Abstract
Background and Aims Floral integration is thought to be an adaptation to promote cross-fertilization, and it is often assumed that it increases morphological matching between flowers and pollinators, increasing the efficiency of pollen transfer. However, the evidence for this role of floral integration is limited, and recent studies have suggested a possible positive association between floral integration and selfing. Although a number of explanations exist to account for this inconsistency, to date there has been no attempt to examine the existence of an association between floral integration and mating system. This study hypothesized that if pollinator-mediated pollen movement among plants (outcrossing) is the main factor promoting floral integration, species with a predominantly outcrossing mating system should present higher levels of floral integration than those with a predominantly selfing mating system.
Methods A phylogenetically informed meta-analysis of published data was performed in order to evaluate whether mating system (outcrossing vs. selfing) accounts for the variation in floral integration among 64 species of flowering plants. Morphometric floral information was used to compare intra-floral integration among traits describing sexual organs (androecium and gynoecium) and those corresponding to the perianth (calix and corolla).
Key Results The analysis showed that outcrossing species have lower floral integration than selfing species. This pattern was caused by significantly higher integration of sexual traits than perianth traits, as integration of the latter group remained unchanged across mating categories.
Conclusions The results suggest that the evolution of selfing is associated with concomitant changes in intra-floral integration. Thus, floral integration of sexual traits should be considered as a critical component of the selfing syndrome.
Keywords: Floral evolution, intra-floral integration, plant mating system, phenotypic integration, selfing syndrome
INTRODUCTION
Floral integration, the magnitude and pattern of covariation among floral parts, has become a model system to understand multivariate patterns of phenotypic evolution (Murren, 2002; Armbruster et al., 2004, 2014; Klingenberg 2008; Ordano et al., 2008; Alcantara et al., 2013). Flowers are particularly well suited for studies of phenotypic evolution because they can be conceived as mechanical devices in which the different parts function together to maximize delivery/receipt of pollen (Faegri and van der Pijl, 1966; Bell, 1985; Fenster et al., 2004; Armbruster et al., 2014). Almost 60 years ago, Berg (1960) proposed that floral integration and the pollination system should be associated as a consequence of the selective pressures promoting successful cross-pollination. She hypothesized that the interaction with specialized pollinators requires precise matching between the pollinator’s body and floral attributes, thus increasing phenotypic integration. In contrast, in her rationale, this selective pressure should be relaxed in species with generalized pollination systems (Armbruster et al., 1999; Gómez et al., 2014; González et al. 2015). Although several attempts have been made to evaluate Berg’s proposal, accumulated evidence is equivocal and we are still far from deciphering the causes of the variation in floral integration (Ordano et al., 2008; Fornoni et al., 2009; Harder, 2009; Bissell and Diggle, 2010; Conner and Lande, 2014; but see González et al., 2015).
Empirical evidence produced during the last decade suggests instead the existence of an association between floral integration and mating system (Pérez et al., 2007; Ferrero et al., 2010; Rosas-Guerrero et al., 2011). Studies comparing species, subspecies or morphs within the genera Ipomoea (Rosas-Guerrero et al., 2011), Schizanthus (Pérez et al., 2007) and Glandora (Ferrero et al., 2010) showed that self-compatible forms express higher levels of floral integration than self-incompatible forms. More recently, Conner and Lande (2014) re-examined Berg’s data and unexpectedly found that correlations among floral traits were lower among species with specialized than generalized pollination systems. Despite the inconsistency between the accumulated empirical evidence and Berg’s expectation (Armbruster et al., 1999; Herrera et al., 2002; Pérez et al., 2007; Meng et al., 2008; Ordano et al., 2008; Bissell and Diggle, 2010; Nattero et al., 2010; Rosas-Guerrero et al., 2011; Baranzelli et al., 2014), there have been no attempts to examine the existence of an association between floral integration and mating system.
The positive relationship between floral integration and selfing may arise for several non-exclusive explanations. (1) If genetic and developmental connections result in high levels of covariation among floral traits, then pollinator-mediated selection favouring cross-pollination may decouple non-functional from functional traits [i.e. promoting intra-floral integration sensu Ordano et al. (2008)], reducing whole-flower integration (Pérez et al., 2007; Baranzelli et al., 2014) (Fig. 1). (2) Linkage disequilibrium derived from recurrent rounds of selfing may also increase genetic correlations among floral traits and consequently floral integration (Pérez et al., 2007; Rosas-Guerrero et al., 2011). (3) Finally, the same rational behind Berg’s hypothesis, that the relative position of the different floral attributes within a flower (floral integration) plays a critical role during cross-pollination (Ganders, 1979; Webb and Lloyd, 1986; Armbruster et al., 2009), can be applied to self-pollination (see Sicard and Lenhard, 2011; Busch and Delph, 2012; Devaux et al., 2014; de Vos et al., 2014). If successful self-fertilization depends on the precise location of the androecium and the gynoecium, then these two attributes should be highly correlated, increasing floral integration (Anderson and Busch, 2006). In accordance with this account, both selective (explanations 1 and 3) and genetic/developmental mechanisms (explanation 2) have been advanced to explain the observation that species with higher prevalence of selfing apparently have higher floral integration than those with a predominantly outcrossing mating system (Pérez et al., 2007; Rosas-Guerrero et al., 2011).
