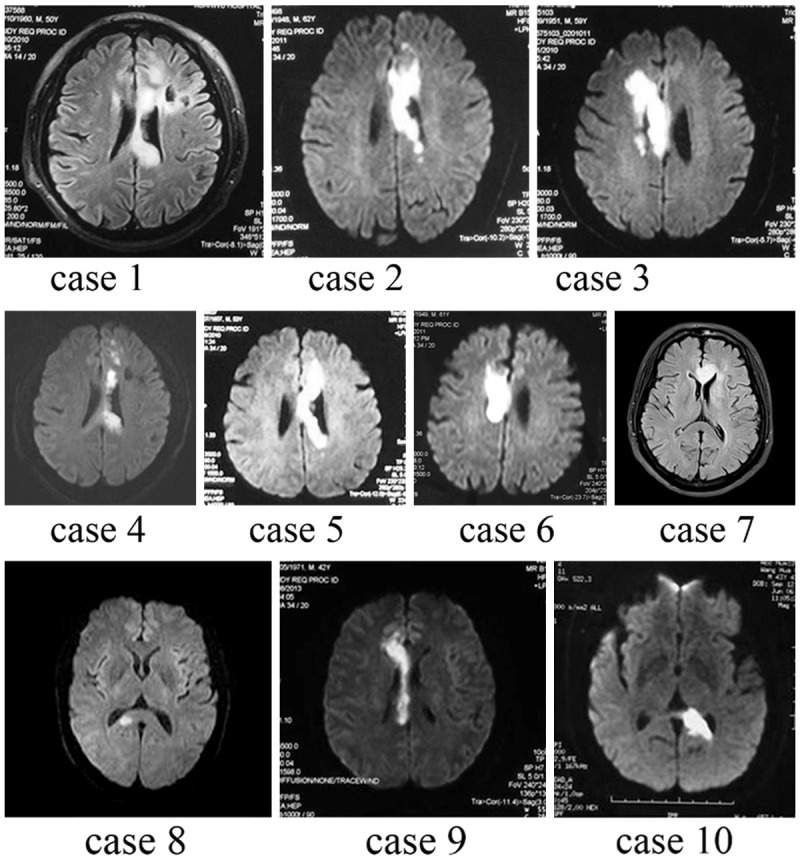
MRI of patients with corpus callosum infarction (case 1-10). All subjects showed infarction in corpus callosum. Since most patients kept the risk factors for atherosclerosis, the other brain tissue nearby the corpus callosum were normally affected by the infarction, that some patients showed lesions going beyond the corpus callosum (patients 1, 3, 4). However, the infarctions basically were in the supply area of the corpus callosum.
Table 2.
Clinical and radiological features of corpus callosum infarction patients
| Case | Age (yrs) | Clinical presentation | MRI finding (corpus callosum) | Vascular assessment results (Artery ultrasound CTA/MRA/DSA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||||||
| LW | Dy. | Diz | Paro. | Mem | Others | Body | Splenium | Genu | ACA | ICA | VA | Others | ||
| 1 | 50 | + | + | -- | -- | -- | left | left | -- | L, M, ste | L MCA, M, ste | |||
| 2 | 63 | + | -- | -- | -- | -- | left | -- | -- | Bi, M, ste | L, M, ste | R, occ | ||
| 3 | 59 | + | -- | + | -- | -- | vertigo | right | -- | right | Bi, M, ste | R MCA, Sev, ste | ||
| 4 | 73 | -- | -- | -- | + | -- | syncope | left | left | -- | L, Sev, ste | L, occ. | L, occ; R, Sev, ste | R MCA, occ |
| 5 | 53 | + | -- | -- | -- | -- | left | -- | -- | |||||
| 6 | 62 | -- | + | -- | -- | + | right | -- | -- | R MCA, Sev, ste; L PCA, Sev, ste | ||||
| 7 | 58 | -- | + | -- | -- | -- | Unsteady gait | -- | -- | left | L, occ | |||
| 8 | 57 | + | + | -- | -- | -- | -- | right | -- | R, Sev, ste | L MCA, M, ste | |||
| 9 | 42 | + | + | -- | -- | -- | Limb numbness | -- | -- | right | R A2, occ | |||
| 10 | 43 | -- | -- | + | -- | + | -- | left | -- | L, Sev, ste | VA-BA M, ste | L MCA, Sev, ste | ||
| Sum | 6 | 5 | 2 | 1 | 2 | 6 | 4 | 3 | 7 | 3 | 3 | |||
Results of neuropsychological tests
For all results of cognitive scores see Table 3. Most scores of cognitive assessment tests in the case group were significantly worse than those of the healthy controls (P<0.05) except for naming ability in MoCA which showed no difference between the two groups. Compared to healthy controls, the general level of MMSE in the case group was lower (P<0.05). In MoCA measurements, individual scores in visuospatial and executive abilities, attention and calculation, language, abstraction, delayed recall and orientation in the case group were significantly lower than in the healthy controls (P<0.05). In addition, scores of WHO-UCLA, digit span and ROCF in the case group were significantly lower than those in the healthy control group (P<0.05).
