Abstract
Background and Aims Nectar robbers affect host fitness in different ways and by different magnitudes, both directly and indirectly, and potentially constitute an important part of pollination interactions. The aim of this study was to assess the effect of nectar robbing on several variables that characterize the reproductive success of Lonicera etrusca, a pollinator-dependent plant with long, tubular flowers that produce abundant nectar.
Methods Using fluorescent powder dye as a proxy for pollen, the distance of pollen dispersal was compared for robbed and non-robbed flowers. Artificial nectar robbing treatments were applied to test its effects on four additional measures of reproductive success, namely the quantity of pollen exported, fruit set, seed/ovule ratio and seed weight.
Key Results Nectar robbing was not found to have any significant negative consequences on female and male components of reproductive success as determined through the five variables that were measured.
Conclusions Although L. etrusca exhibits high levels of nectar robbing and nectar robbers are common floral visitors, no evidence was found of detrimental changes in the components of reproductive success. A combination of morphological and ecological mechanisms is proposed to explain how plants may compensate for the energetic loss caused by the nectar robbers.
Keywords: Lonicera etrusca, plant–animal interactions, plant mating sytems, pollination, reproductive success, floral larceny, nectar robber, floral visitor behaviour, pollen donation, fruit set, seed/ovule ratio, seed weight
INTRODUCTION
Since >87·5 % of angiosperms depend on floral visitors for sexual reproduction (Ollerton et al., 2011), mutualistic pollination interactions are key components for the function of terrestrial ecosystems. Therefore, the current decline of diversity and abundance of pollinators is a threat to the stability of pollination services for crops and wild plants (Potts et al., 2010; Burkle et al., 2013). These organisms are part of complex interaction networks and the consequences for plant reproduction and evolution are highly dynamic and context dependent (Gómez et al., 2007; Burkle and Alarcón, 2011). In plant–pollinator interactions, both groups obtain benefits for their fitness (Waser and Price, 1983; Bronstein, 1994). However, the rewards offered to pollinators may also be exploited by other animals that do not offer benefits in return (Bronstein, 2001).
Nectar robbers are animals that use a hole, slit or tear in the perianth to reach the nectar accumulated within a flower (Inouye, 1980, 1983). The strength and direction of the consequences of this behaviour for plant reproductive success depend on complex arrays of diverse factors that vary in time and space (Irwin and Maloof, 2002; Irwin et al., 2010). Some of those factors involve plant mating systems and the level of pollen limitation, as well as the behaviour, morphology and physiology of pollinators and robbers (Maloof and Inouye, 2000; Burkle et al., 2007; Castro et al., 2009; Navarro and Medel, 2009; Zhang et al., 2014). Net effects for plant reproduction range from negative to positive, and in some systems the consequences are considered to be neutral when no significant differences in plant fitness between robbed and non-robbed flowers are observed (Morris, 1996; Maloof and Inouye, 2000).
In most cases, nectar robbers have negative consequences for male or female success of plants through direct and indirect pathways, and are acknowledged as relevant participants altering pollination services (Irwin et al., 2001, 2010; González-Varo et al., 2013). Some negative direct effects include damage to reproductive organs that affect the flower’s function (McDade and Kinsman, 1980; Traveset et al., 1998; Zhang et al., 2007; Milet-Pinheiro and Schlindwein, 2009). In other plants, the production of additional nectar implies an extraordinary effort that reduces resources for fruit and seed production (Navarro, 2001). Also, robbers may lower male success when they cause significant pollen loss during foraging (Navarro, 1999; Navarro et al., 2008; Irwin et al., 2010). The indirect effects encompass changes in the behaviour of pollinators that negatively affect pollen transfer and fruit or seed production. Negative indirect effects involve territorial defence (Roubik, 1982), changes in the visiting behaviour of pollinators that become secondary robbers in the presence of holes made by primary robbers (Inouye, 1983; Roubik et al., 1985), or a decrease in visit frequency and time spent at the flower (Zimmerman and Cook, 1985; Irwin and Brody, 1998; Irwin, 2000). In all these cases, nectar robbing diminishes the quality of the pollination service, causing a reduction in male success, female success or both (Maloof and Inouye, 2000; Burkle et al., 2007; Irwin et al., 2010).
Under certain circumstances, nectar robbing can potentially be positive for plant reproduction. Robbers can contribute directly to pollination when they systematically contact anthers and stigmas during foraging (Higashi et al., 1988; Zhu et al., 2010), and occasionally robbed flowers have higher fruit or seed set (Navarro, 2000; Zhang et al., 2014). Indirectly, robbing may cause the reduction of nectar standing crop that indirectly compels pollinators to increase flying distances among plants, resulting in a potential increase of outcrossing levels in the population (Zimmerman and Cook, 1985). Unfortunately, information about nectar robbers remains very scarce, scattered and limited to narrow geographic areas, making it difficult to decipher common patterns. Hence, a clear-cut distinction between legitimate visitors as beneficial and robbers as detrimental for the plant fitness needs to be carefully re-evaluated.
