Abstract
Background and Aims Organisms occupying the edges of natural geographical ranges usually survive at the extreme limits of their innate physiological tolerances. Extreme and prolonged fluctuations in environmental conditions, often associated with climate change and exacerbated at species’ geographical range edges, are known to trigger alternative responses in reproduction. This study reports the first observations of adventitious inflorescence-derived plantlet formation in the marine angiosperm Posidonia australis, growing at the northern range edge (upper thermal and salinity tolerance) in Shark Bay, Western Australia. These novel plantlets are described and a combination of microsatellite DNA markers and flow cytometry is used to determine their origin.
Methods Polymorphic microsatellite DNA markers were used to generate multilocus genotypes to determine the origin of the adventitious inflorescence-derived plantlets. Ploidy and genome size were estimated using flow cytometry.
Key Results All adventitious plantlets were genetically identical to the maternal plant and were therefore the product of a novel pseudoviviparous reproductive event. It was found that 87 % of the multilocus genotypes contained three alleles in at least one locus. Ploidy was identical in all sampled plants. The genome size (2 C value) for samples from Shark Bay and from a separate site much further south was not significantly different, implying they are the same ploidy level and ruling out a complete genome duplication (polyploidy).
Conclusions Survival at range edges often sees the development of novel responses in the struggle for survival and reproduction. This study documents a physiological response at the trailing edge, whereby reproductive strategy can adapt to fluctuating conditions and suggests that the lower-than-usual water temperature triggered unfertilized inflorescences to ‘switch’ to growing plantlets that were adventitious clones of their maternal parent. This may have important long-term implications as both genetic and ecological constraints may limit the ability to adapt or range-shift; this seagrass meadow in Shark Bay already has low genetic diversity, no sexual reproduction and no seedling recruitment.
Keywords: Pseudovivipary, reproduction, seagrass, seed abortion, Posidonia australis, seagrass, heat wave, salinity, hybridization, somatic mutation, genetic mosaicism, clonality, evolution
INTRODUCTION
Life-history strategies combining sexual and asexual reproduction can result in stable population structure under variable environmental conditions when the two modes of reproduction are successful in different circumstances (Bengtsson and Ceplitis, 2000; Ofir and Kigel, 2014). At the geographical range limit of species, populations are typically characterized by increased genetic isolation, genetic differentiation, and variability in individual and population performance (Sexton et al., 2009). Survival in peripheral regions is profoundly affected by the frequency of extreme events, and the long-term survival of individuals may depend on their physiological and reproductive capacity (phenotypic plasticity) to survive, rather than evolutionary adaptation (Merila, 2012). Populations often tolerate extreme environmental conditions for short periods of time, but the increasing frequency and extremes of fluctuations associated with climate change can have significant consequences for reproduction and survival (Hedhly et al., 2008; Levin, 2012; Collier et al., 2014). Recently, extreme climatic events have resulted in significant losses to foundation seagrasses in Shark Bay, Western Australia, including vegetative loss and complete reproductive failure (Fraser et al., 2014; Thomson et al., 2015). However, in these circumstances new and novel ways in which to survive, reproduce and disperse often emerge.
Reproduction in seagrasses can occur through several means, although these methods are sometimes confused (Sinclair et al., 2015). Vivipary, defined as the precocious and continuous growth of offspring while still attached to the maternal parent (Goebel, 1905), has been described from fewer than 100 different species of flowering plants, of which approx. 50 % have true vivipary (which involves sexual reproduction), with the remaining species being pseudoviviparous (which does not involve sexual reproduction) (Elmquist and Cox, 1996). Pseudovivipary, in which floral organs are replaced by bulbils or plantlets, provides an asexual means for many terrestrial monocots to reproduce and disperse (Elmquist and Cox, 1996; Coelho et al., 2005; Gordon-Gray et al., 2009; Ofir and Kigel, 2014). Although the molecular mechanism of pseudovivipary remains unknown, its frequent occurrence in extreme environments indicates that only a few key regulators are responsible (Wang et al., 2010). Examples of vivipary and pseudovivipary are observed in both terrestrial and marine environments; species with true vivipary tend to inhabit shallow marine habitats (e.g. mangrove species), while those with pseudovivipary are mostly found in arctic, alpine or arid habitats (Elmquist and Cox, 1996).
True vivipary is known from two seagrass genera, Amphibolis and Thalassodendron (Cymodoceaceae; Kuo and Kirkman, 1990), while pseudovivipary has been reported in only one seagrass family, Posidoniaceae (Ballesteros et al., 2005). In Posidoniaceae, the authors concluded that the plantlets did not originate from fertilized carpels, although no molecular DNA work was performed to determine the origin of the plantlets, so the interpretation remains equivocal. Posidonia spp. do not generally live in extreme environments, but observations of pseudoviviparous plantlets were made in P. oceanica after an intensive flowering episode which followed extreme and sustained high water temperatures in the Mediterranean (Ballesteros et al., 2005).
Most seagrasses (56 % of all species) are dioecious and therefore by definition are outcrossed. However, Posidoniaceae and most Zosteraceae are monoecious (den Hartog, 1970; Les et al., 1997), and outcrossing rates vary, but are generally high (Reusch, 2000; Zipperle et al., 2011; Sinclair et al., 2014a), suggesting that self-pollination is highly regulated. The temporal and spatial pattern in flowering varies greatly among Posidonia meadows (Cambridge, 1975; Díaz-Almela et al., 2006), with extensive overlap during anthesis (Cambridge, 1980; Smith and Walker, 2002; Seddon et al., 2005). Extremely high annual flowering densities (and seed production) have been reported in Posidonia meadows, but in Shark Bay they are very patchy and sporadic (Walker et al., 1988).
Polyploidy and hybridization have been crucial to the evolution of many aquatic plant groups (Les and Philbrick, 1993), with organisms that are now genetically diploid having a palaeopolyploid history (Dufresne et al., 2014). The stages towards polyploid evolution include frequent fertility bottlenecks (shortage of outcrossed pollen) and infrequent events, such as interspecific hybridization and gametic non-reduction hybridization (Ramsey and Schemske, 1998). Ramsey and Schemske (1998) indicate that the triploid bridge pathway can contribute significantly to polyploid formation regardless of the mating system, despite the common claim that triploids are sterile. Ramsey and Schemske (1998) also suggest there is evidence to show that harsh environmental conditions enhance the formation of non-reduced gametes. Any newly establishing polyploids and hybrids can be fixed through clonal growth, with sterile primary hybrid (or back-crossed) genotypes persisting for hundreds or even thousands of years without the presence of the parental species (Prančl et al., 2014). However, the morphological reduction in characters, clonality and extensive phenotypic plasticity in many aquatic species make the identification of hybrids difficult (Prančl et al., 2014).
Under a situation in which clonal growth prevails, mitotic (somatic cell) mutations may be another source of genetic diversity that turn a previously homogeneous meadow into a genetic mosaic consisting of two or more genotypes. Indeed, when clones grow to a large size or age, such as with some seagrasses (e.g. Posidonia oceanica, Arnaud-Haond et al., 2012), mitotic mutations can result in genetic mosaics either within or among ramets (Gill et al., 1995; Reusch and Bostrom, 2011; Arnaud-Haond et al., 2014). We hypothesize that in the almost complete absence of sexual reproduction at the edge of the species’ geographical range, where genetic diversity and connectivity is lowest, natural selection may favour those plants capable of pseudovivipary.