Table 3.
Comparison of cognitive assessment between corpus callosum infarction and control group
| Case Group (n=10) | Healthy Controls (n=10) | t | P | |
|---|---|---|---|---|
| MMSE | 17.7±7.30 | 27.1±1.70 | 3.99 | 0.001* |
| MoCA | 15.5±3.63 | 27.7±1.83 | 9.49 | 0.000* |
| Individual score in MMSE | ||||
| Orientation to time | 2.90±1.10 | 4.20±0.42 | 3.48 | 0.005* |
| Orientation to place | 3.20±1.14 | 4.30±0.48 | 2.82 | 0.015* |
| Registration | 2.20±0.63 | 2.90±0.32 | 3.13 | 0.008* |
| Attention and Calculation | 2.70±1.64 | 4.50±0.53 | 3.31 | 0.007* |
| Recall | 1.00±0.94 | 2.40±0.52 | 4.12 | 0.001* |
| Language | 1.60±0.52 | 2.00±0 | 2.45 | 0.037* |
| Repetition | 3.40±1.84 | 5.90±0.32 | 4.23 | 0.002* |
| Complex commands | 0.60±0.52 | 0.90±0.32 | 1.57 | 0.138 |
| Individual score in MoCA | ||||
| Visuospatial & Executive | 1.40±0.52 | 4.70±0.48 | 21.64 | 0.000* |
| Naming | 2.70±0.48 | 3.00±0.00 | 1.73 | P>0.05 |
| Attention and Calculation | 3.30±1.83 | 5.70±0.48 | 4.00 | 0.003* |
| Language | 1.50±0.53 | 2.80±0.42 | 6.09 | 0.000* |
| Abstraction | 0.80±0.79 | 2.00±0.00 | 2.59 | 0.010* |
| Delayed recall | 1.30±1.58 | 4.20±0.63 | 6.02 | 0.000* |
| Orientation | 3.80±1.14 | 4.90±0.32 | 3.16 | 0.012* |
| WHO-UCLA AVLT | ||||
| Immediate memory | 21.50±13.01 | 50.00±4.22 | 7.31 | 0.000* |
| Delayed memory | 3.00±3.69 | 9.50±0.85 | 5.87 | 0.000* |
| Delayed recall | 6.90±3.67 | 11.90±1.20 | 4.04 | 0.003* |
| WAIS digit span | 9.10±1.10 | 12.80±1.87 | 6.20 | 0.000* |
| ROCF | ||||
| Drawing reproducing | 12.30±1.15 | 15.10±0.88 | 7.20 | 0.000* |
| Immediate memory | 7.80±4.24 | 14.60±1.07 | 4.87 | 0.001* |
| Delayed memory | 4.30±4.42 | 13.80±1.23 | 6.33 | 0.000* |
The difference is significant in statistics (P<0.05) for comparison betweencorpus callosum infarction and control group.
Discussion
Our study aimed to examine to cognitive dysfunctions caused by corpus callosum infarction. Our neuropsychological results were as follows: (1) the MMSE individual factor scores in the case group in the areas of time, orientation, immediate and delayed memory, attention, calculating ability, and repetition were significantly lower than the scores of the control group (see Table 3); (2) the MoCA individual factor scores of the case group in visuospatial and executive ability, attention and calculation, language, abstraction, delayed memory, and orientation were significantly lower than the scores of the controls. (3) the WHO-ULCA, digit span and ROCF tests all showed that patients in the case group had impairment in memory, attention, planning and the ability to learn new things. The assessment results of the MMSE and the MoCA were similar, and which suggests that patients had disabilities in orientation, attention and calculation, delayed memory, language, repetition, etc.