Lonicera etrusca is a host plant for a diversity of legitimate visitors, but a very high proportion of the total visits are by primary nectar robbers (Jordano, 1990; Guitián et al., 1993). As a result, at the end of the blooming season, nearly all mature flowers have one or more holes made by robbers. Considering such high levels of nectar robbing, a reduction in some of the components of reproductive success would be expected. However, through bagging experiments Guitián et al. (1993) found evidence suggesting that nectar robbing does not affect fruit production of this species. Nevertheless, the consequences for plant fitness were measured only for the female component of success, and no proper experimental manipulation was used to exclude nectar robbers. Because total plant fitness is the result of both female and male functions, it is relevant to measure the impacts of nectar robbing on both sexual functions to understand fully its effect on plant reproduction. Nevertheless, few studies have evaluated the impacts on female and male components simultaneously (Zimmerman and Cook, 1985; Maloof, 2001; Temeles and Pan, 2002; Richardson, 2004). In these cases, divergent consequences for female and male components were found. In this study, we experimentally assess the effects of nectar robbing on several variables used to characterize both components of the plant reproductive success to analyse how nectar robbers affect the reproduction of L. etrusca. Such an understanding is essential to achieve a more complete perspective of the complex interplay among plants, pollinators and robbers.
MATERIALS AND METHODS
Study area
The study was conducted in the El Bierzo region, North-west Spain. Two of the populations studied are located in the Natural Park Serra da Enciña da Lastra: Cobas A (567 m a.s.l.; 42°28'19''N, 6°50'17''W) and Cobas B (438 m a.s.l. 42°28'15''N, 6°49'26''W). A third population is located in La Barosa (590 m a.s.l. 42°29'50''N, 6°48'52''W) and the fourth at Carucedo (520 m a.s.l.; 42°29'6''N, 6°45'59''W). The region has a Mediterranean climate, and the landscape is composed of a mosaic of habitats with cultivated lands and native vegetation, such as holm oak woodland (Quercus ilex, Arbutus unedo and Quercus suber), and different Mediterranean shrubland and pasture communities, many of them growing on former cultivated lands.
Study system
Lonicera etrusca Santi (Caprifoliaceae) is a climbing shrub native to the Mediterranean basin. In the northern Iberian Peninsula region, the blooming period starts in May and finishes in June (Guitián et al., 1993). Plants produce on average 882·6 ± 1529 flowers per year (n = 86; Rojas-Nossa, 2015). Floral buds usually open at dusk and the fragrant flowers last 3 d until the sympetalous corolla falls off. The corolla has a white to pinkish colour at anthesis and changes to yellowish-pinkish from the second day on. The flowers present long tubular corollas (32·9 ± 4·6 mm, n = 761; Rojas-Nossa, 2015). Flowers are hermaphroditic with exerted stigmas and five stamens with exerted anthers. After anther dehiscence, pollen remains in the anthers, but it is easily removed through contact with floral visitors. The stigma is receptive at anthesis, and anther maturation occurs on the following day (Guitián et al., 1993). Flowers produce copious nectar that accumulates at the base of the corolla (4·1 ± 2·9 μL, 23 ± 4·1 % sugar concentration). The fruit is a reddish berry with 5–7 seeds measuring about 5 × 3·5 mm. Based on controlled pollination experiments, Guitián et al. (1993) concluded that the species is self-compatible but has an insect-dependent reproductive system. Levels of nectar robbing of L. etrusca were very high during the study. In spring 2010, up to 90·6 % of the flowers in Cobas A (n = 915), 100 % in Cobas B (n = 40) and 96·1 % in La Barosa (n = 7255) were robbed during the blooming season, and significant damage to reproductive organs was infrequent (Rojas-Nossa, 2015).
The hawkmoth Macroglossum stellatarum is probably the main legitimate pollinator of L. etrusca in the Iberian Peninsula (Jordano, 1990; Guitián et al., 1993). Other legitimate visitors include long-tongued bees and bumble-bees, such as Anthophora hispanica, Anthophora acervorum and Bombus vestalis (Guitián et al., 1993), as well as nocturnal Lepidoptera (e.g. Hyloicus pinastri, Sphinx ligustri, Ochropleura flammatra and Ochropleura forcipula; Jordano, 1990). However, the most common visitors to the flowers of L. etrusca are the bumble-bee Bombus terrestris and the carpenter bee Xylocopa violacea (Guitián et al., 1993). These robust hymenopterans are legitimate visitors of a high diversity of plants, but usually behave as nectar robbers in plants with long flowers or hidden nectar (Navarro, 2000; Castro et al., 2009; Goulson et al., 2013; among others). In L. etrusca, both species are exclusively nectar robbers, opening holes with the mouth structures in the base of the corolla [i.e. primary nectar robbers sensu Inouye (1980)], or eventually use existing perforations [i.e. secondary nectar robbers sensu Inouye (1980)] to introduce the tongue and extract nectar held in the base of the flowers. These holes are also used by small bees that collect pollen and behave as secondary robbers. Besides nectarivorous visitors, some species of Diptera and Hymenoptera collect pollen, but in most cases they do not contact the stigma (Jordano, 1990).