In this study, we report the first observations of inflorescence-derived plantlets in the Australian seagrass Posidonia australis. We (1) use microsatellite DNA markers to determine whether these plantlets are genetic copies of their maternal parent plant and thus a result of vegetative reproduction (including pseudovivipary), (2) determine the origin of multiple (more than two) alleles per locus in P. australis samples and test whether these multiple alleles are indicative of genome duplication or genetic mosaicism, (3) describe environmental stressors which may account for alternative reproductive strategies, and (4) utilize the most recent and complete phylogeny for seagrasses to address the issue of the evolution of pseudovivipary.
MATERIALS AND METHODS
Study species
Posidonia australis Hook.f. is a slow-growing marine angiosperm that forms large, dense meadows. It is endemic to temperate Australian waters, and is widespread from Shark Bay, south and eastwards around southern Australia, including the north coast of Tasmania, and to Wallis Lake (central New South Wales). It occurs just below the low water mark to about 15 m water depth. Like all seagrass, P. australis reproduces both vegetatively and sexually. Inflorescences start to form in May (Austral autumn) with pollination occurring in July/August (Austral winter) and fruit release in November/December (Austral summer). Infructescences are positioned above the leaf canopy, with between eight and 20 fruits being produced. Flowering in P. australis occurs frequently and can be prolific (14–145 inflorescences m−2: Cambridge, 1975; Cambridge and Hocking, 1997; Smith and Walker, 2002). Germination of the seed occurs inside the fruit, so there is no dormancy period or formation of a seed bank. Following successful seedling recruitment, P. australis is able to expand through vegetative clonal growth via rhizome extension (ramets). As a result, individual plants that arise from seed (genets) have the capacity to form large clones comprising many, often disconnected shoots (ramets) (Waycott, 1995).
Field site and sampling
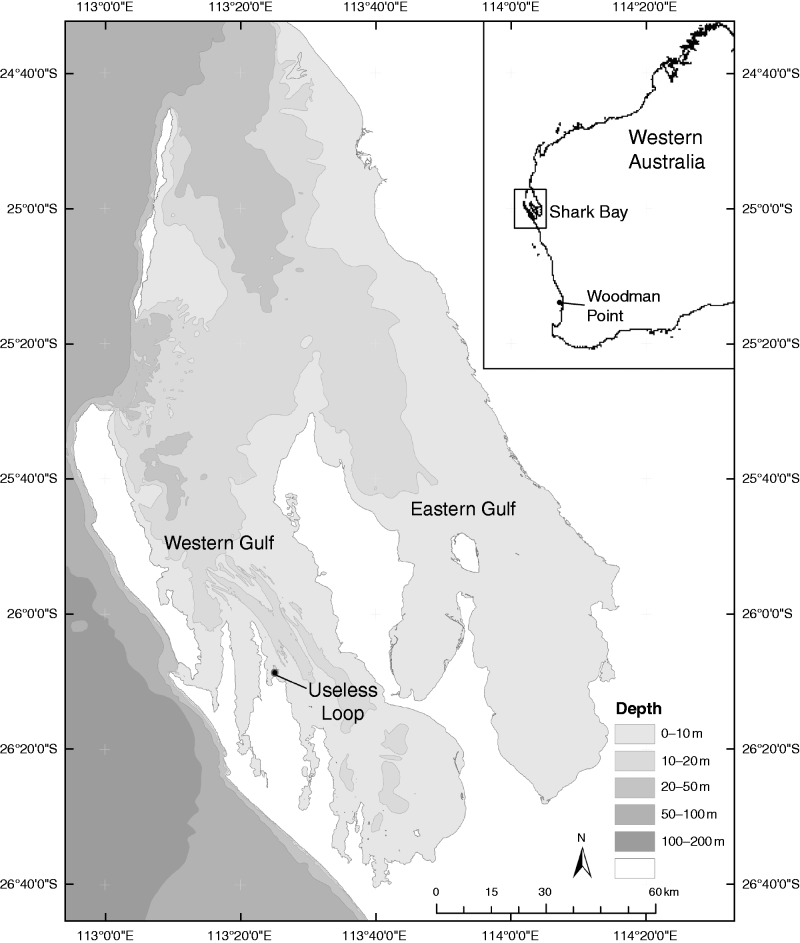
Shark Bay (25°30′S, 113°30′E) was formed by a marine transgression into a coastal environment along the central coast of Western Australia, approx. 7000–8000 years ago (Logan and Cebulski, 1970) and is now recognized as a UNESCO World Heritage Site. It is a unique system at the interface of warm tropical and southern temperate zone ecosystems. The region experiences hot, dry conditions in summer and cooler, wet conditions in winter, with estimates of annual evaporation (2000 mm) exceeding precipitation (200 mm) over the entire year (Logan and Cebulski, 1970; Smith and Atkinson, 1983; Burling et al., 1999). The topography of the seafloor is dominated by a series of barrier ridges and sills (Logan and Cebulski, 1970) limiting exchange with oceanic water. Long water residence times result, especially within the eastern and western gulfs (Fig. 1), and the range in water temperature (12–30 °C) more closely emulates that of a terrestrial landscape, rather than the Indian Ocean. As a result, these features combine to produce hypersaline conditions, with an increasing gradient in salinity southwards into the eastern (>70 p.p.t.) and western (>50 p.p.t.) gulfs. Shark Bay is renowned for containing one of the largest reported seagrass meadows in the world (Walker et al., 1988; Kendrick et al., 2012), and due to its location at a climatic interface, species from both tropical and temperate realms co-occur, resulting in high species diversity (Kendrick et al., 2012). One of the dominant temperate species in Shark Bay, P. australis, is growing at the northern extreme of its geographical range. Although extensive meadows persist, growth rates appear much lower in the phosphorus-deficient system (Walker and McComb, 1988; Atkinson, 1987; Burkholder et al., 2013), reproductive rates are greatly reduced (Walker et al., 1988) and genetic diversity is much lower in comparison with more southerly meadows (Waycott et al., 1997; Sinclair et al., 2014b).
Fig. 1.
Map of Western Australia (inset) showing the location of the Shark Bay World Heritage Site; expanded map of the region with bathymetry and location of Useless Loop.
During two field trips to Shark Bay between January and March 2011, plantlets were observed growing on inflorescence peduncles in P. australis meadows near Useless Loop (UL) in the western gulf of Shark Bay (Fig. 1). A total of 37 basal shoots were sampled from a mature meadow within a 50 -m-diameter area. Of these, 17 were collected as pairs of shoots: a basal (maternal) shoot attached to a subterranean rhizome (‘A’ sample) and a plantlet attached to the basal shoot via a thickened inflorescence stalk or peduncle (‘B’ sample) (Fig. 2B). Each paired sample was placed in a labelled calico bag and kept on ice. On returning to the laboratory, the green leaf area was removed and the leaf-sheath fibre was stripped away to reveal the smooth white leaf meristem on each pair of shoots. Each meristem was cut longitudinally into two pieces and frozen at −80 °C prior to DNA extraction.