The results of the MMSE and MoCA combined with the performance of patients during the process of assessment, indicates that patients had 1) significantly impaired short-term memory; 2) difficulty in concentrating and were easily distracted by other things; 3) possible long-term memory impairments affected by attention. Additionally, when the body of CC was affected, the patient’s vocabulary learning ability showed a much more pronounced and significant decrease in these abilities. All of these results are consistent with the symptoms of patients after corpus callosum tumor surgery that included speech impairment, memory impairment, executive dysfunction, and significant impairment in procedural learning ability [12,13].
As we cited in instruction, the corpus callosum is a major commissural fiber system in the human brain to connect the left and right cerebral hemispheres. It plays a collaborative role in the advanced neuronal activities between the two hemispheres. It primarily connects bilateral motor-speech areas, audiovisual areas, and motor conditioned centers, coordinates perception and cognition from bilateral hemispheres, and plays an important role in transferring movement, sensory information, memory and cognition between the bilateral cerebral hemispheres [2]. Under normal circumstances, the two hemispheres of the brain work in cooperation. The corpus callosum, which consists of neural fibers, is responsible for communicating and transferring information between corresponding parts of the cortex in each hemisphere. If the corpus callosum is cut off, the two cerebral hemispheres will be divided and in isolation, thus the cooperation through the commissural fibers will be lost. As an extreme example, notable split-brain research has shown the dysfunction of memory.
When corpus callosum infarction occurs, communication between bilateral hemispheres is affected which causes cognitive disorders in learning, memory, thinking, and executive function. Current neuropsychological assessments suggest that cognitive disorders caused by CC infarction in early stages are primarily in memory, significant in attention, and accompanied by declines in visuospatial and executive ability, orientation, calculation, etc. Again, the CC is primarily responsible for connecting bilateral information from frontal, temporal, parietal, and occipital cortices. The body of the CC connects the bilateral posterior regions of the frontal and parietal cortices that form the top of the body of the lateral ventricles and transfer motion information. The splenium is the posterior part of the CC which connects parietal and occipital lobes. Specifically, the radiation fibers of the splenium radiate from the CC and extend into the occipital lobe. These fibers in the splenium of the CC play an important role in delivering and integrating textual and pictorial information from the bilateral occipital cortex [15,16]. In this study, CC infarction most frequently occurred in body and/or splenium, causing the pathways in the body and splenium to be broken and interrupted which is linked to attention disorder, and visuospatial and executive function damage as measured by multiple neuropsychological tests [12,13,17].
In our study, the MMSE results indicated that there was no significant difference in the ability of drawing replication between the case group and the control group. On the other hand, the MoCA results indicated that there was decline in visuospatial and executive abilities while naming ability was preserved in case patients. The MMSE scale primarily reflects orientation, memory and language abilities, while only one item checks for visuospatial ability. Compared to the MoCA, the MMSE scale is poorer in its measurement of drawing replication and visuospatial executive ability. Therefore, it makes up for the shortcoming of the MMSE aforementioned by including both tests.
There are several limitations in this study. Firstly, because of the low incidence of corpus callosum infarction, there were only 10 cases that were finally enrolled into this study during the period of two years. So they were not suitable to be satisfied by single or multiple lesions. This needs further research in the future. Secondly, in this study, a brain damaged group was not added to be as control group, since it is not clear that the effect reported is due simply to stoke or acute illness, as opposed to corpus callosum damage. Previous studies had confirmed that cognitive impairment after cerebral infarction was partly associated with lesion location. This research mainly focused on the effect of the white matter fiber on the cognitive change. Therefore, other condition such as subcortical structures, cortex (neurons) being involved was not included minimizing the interference factors.
In summary, our study shows that CC infarctions can cause disorders in cognitive functions, and that an infarction in a different region might cause different levels of reduced cognitive abilities. The infarction in the CC most frequently occurs in the body and/or splenium, and primarily causes disorders in memory accompanied by declines in visuospatial executive abilities, attention and calculation etc.
Acknowledgements
This work was supported by the National Key Department of Neurology funded by Chinese Health and Family Planning Committee; The Ministry of Science and Technology Support Program under Grant 2009BAI77B06; National Key Technology R&D Program of the Ministry of Science and Technology of Beijing under Grant D111107003111006; Beijing 215 Talent Project of Academic Leaders; Beijing “Ten Hundred Thousand” Talent Project of “Ten Telents”.
Disclosure of conflict of interest
None.