Effects of nectar robbing on male reproductive success: distance of pollen dispersal
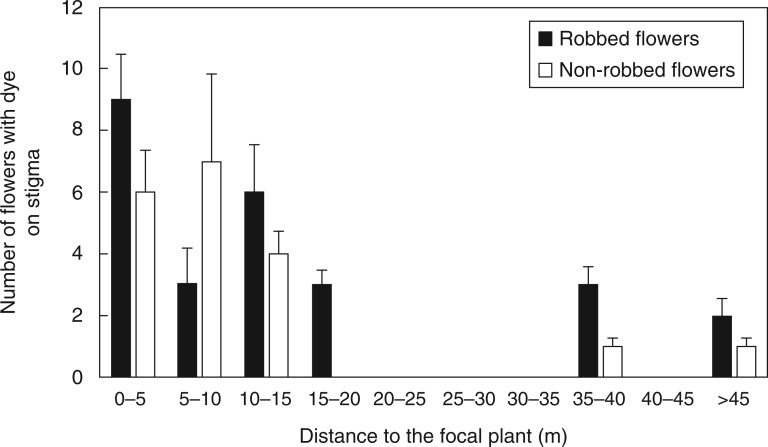
In order to evaluate the effect of nectar robbing on pollen dispersal, we performed an experiment in spring 2011 using fluorescent powdered dyes (Radiant Color, Richmond, CA, USA) as pollen analogues. It has been previously observed that dye transfer closely resembles pollen transfer by insects (see Adler and Irwin, 2006, and references therein). We conducted this experiment in all plants in the population at the Carucedo site. There we chose two focal plants placed in the centre of the population, with a similar size and a high number of floral buds at the same developmental stage 2–3 d before anthesis). We bagged all floral buds present in the focal plants with mosquito netting to prevent visits. When flowers opened, the net was removed and, at each focal plant, two sets of flowers were treated early in the morning every day on three consecutive days (a total of six consecutive days): (1) non-robbed flowers – the corollas of 50 flowers were protected using transparent tape to prevent nectar robbing and pink fluorescent dye was applied on the anthers with a brush; and (2) artificially robbed flowers – the corollas of 50 flowers were manually perforated, nectar was extracted with capillary micropipettes, and yellow fluorescent dye was applied on the anthers. Every day at dusk, we examined all open flowers present in the plants within a radius of 60 m (hereinafter ‘peripheral plants’) from the focal plants using a UV flashlight. For each flower, we recorded the presence of dye as well as the part of the flower where it had been placed. Maximum precision was attained when the pollen substitute was found only on the stigma. Dyes on recipient flowers were carefully removed after each record to avoid recounting the next day. The distance from peripheral plants to the focal plants was measured, and the number of opened flowers on peripheral plants was counted daily. Because we included all plants present in the Carucedo site, 60 m was the maximum distance from the focal plants to any peripheral plant in the population.
Effects of nectar robbing on male reproductive success: quantity of pollen exported
We marked 90 plants in three populations (31 plants in Cobas A, 29 in Cobas B and 30 in La Barosa) at the beginning of the blooming period in 2010. These plants were chosen according to their size and flower production (for practical reasons, all plants with less than eight well-developed floral buds were considered unsuitable for this experiment). Four treatments were applied to floral buds (two flowers per treatment per individual): (1) non-robbed flowers – the corollas were protected from nectar robbing by covering them with transparent plastic tape; (2) robbed flowers – the corolla tube was artificially perforated with a micropipette, approximately in the same way (form and position) as robbers do; (3) mixed treatment – the distal half of the corolla was protected as for treatment (1) and the proximal half was perforated as for treatment (2); and (4) control – unmanipulated flowers. The mixed treatment was useful to analyse whether the presence of the tape used to protect the flowers had an effect on floral visitation. If the properties of the tape (i.e. smell, texture, sheen, etc.) have an effect, the results of the mixed treatment would be different from those of the robbed flowers. Otherwise the results are expected to be similar to the robbing treatment and therefore the non-robbing treatment is reliable. Each treated flower was marked with indelible ink at the base of the calyx so that the mark did not affect the visitation. Threads with different colours were used to facilitate recognition of treated branches within individuals.
To quantify pollen export from flowers under different treatments, anthers were carefully removed 3 d after anthesis by cutting the tip of the filaments to minimize damage to the flower. All anthers of each treated flower were collected and preserved in vials with isotonic solution (ISOTON II Diluent, Beckman Coulter). At the laboratory, anthers were placed on a microscope slide with a drop of ISOTON II, and all pollen grains were manually removed under a magnifying glass. Before analyses, each sample was carefully placed in a plastic vial and immersed in a bath sonicator for 5 min in order to disaggregate the pollen clusters and to detach the grains from any fragments of anther tissue. The pollen sample was filtered with a 100 μm sieve and the volume was maded up to 20 mL with ISOTON II. Pollen grains in 1 mL homogenized sub-samples were counted with a particle counter (Multisizer 3 Coulter Counter, Beckman Coulter). The total number of grains per anther was calculated as the mean of three sub-samples corrected by the dilution factor. The same procedure was performed in anthers of floral buds 1 d before anthesis (90 plants, five buds each), used as a proxy of the pollen contained in freshly opened flowers. The number of exported pollen grains was calculated as the difference between the mean number of grains in anthers of floral buds minus the number of grains remaining in anthers of treated flowers.
Effects of nectar robbing on female reproductive success: fruit set, seed to ovule ratio and seed weight
All the flowers treated in the previous experiment were monitored every 15 d until fruits matured. Mature fruits were collected and kept in 70 % ethanol until dissected in the laboratory. The number of viable seeds, aborted seeds and ovules was counted for each fruit. Seeds that looked viable were extracted from fruits, cleaned and dehydrated in an oven at 50° C until constant weight. Seed weight was measured with an analytical balance (0·01 mg precision).