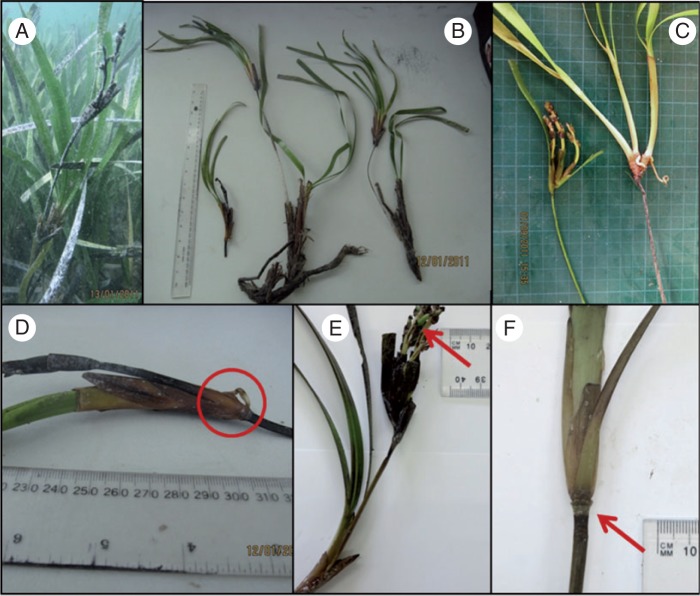
Fig. 2.
Freshly collected P. australis shoots from Useless Loop: (A) pseudoviviparous plantlet still attached to parent plant (January: 2 months after fruit/seed maturity); (B) harvested pseudoviviparous plantlets with inflorescence, basal rhizome with (maternal) shoot and inflorescence-derived plantlet attached via a thickened inflorescence peduncle; (C) inflorescences with multiple immature fruit and single plantlet with roots arising from rhizome (September: 1 month before fruit/seed maturity); (D) close-up of inflorescence bearing a single root (circle); (E) close-up of plantlet with inflorescence bearing immature fruit (arrow) but fruit were non-viable due to aborted seeds; (F) removal of leaf sheath from the inflorescence-derived plantlet reveals proximal end (base) of the peduncle attached to the maternal parent plant at the apex of a shoot (arrow). Photos by R. Hovey and J. Statton.
Environmental variables
Salinity has been monitored at 11 sites around Shark Bay Salt operations at Useless Loop since 2004. These data were obtained to see if there were any significant fluctuations that may have impacted on the P. australis life cycle. Mean monthly satellite-derived sea surface temperatures (SSTs) between 2001 and 2011 have been published for the eastern Gulf in Shark Bay (Fraser et al., 2014; Thomson et al., 2015). Reynolds monthly SST anomaly charts were available for October 2010 to May 2011 (Pearce and Feng, 2013).
DNA extraction and genotyping
Total genomic DNA was extracted from frozen shoot meristems using a modified PVP–SDS method [polyvinylpyrrolidone (PVP) and sodium dodecyl sulfate (SDS)] (Sinclair et al., 2009, 2014a). Multilocus genotypes were generated using seven polymorphic microsatellite loci (PaA1, PaA105, PaA120, PaB6, PaB8, PaB112, PaD113) using PCR conditions described by Sinclair et al. (2009). Allele frequencies were calculated using GENODIVE version 2.0b27 (Meirmans and van Tienderen, 2004), which handles genotypes with more than two alleles per locus. The assignment of clones was determined using a stepwise mutation model, with a threshold of 3. We used Nei’s corrected diversity index to test for clonal structure in GENODIVE. Clonal diversity (R) within the meadow was expressed as the proportion of distinguishable multilocus genets (MLG-1) divided by the number of ramets sampled (N-1) (Ellstrand and Roose, 1987).
Multilocus genotypes (MLGs) were compared between the 17 pairs of maternal (basal) shoots and plantlets to determine whether the plantlets were the result of vivipary (different MLG as a result of recombination through sexual reproduction) or pseudovivipary (same MLG due to reversion of flowers to vegetative growth after failing to pollinate). MLGs were also compared among the basal shoots (n = 37) to determine whether a single clone or multiple plants were involved in the production of plantlets.
Five microsatellite loci produced three alleles in the UL population (PaA1, PaA105, PaA120, PaB8, PaB112). These loci typically display (homozygous or heterozygous) di-allelic genotypes expected from normally segregating multilocus microsatellite loci (Sinclair et al., 2009, 2014a, b). However, in the samples from UL, Shark Bay, an additional allele was observed in most samples for between one and three loci (Supplementary Data, Table S1).
Determination of ploidy and genome size using flow cytometry
Comparison of ploidy and genome size (2C value) between samples from UL and Woodman Point (WP, Fig. 1, where no additional alleles were detected in MLGs) was done to determine whether a full genome duplication had occurred and could account for the additional alleles. Flow cytometry (FCM) has become the prevailing method of choice for measuring nuclear DNA content in plants (Dolezel et al., 2007; e.g. Koce et al., 2003; Prančl et al., 2014). Freshly collected P. australis meristem tissue (n = 10 each from WP and UL) was used to determine ploidy. In addition, frozen material from the unique genotyped clones from UL (n = 13) were compared with these samples to confirm ploidy. Suspensions of intact nuclei were prepared using approx. 50 mg of latitudinally sectioned plant tissue following the protocol described by Dolezel et al. (2007), using the Tris.MgCl2 buffer plus 1 % PVP. A 20 -µm nylon mesh (Plastok, Birkenhead, UK) was used to filter the homogenate, before staining the nuclei with 9 µg ml−1 propidium iodide (Sigma-Aldrich, Castle Hill, Australia). A subset of three freshly collected samples per site were chopped with an internal size standard and treated with 50 µg mL−1 RNase to obtain accurate estimates of the genome size. These six samples were prepared and measured on three separate occasions to account for fluctuation. Solanum lycopersicum L. ‘Stupické polné rané’ (Dolezel et al., 2007) was selected as the reference standard (2C = 1·96 pg DNA). Samples were run on a Becton Dickinson FACScan flow cytometer with the fluorescence intensity of a minimum of 6000 particles recorded.
Detection of genetic mosaicism
Genetic mosaicism may be an alternative explanation for the additional alleles, as was recently described in northern hemisphere seagrasses, Zostera marina (Reusch and Bostrom, 2011), and suggested through the spectrum of allelic distance for Cymodocea nodosa and P. oceanica at the meadow scale (Arnaud-Haond et al., 2007; Rozenfeld et al., 2007). We took an individual leaf from two different shoots at UL (one individual showed three alleles at locus PaA105 only and the second at locus PaB8 only) to determine whether the origin of these additional alleles was the result of genetic mosaicism. Each single leaf blade was cut into 19–20 longitudinal sections under a microscope in an attempt to isolate individual cell lines (following protocols in Reusch and Bostrom, 2011). Each section was approx. 1 mm wide and 60 mm long. DNA extractions were performed as described above, except extraction volumes were reduced due to very small amounts of tissue being used. PCRs were also performed as described above for the five polymorphic loci, but with an additional five PCR cycles. The PCRs resulted in clear, scoreable amplification products that were comparable to those obtained with our standard shoot genotyping procedures.