References
- 1.Verger K, Junque C, Levin HS, Jurado MA, Perez-Gomez M, Bartres-Faz D, Barrios M, Alvarez A, Bartumeus F, Mercader JM. Correlation of atrophy measures on MRI with neuropsychological sequelae in children and adolescents with traumatic brain injury. Brain Inj. 2001;15:211–221. doi: 10.1080/02699050010004059. [DOI] [PubMed] [Google Scholar]
- 2.Caille S, Sauerwein HC, Schiavetto A, Villemure JG, Lassonde M. Sensory and motor interhemispheric integration after section of different portions of the anterior corpus callosum in nonepileptic patients. Neurosurgery. 2005;57:50–59. doi: 10.1227/01.neu.0000163089.31657.08. discussion 50-59. [DOI] [PubMed] [Google Scholar]
- 3.Scepkowski LA, Cronin-Golomb A. The alien hand: cases, categorizations, and anatomical correlates. Behav Cogn Neurosci Rev. 2003;2:261–277. doi: 10.1177/1534582303260119. [DOI] [PubMed] [Google Scholar]
- 4.Glickstein M, Berlucchi G. Classical disconnection studies of the corpus callosum. Cortex. 2008;44:914–927. doi: 10.1016/j.cortex.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Doody RS, Jankovic J. The alien hand and related signs. J Neurol Neurosurg Psychiatry. 1992;55:806–810. doi: 10.1136/jnnp.55.9.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasow DL, Destian S, Braun C, Quintas JC, Kagetsu NJ, Johnson CE. Corpus callosum infarcts with atypical clinical and radiologic presentations. AJNR Am J Neuroradiol. 2000;21:1876–1880. [PMC free article] [PubMed] [Google Scholar]
- 7.Suwanwela NC, Leelacheavasit N. Isolated corpus callosal infarction secondary to pericallosal artery disease presenting as alien hand syndrome. J Neurol Neurosurg Psychiatry. 2002;72:533–536. doi: 10.1136/jnnp.72.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim YD, Lee ES, Lee KS, Kim JS. Callosal alien hand sign following a right parietal lobe infarction. J Clin Neurosci. 2010;17:796–797. doi: 10.1016/j.jocn.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Yuan JL, Wang SK, Guo XJ, Hu WL. Acute infarct of the corpus callosum presenting as alien hand syndrome: evidence of diffusion weighted imaging and magnetic resonance angiography. BMC Neurol. 2011;11:142. doi: 10.1186/1471-2377-11-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brion S, Jedynak CP. [Disorders of interhemispheric transfer (callosal disonnection). 3 cases of tumor of the corpus callosum. The strange hand sign] . Rev Neurol (Paris) 1972;126:257–266. [PubMed] [Google Scholar]
- 11.Gazzaniga MS. Forty-five years of split-brain research and still going strong. Nat Rev Neurosci. 2005;6:653–659. doi: 10.1038/nrn1723. [DOI] [PubMed] [Google Scholar]
- 12.Sauerwein HC, Lassonde M. Cognitive and sensori-motor functioning in the absence of the corpus callosum: neuropsychological studies in callosal agenesis and callosotomized patients. Behav Brain Res. 1994;64:229–240. doi: 10.1016/0166-4328(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 13.Peltier J, Roussel M, Gerard Y, Lassonde M, Deramond H, Le Gars D, De Beaumont L, Godefroy O. Functional consequences of a section of the anterior part of the body of the corpus callosum: evidence from an interhemispheric transcallosal approach. J Neurol. 2012;259:1860–1867. doi: 10.1007/s00415-012-6421-x. [DOI] [PubMed] [Google Scholar]
- 14.Kunitz SC, Gross CR, Heyman A, Kase CS, Mohr JP, Price TR, Wolf PA. The pilot Stroke Data Bank: definition, design, and data. Stroke. 1984;15:740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 15.Binder JR, Mohr JP. The topography of callosal reading pathways. A case-control analysis. Brain. 1992;115:1807–1826. doi: 10.1093/brain/115.6.1807. [DOI] [PubMed] [Google Scholar]
- 16.Molko N, Cohen L, Mangin JF, Chochon F, Lehericy S, Le Bihan D, Dehaene S. Visualizing the neural bases of a disconnection syndrome with diffusion tensor imaging. J Cogn Neurosci. 2002;14:629–636. doi: 10.1162/08989290260045864. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Yamadori A, Endo K, Fujii T, Ezura M, Takahashi A. Dissociation of letter and picture naming resulting from callosal disconnection. Neurology. 1998;51:1390–1394. doi: 10.1212/wnl.51.5.1390. [DOI] [PubMed] [Google Scholar]