Analyses
To test the effect of robbing on the distance of pollen dispersal, we used a Generalized Linear Mixed Model (GLMM). We fitted the model using a Poisson error structure and log link function. The model included the treatment (robbed/non-robbed), the distance of the sampled plant to the treated plant, and the interaction term as fixed effects. The response variable was the number of flowers that were found with the respective dye on the stigma per sampled (peripheral) plant. The identity of the sampled plant and the date were included as random effects in the model. The number of flowers inspected per sampled plant (log transformed) was included as an offset term in the model. Prior to fitting the model, we z-transformed the distances to a mean of zero and a standard deviation of one. To determine the overall effect of treatment (i.e. the impact of treatment and/or its interaction with distance), we compared the fit between the full model and the null model (i.e. a model lacking treatment and its interaction with distance but comprising all other effects and terms present in the full model). This comparison was based on a likelihood ratio test. We inspected a mean of 780 ± 158 flowers per day from 40 plants.
To analyse the effect of nectar robbing on the quantity of pollen exported, we followed several steps. First we used a one-way analysis of variance (ANOVA) to compare differences in the quantity of pollen that was present in closed anthers among individuals. Then, we tested differences in pollen production per anther within individuals using a General Linear Model (GLM), including the individual identity as a random effect. Finally, to determine the potential influence of the four treatments (robbed, non-robbed, mixed and control) on the quantity of pollen donated, we used a GLMM. We included the treatment as a fixed effect. Plant identity, population and date were included as random effects. We used the estimated number of pollen grains exported per flower as the response variable. We derived P-values using Markov chain Monte Carlo randomizations (PMCMC). We tested the significance of the random effects by removing them from the full model as described above and compared the fits of the two models using likelihood ratio tests. The sample size for this analysis was 220 flowers from 69 plants of three populations on ten different days.
To analyse the effect of control, mixed, non-robbed and robbed treatments on fruit set, number of viable seeds and total weight of viable seeds, we used GLMM. We included the treatment, plant height, plant diameter (square-root transformed), the total number of flowers produced by the plant (log transformed) and their interaction as fixed effects for fruit set and number of viable seeds models. For the total weight of viable seeds model, we used treatment, total number of flowers produced by the plant (log transformed), tube length and volume of nectar per flower as fixed effects. In all these models, the plant identity was used as a random effect. Since the response for the first model (fruit set) was binary (mature vs. aborted), the model was fitted with binomial error structure and logit link function. The sample size for this analysis was 541 fruits from 64 plants. To analyse the effect of treatments on the number of viable seeds, we used a Poisson error structure and log link function. To control for the number of ovules per ovary, we included it as an offset term (log transformed) in the model. To test specifically for the effect of treatment, we compared the full model with a null model lacking treatment but comprising all other terms in the full model using a likelihood ratio test. The sample size for this analysis was 105 fruits from 46 plants. To analyse the effect of treatments on the weight of the seeds, we used a Gaussian error function and identity link to fit the model. Prior to running the model, we square-root transformed the seed weight and tube length (after subtracting the minimum tube length) and log transformed the total number of flowers per plant and plant volume. Then, we z-transformed the predictors to a mean of zero and a standard deviation of one. The sample size was 87 seeds from 39 plants. All data were included in the analyses. We considered 0·05 as the level of significance. The models were fitted in R software using the packages lme4 (Bates et al., 2011) and languageR (Baayen, 2011).
RESULTS
Distance of pollen dispersion
Both robbed and non-robbed flowers dispersed more dye within the first 10 m than at any other distance (Fig. 1), and the probability of pollen transfer decreased with the distance between treated and sampled plants (estimate ± s.e. = –1·01 ± 0·15, z = –6·76, P < 0·001). The full-null model comparison revealed that the frequency of dye deposition on stigmas of peripheral plants was not influenced by robbing (χ2 = 2·01, d.f. = 2, P = 0·366).
Fig. 1.
Patterns of fluorescent dye transport from robbed and non-robbed flowers as a function of the distance from the focal plant (pollen donor).