RESULTS
Description of plantlets
Plantlets were observed growing on inflorescences within the P. australis meadow at UL in January 2011 (Fig. 2A). The plantlets were attached to the upper inflorescence peduncle. The proximal end (base) of the peduncle was attached to the maternal parent plant at the apex of a shoot, as expected for an inflorescence. In a typical inflorescence, several branchlets form off the main peduncle, with each containing multiple flowers. The entire peduncle abscises once the fruit have matured and been released. However, in this case it continued to grow, with the most basal branchlet forming a plantlet, while the upper branchlets contained multiple flowers (and immature fruit) (Fig. 2B, C, E). Each plantlet contained between one and three shoots, with most bearing rudimentary or developed roots that were short (<30 mm) and unbranched (Fig. 2C, D). Old leaf sheaths were present on the plantlets, but the leaves were lost (Fig. 2B). The apical region of the inflorescence contained multiple branchlets with immature fruit, which should be almost mature by October (Austral spring, Fig. 2E). Sectioning of the fruit showed they had aborted (no seed present). Removal of the leaf sheath from a plantlet showed the proximal end (base) of the peduncle attached to the maternal parent plant at the apex of a shoot (Fig. 2F), in the same way the inflorescence peduncle attaches to the basal shoot apex. It was also noted that the peduncle was shorter than usual, with inflorescences situated within the leaf canopy, rather than above the canopy, which is a feature that separates P. australis from other Posidonia species.
The UL site was subsequently revisited during August 2011 and again in late October 2011. Low flowering density was observed (∼10 inflorescences m−2) in August 2011, but all trace of the plantlets was gone, including peduncles, and no detached plantlets were observed in the vicinity. In October 2011, a survey of reproductive output covering 7 km of nearshore P. australis meadows (<5 m depth) showed widespread fruit development, but sectioning of the fruit found no seeds had developed.
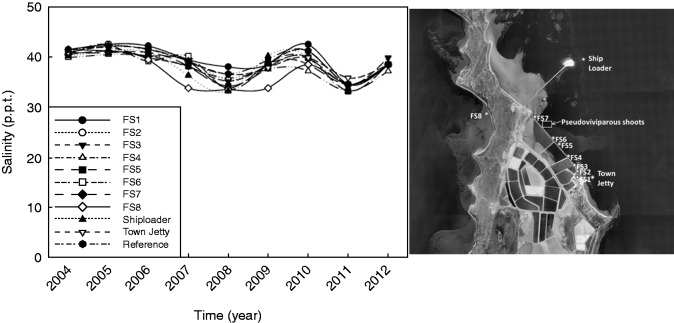
Environmental trends at UL
Salinity records for UL between 2004 and 2012 show significant fluctuations over time (Fig. 3). All sites were generally well above the marine water salinity range of 32–37 p.p.t., with 2008 and 2011 being the only years in which salinity levels were recorded within this range. A large rainfall event in December 2010 with prolonged flooding (Fraser et al., 2014) probably explains the lower salinity level in 2011. The satellite-derived SST charts published in Fraser et al. (2014) and Thomson et al. (2015) show an unusually low water temperature in July 2010, approx. 1.5 °C below mean temperature (Pearce and Feng, 2013). An unprecedented marine heat wave followed between January and May 2011, driven by a record strength Leeuwin current (Pearce and Feng, 2013). At its peak in February 2011, the SST at Shark Bay was nearly 5 °C higher than the mean temperature.
Fig. 3.
Salinity (p.p.t.) for 11 sites at Useless Loop between 2004 and 2012. Data courtesy of Shark Bay Salt Pty. Ltd.
Genetic diversity and evidence for pseudovivipary
Five of the seven microsatellite loci were polymorphic in the P. australis samples from UL (Table 1). Levels of genetic diversity were low, with 3–5 alleles per locus. Clonal diversity was also low (R = 0·33), with significant clonal structure (observed diversity = 0·73, P < 0·001), indicating the observed clonal diversity is due to sexual reproduction. The MLGs for all 17 basal shoot and plantlet pairs were identical, indicating that they are the result of pseudovivipary (vegetative growth within a floral structure; Fig. 4A), rather than precocious germination or vivipary (sexual reproduction). Six different clones were involved in the pseudoviviparous production of the inflorescence-derived plantlets, with one clone being sampled 13 times, indicating that at least one of the clones was extensive.
Table 1.
Allele frequency data for seven microsatellite loci based on 37 basal shoot samples of P. australis from Useless Loop, Shark Bay
| Locus | Allele/n | Useless Loop | MLG | R | Na | Ho (%) |
|---|---|---|---|---|---|---|
| PaA1 | n | 36 | 4 | 0·09 | 5 | 100·0 |
| 242 | 0·444 | |||||
| 265 | 0·037 | |||||
| 271 | 0·049 | |||||
| 273 | 0·444 | |||||
| 275 | 0·025 | |||||
| PaA105 | n | 37 | 4 | 0·08 | 4 | 78·4 |
| 215 | 0·067 | |||||
| 225 | 0·506 | |||||
| 227 | 0·258 | |||||
| 229 | 0·169 | |||||
| PaA120 | n | 37 | 4 | 0·08 | 3 | 81·1 |
| 194 | 0·537 | |||||
| 196 | 0·375 | |||||
| 198 | 0·087 | |||||
| PaB6 | n | 24 | 1 | 0·00 | 1 | 0·0 |
| 245 | 1·000 | |||||
| PaB8 | n | 37 | 2 | 0·03 | 3 | 100·0 |
| 204 | 0·430 | |||||
| 218 | 0·430 | |||||
| 220 | 0·140 | |||||
| PaB112 | n | 37 | 3 | 0·06 | 3 | 81·1 |
| 185 | 0·395 | |||||
| 189 | 0·579 | |||||
| 195 | 0·026 | |||||
| PaD113 | n | 35 | 1 | 0·00 | 1 | 0·0 |
| 195 | 1·000 | |||||
| Overall loci | 37 | 13 | 0·33 | 4 | 62·9 | |
Genetic diversity indices by single locus and combined loci. n = number of shoots genotyped, MLG = number of multilocus genotypes, R = clonal diversity, Na = total number of alleles, Ho = observed heterozygosity.
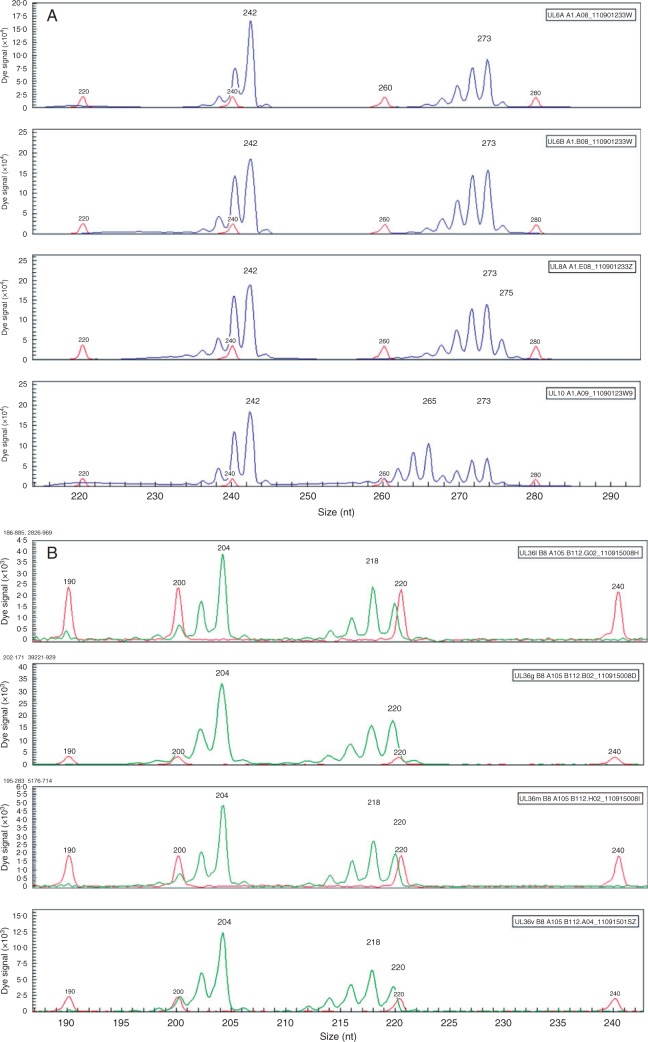
Fig. 4.
Posidonia australis genotypes (in base pairs) for (A) locus PaA1 showing the same genotype for a basal maternal shoot and inflorescence-derived plantlet pair (242/273), and two samples with an additional allele (242/273/275 and 242/265/273); and (B) locus PaB8 genotypes from a single shoot sample showing the composite lineage (204/218/220) and three longitudinal leaf sections with the isolation of a bi-allelic genotype (204/220).
Of the 54 samples genotyped (37 basal shoots and 17 plantlets), 46 samples (85 %) exhibited three alleles per locus for between one and three loci (Supplementary Data, Table S1). These additional alleles were observed at different frequencies across five of the seven loci (PaA1, PaA105, PaA120, PaB8 and PaB112), and usually involved a single stepwise mutation from the larger of the two alleles from the most common diploid genotype in the meadow, in all but two instances. For example, in locus PaB8, the common genotype was 204/218 and the mosaic genotype was 204/218/220. This follows the typical (stepwise mutation) model for di-nucleotide microsatellite mutations resulting in genetic mosaicism (O’Connell and Ritland, 2004). Seventeen basal shoot samples had three alleles at a single locus, while nine had three alleles at two loci, and only three had three alleles at three loci. All alleles have been detected within the UL meadow, or other P. australis meadows, with usual diploid genotypes.
There were no differences in observed ploidy between freshly collected samples at WP and UL, and the unique clones at UL (obtained from frozen plant material), with all samples within 10 % of the mean value. The genome size (2C value) was measured as 4·66 ± 0·05 pg DNA at UL and 4·71 ± 0·04 pg DNA at WP, with no significant difference between the two sites (t-test: 0·754, P = 0·538). Two different genotypes were detected in longitudinal leaf sections from a single ramet, providing evidence for a genetic mosaic. Two different genotypes were obtained for locus PaB8: the three-allele genotype (204/218/220) for the basal shoot and 19 longitudinal leaf sections, and a two-allele genotype (204/220) for one longitudinal leaf section (Fig. 4B). Interestingly, we did not detect the common 204/218 genotype (Table S1). The same genotypes were obtained for all samples (basal shoot and 19 longitudinal leaf sections) from the second shoot (locus PaA105, genotype = 225/227/229).
DISCUSSION
Here we show the occurrence of pseudovivipary and genetic mosaicism in P. australis, based on morphological and genetic data. Pseudovivipary was also reported from the congeneric P. oceanica (Ballesteros et al., 2005), although the interpretation was equivocal, as it was not confirmed with genetic data. The occurrence of pseudovivipary in multiple unrelated angiosperm families supports multiple independent evolutionary origins, in some cases through convergent evolutionary processes (Elmquist and Cox, 1996). However, we suggest pseudovivipary in Posidoniaceae was retained from ancestral monocots, but this has yet to be observed among other taxa within the genus. The two examples suggest it is facultative and probably occurs in response to extreme environmental conditions.
Pseudoviviparous plantlets in P. australis were observed growing from inflorescence peduncles located in the upper portion of the seagrass canopy. This observation is consistent with a reversion of the floral spikelet to a leafy vegetative shoot (E. Díaz-Almela, pers. comm.). In addition, both immature fruit and pseudoviviparous plantlets were observed on the same inflorescence, suggesting the plant can take advantage of long-distance dispersal of seeds and local dispersal of plantlets (Ballesteros et al., 2005). Flowering is typically composed of a series of irreversible events, although reversion from floral to vegetative growth is frequently observed in nature (Tooke et al., 2005). In P. australis, the entire basal branchlet containing multiple flowers appears to be replaced by a single plantlet, while pseudoviviparous plantlets replace individual flowers within the P. oceanica inflorescence (Ballesteros et al., 2005). This suggests the ‘decision’ to grow vegetatively may occur much earlier in the development of the P. australis inflorescence than in P. oceanica.
Production of pseudoviviparous plantlets has been studied more extensively in terrestrial species, and is shown to be under both genetic and environmental control (Wang et al., 2010; Chiurugwi et al., 2011). Two significant climatic events probably impacted P. australis reproduction at UL: (1) low water temperature in July 2010 (approx. 1.5 °C below normal) followed by (2) a prolonged marine heat wave during summer/autumn 2011 (5 °C above normal). These temperature fluctuations may have contributed to the very limited flowering (and pollen shortage) in winter 2010, and a mass flowering event following the 2011 heat wave, similar to P. oceanica, although nothing was concluded about the signal triggering plantlet production, as the link between warm water and pseudovivipary was only circumstantial (Ballesteros et al., 2005; Díaz-Almela et al., 2007).
It is not known when or how the P. australis plantlets released from the parent plants, and whether they were able to recruit elsewhere (as they had disappeared on revisiting the site). The lack of observations of plantlets in the surrounding meadow (unlike P. oceanica) suggests that longer distance dispersal may be an option. Reproductive failure is very high in P. oceanica due to high predation and seed abortion rates (87 %, Balestri and Cinelli, 2003). This is in contrast to much lower reported rates in P. australis (<5 %, Sinclair et al., 2014a). However, an attempt to study the mating system in P. australis at UL could not be performed due to 100 % seed abortion. Immature developing fruit and inflorescence-derived plantlets were again observed in October 2014. The fruit were immature and seed development had ceased, consistent with environmental and genetic factors being responsible for ongoing reproductive failure in this meadow.