Quantity of pollen donated
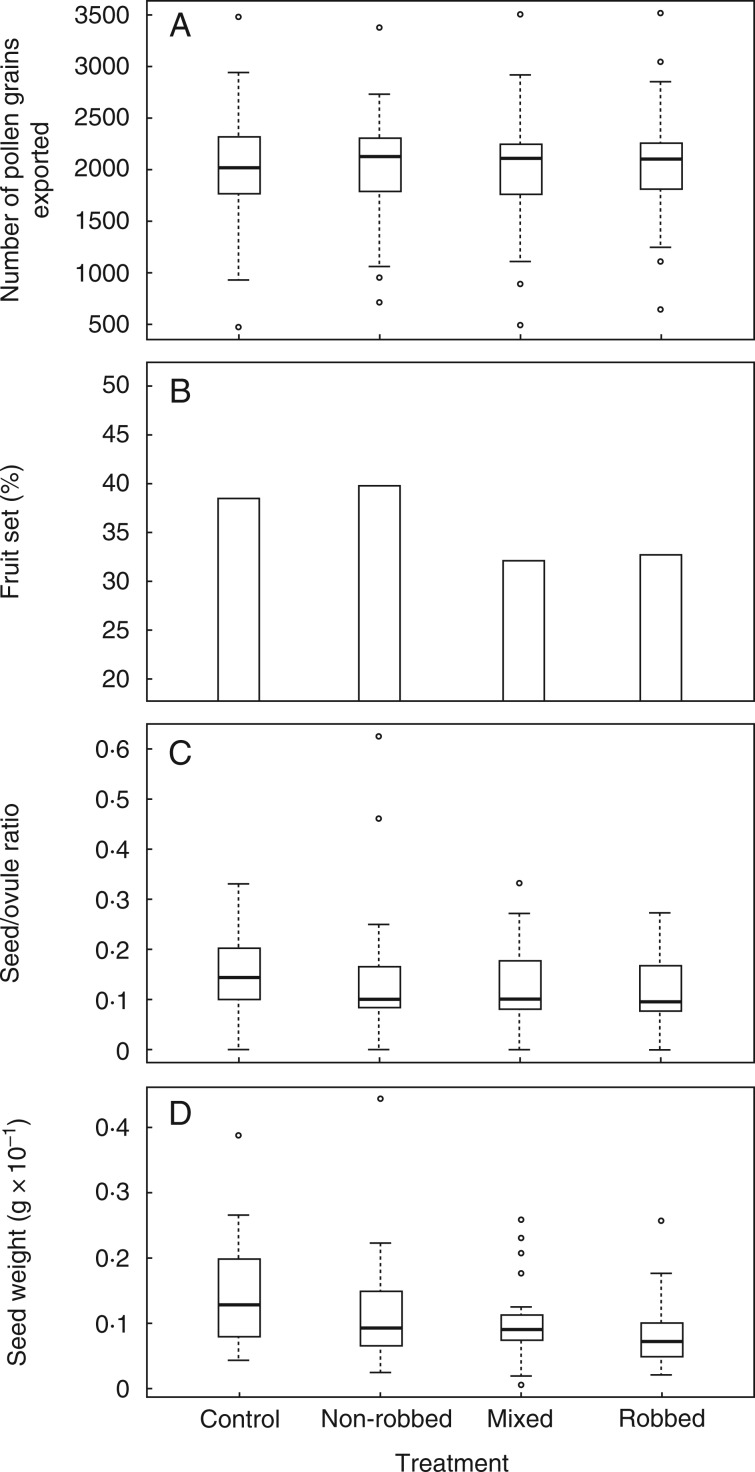
We found differences in the quantity of pollen present in closed anthers among plants (F73, 317 = 3·06, P < 0·001) but not among flowers within plants (F5, 53 = 0·88, P = 0·5). The GLMM revealed that robbed flowers exported similar quantities of pollen in comparison with non-robbed flowers, mixed treatment or control flowers (Fig. 2A, PMCMC = 0·89). Other variables, such as the identity of the individual plant (χ2 = 201·23, d.f. = 1, P < 0·001) and date when the anthers were collected (χ2 = 1651·53, d.f. = 1, P < 0·001), had significant effects on the probability of pollen donation.
Fig. 2.
Effect of different experimental robbing and control treatments on male and female components of the reproductive success of Lonicera etrusca. (A) Quantity of pollen grains exported by the treated flowers. (B) Percentage of fruits produced by the treated flowers. (C) Seed/ovule ratio of the treated flowers. (D) Total dry weight of the viable seeds per fruit from the treated flowers. Whisker plots represent medians (horizontal lines), quartiles (boxes), 2.5–97.5 percentiles (vertical lines) and outliers (open circles).
Fruit set
Fruit set was similar among treatments (χ2 = 2·83, d.f. = 3, P = 0·419). More than 30 % of robbed flowers were able to produce mature fruits (Fig. 2B). The probability that mature fruits were produced was lower in Cobas A than in Cobas B and La Barosa populations (χ2 = 6·01, d.f. = 2, P = 0·049).
Seed/ovule ratio
Although L. etrusca presented an average of 10·6 ovules per flower (s.d. = 1·7, n = 92), most of the fruits produced only one or two viable seeds (1·6 ± 0·8, n = 88). Non-robbed flowers occasionally produced fruits with five and six seeds, but the average seed/ovule ratio was very similar among treatments (Fig. 2C). As for the fruit set, there was no significant effect of the treatment on the number of seeds per fruit (χ2 = 2·30, d.f. = 3, P = 0·51).
Seed weight
Seed weight did not differ significantly among treatments (PMCMC = 0·110; see Fig. 2D).
DISCUSSION
Nectar robbing did not reduce male or female functions in L. etrusca. Robbed flowers have similar patterns of pollen export (in terms of quantity and distance) in comparison with non-robbed flowers. Also, fruit set, seed/ovule ratio and seed weight did not differ between robbed and non-robbed flowers. These results suggest that despite the high levels of nectar robbing occurring in the studied populations, the interaction does not have negative consequences for the reproduction of the plant through direct or indirect mechanisms. We believe that such a ‘neutral’ consequence of nectar robbing is not due to a lack of effects, but rather to the ability of the plant to obtain some benefit from the interaction with robbers.
Only one previous study reported similar effects, referred to by the author as ‘neutral’, for both components of fitness in Mertensia paniculata (Morris, 1996). These results were attributed to the particular behaviour of the bumble-bees, which robbed nectar during the female phase of the flowers but collected pollen during the male phase, performing cross-pollination thanks to this mixed behaviour. Nevertheless, the marked dichogamy present in M. paniculata and the changes in behaviour of robbers observed by Morris (1996) do not occur in L. etrusca. In this species, primary robbers forage exclusively for nectar, and male and female phases overlap for an important part of the flowers’ life (Jordano, 1990; Guitián et al., 1993). Therefore, other mechanisms must be responsible for the patterns observed in our study.
In several pollination systems, nectar robbing diminishes the functionality of flowers by shortening floral life span, causing considerable damage to floral tissues and altering attractiveness to pollinators (Traveset et al., 1998; Temeles and Pan, 2002; Rojas-Nossa, 2007; Zhang et al., 2007). Changes in attractiveness cause avoidance of robbed flowers or plants, or a decrease in visit frequencies by legitimate visitors that reduces pollination services (Zimmerman and Cook, 1985; Irwin and Brody, 1998; Irwin, 2000; Navarro, 2001). As a consequence, female and male components of reproduction are negatively affected, presenting a reduction in the quantity of sired seeds, pollen removal or distance of pollen dispersal (Irwin and Brody, 2000; Zhang et al., 2007; Castro et al., 2008; Irwin et al., 2010). However, since L. etrusca present a self-compatible reproductive system but pollen from another flower of the same or another plant is required to produce fruits and seeds (Guitián et al., 1993), our results suggest that robbed flowers receive a similar pollination service in comparison with non-robbed flowers. This indicates that robbing does not generate a reduction in the functionality of the flowers and that the behaviour of legitimate visitors does not change as a consequence of nectar robbing.
In some systems in which nectar robbers do not cause significant changes in plant reproduction, it is because they often perform pollination as well (Graves, 1982; Arizmendi et al., 1996; Morris, 1996). In those cases, the visiting behaviour of robbers and morphological adjustments between flowers and insects allow effective pollination that maintains or even enhances plant reproductive success (Higashi et al., 1988; Navarro, 2000; Zhu et al., 2010; Zhang et al., 2014). In Mediterranean ecosystems, the primary robbers X. violacea and B. terrestris pollinate the flowers of Anthyllis vulneraria and increase fruit production (Navarro, 2000). These robust bees systematically contact reproductive flower parts while moving from one flower to the next in inflorescences or during nectar robbing. The same could be occurring in L. etrusca, facilitated by the arrangement of the flowers in compact inflorescences that allows the pollination process when the insects crawl between flowers to pierce the base of the corolla and take the nectar. Similarly, in other systems, there appears to be a relationship between pollination by robber birds and the arrangement of flowers in dense inflorescences that allow pollen transfer while the birds pierce the flower and extract nectar (Graves, 1982; Arizmendi et al., 1996; Fumero-Cabán and Meléndez-Ackerman, 2007). Additionally, the robbed flowers of L. etrusca also receive visits from secondary nectar robbers that extract nectar through the perforations made by primary robbers (Jordano, 1990). These visitors (such as Megachile and Lassioglossum bees) commonly forage for nectar and collect pollen from anthers during the same visit. Therefore, the plant could indirectly benefit by primary nectar robbing, because it allows the addition of potential pollen vectors to the system (Zimmerman and Cook, 1985; Morris, 1996; Irwin et al., 2001; Richardson, 2004; Newman and Thompson, 2005). The floral morphology, particularly the exertion of stigma and anthers, might facilitate pollination by a higher diversity of animals than expected by the ‘sphingophilous syndrome’ characteristic of several species of the Lonicera (Miyake and Yahara, 1998; Miyake et al., 1998).
Based on the evidence obtained in this study, we consider that in several systems nectar robbers are an important part of mutualistic plant–animal interactions, and a combination of mechanisms allows plants to compensate for the energetic investment in nectar exploited by robbers. We hypothesize that some of those mechanisms are as follows: (a) nectar robbers do not significantly damage the reproductive structures or reduce flower life span or other components of floral attractiveness as they do in other systems; (b) considering that robbed flowers receive enough cross-pollen to form viable seeds and develop mature fruits, this is an indication that floral functionality and the behaviour of legitimate visitors are not significantly affected by robbers. This could be favoured by the foraging patterns of robbers and legitimate visitors, because robbing is usually performed at midday, after the visits of crepuscular sphingids that occur immediately after anthesis in the late afternoon (Jordano, 1990; Guitián et al., 1993); moreover, (c) the primary robbers (X. violacea and B. terrestris) could also act as pollinators of this plant species. Pollination performed by robbers is facilitated by the arrangement of flowers in inflorescences and exerted reproductive structures that allow contact with the insect’s body during nectar robbing. Finally, (d) primary robbers make a new resource accessible to small bees that behave as secondary nectar robbers and in turn they can contribute to pollination when gathering pollen during the same visit (Newman and Thomson, 2005). Each of these scenarios is plausible, but the feasibility and relative importance of each still needs to be carefully evaluated.
ACKNOWLEDGEMENTS
We are grateful for the valuable assistance of D. Boisits, A. Boisits, V. Ferrero, A. Vale, S. Nicolás and the staff of Parque Natural Serra da Enciña da Lastra during the field work. Comments by J. Karron, M.C. Castellanos, R. Pérez-Barrales, P. Guitián, D. Rojas, F. Maestre and two anonymous referees contributed to improve the manuscript. This work was supported by the University of Vigo [pre-doctoral scholarship to S.V.R.N.], Spanish DGICYT [CGL2009-10466 and CGL2013-45941], FEDER funds from the European Union and the Xunta de Galicia [INCITE09-3103009PR].