Little is known about self-incompatibility mechanisms in seagrasses, although Balestri and Cinelli (2003) suggested the high seed abortion rates in P. oceanica were due to post-fertilization factors. The reproductive failure in P. australis through post-zygotic abortion of embryos may be due to underlying genetic issues, as it is an obligate outcrosser (Sinclair et al., 2014a). Overall, genetic diversity is low at UL, a trait commonly associated with range-edge populations, with prolonged and nearly exclusive clonal growth through environmental suppression of sexual reproduction, which can ultimately lead to local sexual extinction and monoclonal populations of species (Honnay and Bossuyt, 2005).
The presence of three alleles at microsatellite loci was unexpected, as all Posidonia spp. have a somatic diploid chromosome number of 2 n = 20 (den Hartog et al., 1987; Kuo et al., 1990) based on karyotyping, so there is no evidence that speciation in Posidonia, which is relatively prolific in comparison with other seagrass genera, is accompanied by a change in chromosome number (Kuo et al., 1990). Therefore, we interpret our results of no observable difference in ploidy or genome size between WP and UL samples to mean that the additional alleles observed in the MLGs at UL are not the result of a complete genome duplication. The P. australis genome (2C value = 4·66–4·71 pg DNA) is considerably smaller than that of P. oceanica (2C value = 7·27 pg DNA, Koce et al., 2003), perhaps reflecting a long period of divergence from a common ancestor, which pre-dates closure of the Tethys Sea at approx. 65 Mya (Aires et al., 2011, and references within).
We suggest genetic mosaicism is a possible explanation for additional alleles detected at multiple loci in P. australis. Over 3500 P. australis samples have been genotyped from meadows throughout the species range (Sinclair et al., 2014a, b; E. A. Sinclair et al., unpubl. res.) with multiple (more than two) alleles rarely observed; only when we detected many samples with multiple alleles within a meadow was it clear that there was a pattern in which PCR artefacts may not be the only explanation. Genetic mosaicism observed at neutral microsatellite markers suggests the possibility of within-clone variation at selectively relevant loci and supports the notion that members of clones are seldom genetically identical. Genetic mosaicism was recently reported in the northern hemisphere seagrass Zostera marina (Reusch and Bostrom, 2011), with more genetic mosaics found in meadows at the distributional range limits where sexual reproduction was rare or absent.
A hybridization hypothesis (and creation of sterile back-crossed clones) is also consistent with the observed pattern of seed abortion. The presence of additional alleles in the UL clones suggests they may be sexually incompatible with the lineage(s) they were derived from. Posidonia coriacea is a likely candidate for hybridization with P. australis, as it co-occurs in the region, and is known to hybridize elsewhere (E. A. Sinclair et al., unpubl. res.). In this scenario, a diploid F1 hybrid plant would be fertile and able to backcross to either parental species. Additional alleles in the backcross hybrid may be caused by unreduced (diploid) pollen combining with the haploid pollen from either parental species. These backcross hybrids are probably sterile, as supported by complete seed abortion and the presence of pseudoviviparous shoots. The putative triploid hybrid (three alleles in UL samples) has a similar genome size as the putative diploid from WP. The smaller than expected genome size recovered may be due to (1) ancient polyploidization followed by diploidization that has acted to reduce the genome size of these hybrids, or (2) the other parent of the hybrid (P. coriacea) has a significantly smaller genome size than P. australis. Further research is required to distinguish between these scenarios. In either case, the genetic signature would be able to persist through time because clones are long-lived and slow-growing. The shorter peduncles (held within the leaf canopy) also provide additional support for this hypothesis. Hybridization has been recently reported in two other seagrass genera, Ruppia (Ito et al., 2010) and Halodule (Ito and Tanaka, 2011).
Evolution and climate change
The emergence of pseudovivipary as a reproductive mode within Posidoniaceae raises interesting evolutionary questions, such as is this a derived trait or has it been retained from the terrestrial or aquatic–freshwater ancestors? Why is vegetative reproduction so common in aquatic plants? Is it easier, or is it in response to failed sexual reproduction? Have all aquatic groups retained the ability to reproduce via pseudovivipary, but it is only observed under a particular set of environmental stressors? If so, it would suggest that pseudovivipary is an ‘adaptive plastic’ trait (see Sexton et al., 2009), which may be far less common in water (or less likely to be observed) than on land.
Researchers have speculated as to the evolutionary role of mosaicism (reviewed by Pineda-Krch and Lehtila, 2004), with benefits including increased genetic variation. If genetic diversity is able to increase through somatic mutations, then pseudoviviparous plantlets may be a quick fix with colonizing potential, or a ‘last ditch’ effort where somatic mutations (and environmental stressors) have contributed to reproductive failure. Reproductive instability is a potential and significant cost associated with somatic mutation, so the failure of pollination through non-viable pollen production or premature abortion of inbred seeds construes no evolutionary advantage. The negative effects of infertility in genetic mosaics may eventually outweigh the benefits of (limited) dispersal by a few plantlets. The meadow probably suffers from inbreeding through (1) pollen limitation – flowers were not pollinated due to a shortage of outcrossed pollen, and/or (2) pollen sterility – potentially as a result of genetic mosaics or haplo-diploidy involving hybridization with a congener. This ultimately leads to reduced demographic resilience for P. australis via lower fitness and genetic variability (Waycott et al., 1997). Maintaining genetic diversity in the UL meadow is important for long-term resilience and adaptation in the face of global warming (Ehlers et al., 2008; Monro and Poore, 2009; Bergmann et al., 2010). The long-term consequences for P. australis and other range edge populations of marine and terrestrial species are under threat: additional environmental stress is shifting populations rapidly to predominantly clonal reproduction, with no genetic recombination to find potentially more suited gene combinations and little or no plant turnover within or among populations.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of Table S1: details of the 54 samples for multilocus genotypes.
ACKNOWLEDGEMENTS
This project was funded by two ARC Linkage grants to G.A.K. and K.W.D. (LP100200429, LP130100918) at the University of Western Australia, with industry partners Cockburn Cement Ltd, Department of Parks and Wildlife (previously Environment and Conservation), Botanic Gardens and Parks Authority, and Shark Bay Salt. Thanks to Mark Wallace, Matt Barrett, Tony Scalzo and Thorston Reusch for technical support and discussions, and Sophie Arnaud-Haond and one anonymous reviewer for their constructive comments.