LITERATURE CITED
- Adler LS, Irwin RE. 2006. Comparison of pollen transfer dynamics by multiple floral visitors: experiments with pollen and fluorescent dye. Annals of Botany 97: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arizmendi M, Dominguez C, Dirzo R. 1996. The role of an avian nectar robber and of hummingbird pollinators in the reproduction of two plant species. Functional Ecology 10: 119–127. [Google Scholar]
- Baayen RH. 2011. languageR: data sets and functions with ‘Analyzing linguistic data: a practical introduction to statistics’. R package version, 1. https://cran.r-project.org/web/packages/languageR/index.html [Google Scholar]
- Bates D, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes. R package version 0.999375-42. http://CRAN.R-project.org/package=lme4 [Google Scholar]
- Bronstein JL. 1994. Conditional outcomes in mutualistic interactions. Trends in Ecology and Evolution 9: 214–217. [DOI] [PubMed] [Google Scholar]
- Bronstein JL. 2001. The exploitation of mutualisms. Ecology Letters 4: 277–287. [Google Scholar]
- Burkle LA, Alarcón R. 2011. The future of plant–pollinator diversity: understanding interaction networks across time, space, and global change. American Journal of Botany 98: 528–538. [DOI] [PubMed] [Google Scholar]
- Burkle LA, Irwin RE, Newman DA. 2007. Predicting the effects of nectar robbing on plant reproduction: implications of pollen limitation and plant mating system. American Journal of Botany 94: 1935–1943. [DOI] [PubMed] [Google Scholar]
- Burkle LA, Marlin JC, Knight TM. 2013. Plant–pollinator interactions over 120 years: loss of species, co-occurrence, and function. Science 339: 1611–1615. [DOI] [PubMed] [Google Scholar]
- Castro S, Silveira P, Navarro L. 2008. Consequences of nectar robbing for the fitness of a threatened plant species. Plant Ecology 199: 201–208. [Google Scholar]
- Castro S, Silveira P, Navarro L. 2009. Floral traits variation, legitimate pollination, and nectar robbing in Polygala vayredae (Polygalaceae). Ecological Research 24: 47–55. [Google Scholar]
- Fumero-Cabán JJ, Meléndez-Ackerman EJ. 2007. Relative pollination effectiveness of floral visitors of Pitcairnia angustifolia (Bromeliaceae). American Journal of Botany 94: 419–424. [DOI] [PubMed] [Google Scholar]
- Gómez JM, Bosch J, Perfectti F, Fernández J, Abdelaziz M. 2007. Pollinator diversity affects plant reproduction and recruitment: the tradeoffs of generalization. Oecologia 153: 597–605. [DOI] [PubMed] [Google Scholar]
- González-Varo JP, Biesmeijer JC, Bommarco R, et al. 2013. Combined effects of global change pressures on animal-mediated pollination. Trends in Ecology and Evolution 28: 524–530. [DOI] [PubMed] [Google Scholar]
- Goulson D, Park KJ, Tinsley MC, Bussière LF, Vallejo-Marin M. 2013. Social learning drives handedness in nectar-robbing bumblebees. Behavioral Ecology and Sociobiology 67: 1141–1150. [Google Scholar]
- Graves GR. 1982. Pollination of Tristerix mistletoe (Loranthaceae) by Diglossa (Aves, Thraupidae). Biotropica 14: 316. [Google Scholar]
- Guitián P, Guitián J, Navarro L. 1993. Pollen transfer and diurnal versus nocturnal pollination in Lonicera etrusca. Acta Oecologica 14: 219–227. [Google Scholar]
- Higashi S, Ohara M, Arai H, Matsuo K. 1988. Robber-like pollinators: overwintered queen bumblebees foraging on Corydalis ambigua. Ecological Entomology 13: 411–418. [Google Scholar]
- Inouye DW. 1980. The terminology of floral larceny. Ecology 61: 1251–1253. [Google Scholar]
- Inouye DW. 1983. The ecology of nectar robbing. In: Elias TS, Bentley BL, eds. The biology of nectaries. New York: Columbia University Press, 153–173. [Google Scholar]
- Irwin RE. 2000. Hummingbird avoidance of nectar-robbed plants: spatial location or visual cues. Oikos 91: 499–506. [Google Scholar]
- Irwin RE, Brody AK. 1998. Nectar robbing in Ipomopsis aggregata: effects on pollinator behavior and plant fitness. Oecologia 116: 519–527. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Brody AK. 2000. Consequences of nectar robbing for realized male function in a hummingbird-pollinated plant. Ecology 81: 2637–2643. [Google Scholar]
- Irwin R, Maloof J. 2002. Variation in nectar robbing over time, space, and species. Oecologia 133: 525–533. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Brody AK, Waser NM. 2001. The impact of floral larceny on individuals, populations, and communities. Oecologia 129: 161–168. [DOI] [PubMed] [Google Scholar]
- Irwin RE, Bronstein JL, Manson JS, Richardson L. 2010. Nectar robbing: ecological and evolutionary perspectives. Annual Review of Ecology, Evolution, and Systematics 41: 271–292. [Google Scholar]
- Jordano P. 1990. Biología de la reproducción de tres especies del género Lonicera (Caprifoliaceae) en la Sierra de Cazorla. Anales del Jardín Botánico de Madrid 48: 31–52. [Google Scholar]
- Maloof JE. 2001. The effects of a bumble bee nectar robber on plant reproductive success and pollinator behavior. American Journal of Botany 88: 1960–1965. [PubMed] [Google Scholar]
- Maloof JE, Inouye DW. 2000. Are nectar robbers cheaters or mutualists? Ecology 81: 2651–2661. [Google Scholar]
- McDade LA, Kinsman S. 1980. The impact of floral parasitism in two neotropical hummingbird-pollinated plant species. Evolution 34: 944–958. [DOI] [PubMed] [Google Scholar]
- Milet-Pinheiro P, Schlindwein C. 2009. Pollination in Jacaranda rugosa (Bignoniaceae): euglossine pollinators, nectar robbers and low fruit set. Plant Biology 11: 131–141. [DOI] [PubMed] [Google Scholar]
- Miyake T, Yahara T. 1998. Why does the flower of Lonicera japonica open at dusk? Canadian Journal of Botany 76: 1806–1811. [Google Scholar]
- Miyake T, Yamaoka R, Yahara T. 1998. Floral scents of hawkmoth-pollinated flowers in Japan. Journal of Plant Research 111: 199–205. [Google Scholar]
- Morris WF. 1996. Mutualism denied? Nectar-robbing bumble bees do not reduce female or male success of bluebells. Ecology 77: 1451–1462. [Google Scholar]
- Navarro L. 1999. Pollination ecology and effect of nectar removal in Macleania bullata (Ericaceae). Biotropica 31: 618–625. [Google Scholar]
- Navarro L. 2000. Pollination ecology of Anthyllis vulneraria subsp. vulgaris (Fabaceae): nectar robbers as pollinators. American Journal of Botany 87: 980–985. [PubMed] [Google Scholar]
- Navarro L. 2001. Reproductive biology and effect of nectar robbing on fruit production in Macleania bullata (Ericaceae). Plant Ecology 152: 59–65. [Google Scholar]
- Navarro L, Medel R. 2009. Relationship between floral tube length and nectar robbing in Duranta erecta L. (Verbenaceae). Biological Journal of the Linnean Society 96: 392–398. [Google Scholar]
- Navarro L, Guitián P, Ayensa G. 2008. Pollination ecology of Disterigma stereophyllum (Ericaceae) in Southwestern Colombia. Plant Biology 10: 512–518. [DOI] [PubMed] [Google Scholar]
- Newman DA, Thomson JD. 2005. Interactions among nectar robbing, floral herbivory, and ant protection in Linaria vulgaris. Oikos 110: 497–506. [Google Scholar]
- Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120: 321–326. [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution 25: 345–353. [DOI] [PubMed] [Google Scholar]
- Richardson S. 2004. Are nectar-robbers mutualists or antagonists? Oecologia 139: 246–254. [DOI] [PubMed] [Google Scholar]
- Rojas-Nossa SV. 2007. Estrategias de extracción de néctar por pinchaflores (Aves: Diglossa y Diglossopis) y sus efectos sobre la polinización de plantas de los altos Andes. Ornitología Colombiana 5: 21–39. [Google Scholar]
- Rojas-Nossa SV. 2015. Ecological and evolutionary implications of nectar robbing. PhD thesis, University of Vigo, Spain. [Google Scholar]
- Roubik DW. 1982. The ecological impact of nectar-robbing bees and pollinating hummingbirds on a tropical shrub. Ecology 63: 354–360. [Google Scholar]
- Roubik DW, Holbrook NM, Parra GV. 1985. Roles of nectar robbers in reproduction of the tropical treelet Quassia amara (Simaroubaceae). Oecologia 66: 161–167. [DOI] [PubMed] [Google Scholar]
- Temeles EJ, Pan IL. 2002. Effect of nectar robbery on phase duration, nectar volume, and pollination in a protandrous plant. International Journal of Plant Sciences 163: 803–808. [Google Scholar]
- Traveset A, Willson MF, Sabag C. 1998. Effect of nectar-robbing birds on fruit set of Fuchsia magellanica in Tierra del Fuego: a disrupted mutualism. Functional Ecology 12: 459–464. [Google Scholar]
- Waser NM, Price MV. 1983. Optimal and actual outcrossing in plants, and the nature of plant–pollinator interaction. In: Jones C, Little R, eds. Handbook of experimental pollination biology. New York: Van Nostrand Reinhold Company Inc, 341–359. [Google Scholar]
- Zhang YW, Robert G, Wang Y, Guo YH. 2007. Nectar robbing of a carpenter bee and its effects on the reproductive fitness of Glechoma longituba (Lamiaceae). Plant Ecology 193: 1–13. [Google Scholar]
- Zhang YW, Zhao JM, Inouye DW. 2014. Nectar thieves influence reproductive fitness by altering behaviour of nectar robbers and legitimate pollinators in Corydalis ambigua (Fumariaceae). Journal of Ecology 102: 229–237. [Google Scholar]
- Zhu XF, Wan JP, Li QJ. 2010. Nectar robbers pollinate flowers with sexual organs hidden within corollas in distylous Primula secundiflora (Primulaceae). Biology Letters 6: 785–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Cook S. 1985. Pollinator foraging, experimental nectar-robbing and plant fitness in Impatiens capensis. American Midland Naturalist 113: 84–91. [Google Scholar]