LITERATURE CITED
- Aires T, Marbà N, Cunha RL, et al. 2011. Evolutionary history of the seagrass genus Posidonia. Marine Ecology Progress Series 421: 117–130. [Google Scholar]
- Arnaud-Haond S, Duarte CM, Alberto F, Serrão EA. 2007. Standardizing methods to address clonality in population studies. Molecular Ecology 16: 5115–5139. [DOI] [PubMed] [Google Scholar]
- Arnaud-Haond S, Duarte CM, Díaz-Almela E, Marbà N, Sintes T, Serrão EA. 2012. Implications of extreme life span in clonal organisms: millenary clones in meadows of the threatened seagrass Posidonia oceanica. PLoS ONE 7: e30454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaud-Haond S, Moalic Y, Hernandez-Garcia E, et al. 2014. Disentangling the influence of mutation and migration in clonal seagrasses using the genetic diversity spectrum for microsatellites. Journal of Heredity 105: 532–541. [DOI] [PubMed] [Google Scholar]
- Atkinson MJ. 1987. Low phosphorus sediments in a hypersaline marine bay. Estuarine, Coastal and Shelf Science 24: 335–347. [Google Scholar]
- Balestri E, Cinelli F. 2003. Sexual reproductive success in Posidonia oceanica. Aquatic Botany 75: 21–32. [Google Scholar]
- Ballesteros E, Cebrian E, Garcia-Rubies A, Alcoverro T, Romero J, Font X. 2005. Pseudovivipary, a new form of asexual reproduction in the seagrass Posidonia oceanica. Botanica Marina 48: 175–177. [Google Scholar]
- Bengtsson BO, Ceplitis A. 2000. The balance between sexual and asexual reproduction in plants living in variable environments. Journal of Evolutionary Biology 13: 415–422. [Google Scholar]
- Bergmann N, Winters G, Rauch G, et al. 2010. Population-specificity of heat stress gene induction in northern and southern eelgrass Zostera marina populations under simulated global warming. Molecular Ecology 19: 2870–2883. [DOI] [PubMed] [Google Scholar]
- Burkholder DA, Fourqurean JW, Heithaus MR. 2013. Spatial pattern in seagrass stoichiometry indicates both N-limited and P-limited regions of an iconic P-limited subtropical bay. Marine Ecology Progress Series 472: 101–115. [Google Scholar]
- Burling M, Ivey GN, Pattiaratchi C. 1999. Convectively driven exchange in a shallow coastal embayment. Continental Shelf Research 19: 1599–1616. [Google Scholar]
- Cambridge ML. 1975. Seagrasses of south-western Australia with special reference to the ecology of Posidonia australis Hook f. in a polluted environment. Aquatic Botany 1: 149–161. [Google Scholar]
- Cambridge ML. 1980. Ecological studies of seagrasses of south western Australia with particular reference to Cockburn Sound. PhD Thesis, The University of Western Australia, Australia. [Google Scholar]
- Cambridge ML, Hocking PJ. 1997. Annual primary production and nutrient dynamics of the seagrasses Posidonia sinuosa and Posidonia australis in south-western Australia. Aquatic Botany 59: 277–295. [Google Scholar]
- Chiurugwi T, Beaumont MA, Wilkinson MJ, Battey NH. 2011. Adaptive divergence and speciation among sexual and pseudoviviparous populations of Festuca. Heredity 106: 854–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho FF, Neves ACO, Capelo C, Figueira JEC. 2005. Pseudovivipary in two rupestrian endemic species (Leiothrix spiralis and Leiothrix vivipara). Current Science 88: 1225–1226. [Google Scholar]
- Collier CJ, Villacorta-Rath C, van Dijk JK, Takahashi M, Waycott M. 2014. Seagrass proliferation precedes mortality during hypo-salinity events: a stress-induced morphometric response. PloS ONE 9: e94014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hartog CKJ. 1970. The sea-grasses of the world. Amsterdam: North-Holland Publishing Company. [Google Scholar]
- den Hartog CKJ, Hennen J, Noten TMPA, van Wijk RJ. 1987. Chromosome numbers of the European seagrasses. Plant Systematics and Evolution 156: 55–60. [Google Scholar]
- Díaz-Almela E, Marbà N, Álvarez E, Balestri E, Ruiz-Fernández JM, Duarte CM. 2006. Patterns of seagrass (Posidonia oceanica) flowering in the Western Mediterranean. Marine Biology 148: 723–742. [Google Scholar]
- Díaz-Almela E, Marbà N, Duarte CM. 2007. Consequences of Mediterranean warming events in seagrass (Posidonia oceanica) flowering records. Global Change Biology 13: 224–235. [Google Scholar]
- Dolezel J, Greilhuber J, Suda J. 2007. Estimation of nuclear DNA content in plants using flow cytometry. Nature Protocols 2: 2233–2244. [DOI] [PubMed] [Google Scholar]
- Dufresne F, Stift M, Vergilino R, Mable BK. 2014. Recent progress and challenges in population genetics of polyploid organisms: an overview of current state-of-the-art molecular and statistical tools. Molecular Ecology 23: 40–69. [DOI] [PubMed] [Google Scholar]
- Ehlers A, Worm B, Reusch THB. 2008. Importance of genetic diversity in eelgrass Zostera marina for its resilience to climate warming. Marine Ecology Progress Series 355: 1–7. [Google Scholar]
- Ellstrand NC, Roose ML. 1987. Patterns of genotypic diversity in clonal plant species. American Journal of Botany 74: 123–131. [Google Scholar]
- Elmquist T, Cox PA. 1996. The evolution of vivipary in flowering plants. Oikos 77: 3–9. [Google Scholar]
- Fraser MW, Kendrick GA, Statton J, et al. 2014. Extreme climate events lower resilience of foundation seagrass at edge of biogeographical range. Journal of Ecology 102: 1528–1536. [Google Scholar]
- Gill DE, Chao L, Perkins SL, Wolf JB. 1995. Genetic mosaicism in plants and clonal animals. Annual Review of Ecology and Systematics 26: 423–444. [Google Scholar]
- Goebel KE. 1905. Organography of plants. New York: Hafner Publishing Company. [Google Scholar]
- Gordon-Gray KD, Baijnath H, Ward CJ, Wragg PD. 2009. Studies in Cyperaceae in southern Africa 42: Pseudo-vivipary in South African Cyperaceae. South African Journal of Botany 75: 165–171. [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. 2008. Global warming and sexual plant reproduction. Trends in Plant Science 14: 30–36. [DOI] [PubMed] [Google Scholar]
- Honnay O, Bossuyt B. 2005. Prolonged clonal growth: escape route or route to extinction? Oikos 108: 427–432. [Google Scholar]
- Ito Y, Tanaka N. 2011. Hybridization in a tropical seagrass genus, Halodule (Cymodoceaceae), inferred from plastid and nuclear DNA phylogenies. Telopea 13: 219–231. [Google Scholar]
- Ito Y, Ohi-Toma T, Murata J, Tanaka N. 2010. Hybridization and polyploidy of an aquatic plant, Ruppia (Ruppiaceae), inferred from plastid and nuclear DNA phylogenies. American Journal of Botany 97: 1156–1167. [DOI] [PubMed] [Google Scholar]
- Kendrick GA, Fourqurean JWB, Fraser MW, et al. 2012. Science behind management of Shark Bay and Florida Bay, two P-limited subtropical systems with different climatology and human pressures. Marine and Freshwater Research 63: 941–951 [Google Scholar]
- Koce JD, Vilhar B, Bohanec B, Dermastia M. 2003. Genome size of Adriatic seagrasses. Aquatic Botany 77: 17–25. [Google Scholar]
- Kuo J, Kirkman H. 1990. Anatomy of viviparous seagrasses: seedlings of Amphibolis and Thalassodendron and their nutrient supply. Botanica Marina 33: 117–126. [Google Scholar]
- Kuo J, James SH, Kirkman H, den Hartog CKJ. 1990. Chromosome numbers and their systematic implications in Australian marine angiosperms the Posidoniaceae. Plant Systematics and Evolution 171: 199–204. [Google Scholar]
- Les DH, Philbrick CT. 1993. Studies of hybridization and chromosome number variation in aquatic angiosperms: evolutionary implications. Aquatic Botany 44: 181–228. [Google Scholar]
- Les DH, Cleland MA, Waycott M. 1997. Phylogenetic studies in Alismatidae, II: Evolution of marine angiosperms (seagrasses) and hydrophily. Systematic Botany, 22: 443–463. [Google Scholar]
- Levin DA. 2012. Mating system shifts on the trailing edge. Annals of Botany 109: 613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan BW, Cebulski DE. 1970. Sedimentary environments of Shark Bay, Western Australia. In: Logan BW, ed. Carbonate sedimentation and environments, Shark Bay, Western Australia. Tulsa, OK: The American Association of Petroleum Geologists. [Google Scholar]
- Meirmans PG, van Tienderen PH. 2004. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes 4: 792–794. [Google Scholar]
- Merila J. 2012. Evolution in response to climate change: in pursuit of the missing evidence. Bioessays 34: 811–818. [DOI] [PubMed] [Google Scholar]
- Monro K, Poore AGB. 2009. The potential for evolutionary responses to cell-lineage selection on growth form and its plasticity in a red seaweed. The American Naturalist 173: 151–163. [DOI] [PubMed] [Google Scholar]
- O’Connell LM, Ritland K. 2004. Somatic mutations at microsatellite loci in western red cedar (Thuja plicata: Cupressaceae). Journal of Heredity 95: 172–176. [DOI] [PubMed] [Google Scholar]
- Ofir M, Kigel J. 2014. Temporal and intra-clonal variation of flowering and pseudovivipary in Poa bulbosa. Annals of Botany 113: 1249–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce AF, Feng M. 2013. The rise and fall of the “marine heat wave” off Western Australia during the summer of 2010/2011. Journal of Marine Systems 111–112: 139–156. [Google Scholar]
- Pineda-Krch M, Lehtila K. 2004. Costs and benefits of genetic heterogeneity within organisms. Journal of Evolutionary Biology 17: 1167–1177. [DOI] [PubMed] [Google Scholar]
- Prančl J, Kaplan Z, Travnıcek P, Jarolımova V. 2014. Genome size as a key to evolutionary complex aquatic plants: polyploidy and hybridization in Callitriche (Plantaginaceae). PLoS ONE 9: e105997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey J, Schemske DW. 1998. Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Reviews in Ecology and Systematics 29: 467–501. [Google Scholar]
- Reusch TBH. 2000. Pollination in the marine realm: microsatellites reveal high outcrossing rates and multiple paternity in eelgrass Zostera marina. Heredity 85: 459–464. [DOI] [PubMed] [Google Scholar]
- Reusch TBH, Bostrom C. 2011. Widespread genetic mosaicism in the marine angiosperm Zostera marina is correlated with clonal reproduction. Evolutionary Ecology 25: 899–913. [Google Scholar]
- Rozenfeld AF, Arnaud-Haond S, Hernandez-Garcia E, et al. 2007. Spectrum of genetic diversity and networks of clonal organisms. Journal of the Royal Society Interface 4: 1093–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seddon S, Wear RJ, Venema S, Miller DJ. 2005. Seagrass rehabilitation in Adelaide metropolitan coastal waters. II. Development of donor bed independent methods using Posidonia seedlings. Prepared for the Coastal Protection Branch, Department for Environment and Heritage. SARDI Aquatic Sciences Publication No. RD004/0038-2. Adelaide: SARDI Aquatic Sciences. [Google Scholar]
- Sexton JP, McIntyre PJ, Angert AL, Rice KJ. 2009. Evolution and ecology of species range limits. Annual Review of Ecology, Evolution, and Systematics 40: 415–436. [Google Scholar]
- Sinclair EA, Anthony J, Coupland GT, et al. 2009. Characterisation of polymorphic microsatellite markers in the widespread Australian seagrass, Posidonia australis Hook. f. (Posidoniaceae), with cross-amplification in the sympatric P. sinuosa. Conservation Genetics Resources 1: 273–276. [Google Scholar]
- Sinclair EA, Gecan I, Krauss SL, Kendrick GA. 2014a. Against the odds: complete outcrossing in a monoecious clonal seagrass. Annals of Botany 113: 1185–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair EA, Krauss SL, Anthony J, Hovey R, Kendrick GA. 2014b. The interaction of environment and genetic diversity within meadows of the seagrass Posidonia australis (Posidoniaceae). Marine Ecology Progress Series 506: 87–98. [Google Scholar]
- Sinclair EA, Hovey R, Statton J, Fraser MW, Cambridge ML, Kendrick GA. 2015. Comment on ‘Seagrass viviparous propagules as a potential long-distance dispersal mechanism’ by A. C. G. Thomson et al. 2014. Estuaries and Coasts doi:10.1007/s12237-015-9941-7. [Google Scholar]
- Smith SV, Atkinson MJ. 1983. Mass balance of carbon and phosphorus in Shark Bay, Western Australia. Limnology and Oceanography 284: 625–639. [Google Scholar]
- Smith NM, Walker DI. 2002. Canopy structure and pollination biology of the seagrasses Posidonia australis and P. sinuosa (Posidoniaceae). Aquatic Botany 74: 57–70. [Google Scholar]
- Thomson JA, Burkholder DA, Heithaus MR, et al. 2015. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Global Change Biology 21: 1463–1474. [DOI] [PubMed] [Google Scholar]
- Tooke F, Ordidge M, Chiurugwa T, Battey N. 2005. Mechanisms and function of flower and inflorescence reversion. Journal of Experimental Botany, 56: 2587–2599. [DOI] [PubMed] [Google Scholar]
- Walker DI, McComb AJ. 1988. Seasonal variation in the production biomass and nutrient status of Amphibolis antarctica (Labill.) Sonder ex Aschers. and Posidonia australis Hook. f. in Shark Bay, Western Australia. Aquatic Botany 31: 259–276. [Google Scholar]
- Walker DI, Kendrick GA, McComb AJ. 1988. The distribution of seagrass species in Shark Bay Western Australia, with notes on their ecology. Aquatic Botany 30: 305–318. [Google Scholar]
- Wang K, Ding Tang D, Hong L, et al. 2010. DEP and AFO regulate reproductive habit in rice. PLoS Genetics 6: e1000818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waycott M. 1995. Assessment of genetic variation and clonality in the seagrass Posidonia australis using RAPD and allozyme analysis. Marine Ecology Progress Series 116: 289–295. [Google Scholar]
- Waycott M, James SH, Walker DI. 1997. Genetic variation within and between populations of Posidonia australis, a hydrophilous, clonal seagrass. Heredity 79: 408–417. [Google Scholar]
- Zipperle AM, Coyer JA, Reise K, Stam WT, Olsen JL. 2011. An evaluation of small-scale genetic diversity and the mating system in Zostera noltii on an intertidal sandflat in the Wadden Sea. Annals of Botany 107: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.