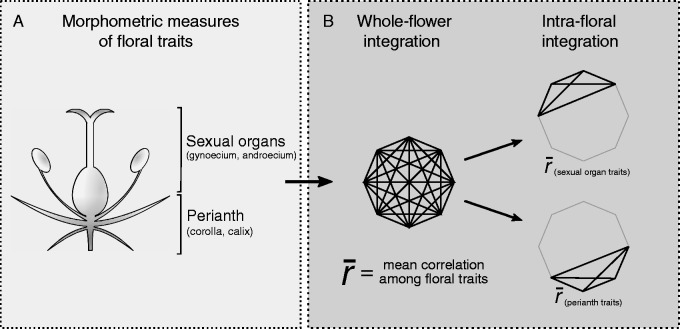
Fig. 1.
Schematic representation of the protocol to estimate floral integration based on morphometric measures of floral traits. (A) The inner whorls of a flower corresponding to sexual organs (gynoecium and androecium), and the outer cycles corresponding to perianth traits (corolla and calix). (B) An illustration of how whole-flower and intra-floral integration are obtained. Polygons represent the correlation matrix among the whole set of floral traits. Vertices represent each trait, and lines connecting vertices indicate estimated correlations. Whole-flower integration is estimated as the mean correlation value among all floral traits. The same rationale is followed to estimate integration for a sub-set of floral traits (intra-floral integration). In this case, intra-floral integration was estimated among sexual organs traits and among perianth traits.
We hypothesized that if pollinator-mediated pollen movement among plants (outcrossing) is the main factor promoting floral integration, species with a predominantly outcrossing mating system should present higher levels of floral integration than those with a predominantly selfing mating system. In contrast, if pollen movement within the flowers is the main factor promoting floral integration, species that experience high levels of selfing should present higher floral integration than outcrossers. Thus, while Berg’s hypothesis highlights the functional value of floral integration to increase the efficiency of pollen movement among plants, the available evidence suggests that pollen movement within the flower could also influence this complex attribute.
In this study, we applied a phylogenetically informed meta-analysis to evaluate a possible association between mating system and floral integration. Specifically, we tested whether species with a predominantly outcrossing mating system have lower floral integration than those with a mixed mating system or high levels of selfing. In addition, because the evolution of selfing can affect the position of sexual organs within the flower (Sicard and Lenhard, 2011), we compared levels of floral integration among these three categories using sub-sets of morphometric floral traits of sexual organs (androecium and gynoecium) and those corresponding to perianth traits (calix and corolla). If correlations among sexual traits condition the occurrence of selfing, higher floral integration in this module should be observed among species that evolved toward selfing than those with an outcrossing mating system (explanation 3). Conversely, if linkage disequilibrium promoted by selfing increases correlations among traits (explanation 2), both the integration among sexual traits and those of perianth traits should be equally higher among species that evolved toward selfing than those with an outcrossing mating system. In addition, if higher floral integration is promoted by linkage disequilibrium driven by selfing, self-compatible species could have higher levels of integration than self-incompatible species.
MATERIALS AND METHODS
Data collection
We performed a literature search using the Thomson Reuters Web of Science online service to obtain all published studies reporting phenotypic correlation matrices of floral traits and/or mean correlation values calculated from them (Supplementary Data References and Table S1). The literature search was performed using the following key word combination: floral, morphology, traits, correlation, phenotypic, genotypic and integration, with active lemmatization for all available document types, languages and years (to August 2014). Reference lists of retrieved studies were examined to detect additional published cases. We used the absolute mean correlation coefficient among all pairs of floral traits as an estimator of the magnitude of floral integration (‘effect size’) (Conner and Sterling, 1995) (Fig. 1), since the correlation coefficient is the unique estimator of phenotypic integration suitable for direct use in meta-analysis. However, previous simulations showed that the mean correlation value of a correlation matrix is strongly and positively correlated with other estimators of phenotypic integration (Ordano et al., 2008; Pavlicev et al., 2009; Haber, 2011). Since we used the estimated integration values at the species level, statistical contrasts are free from non-independence within each floral matrix.
When the mean correlation of floral traits was unavailable, we calculated it from phenotypic or genotypic matrices. When studies reported both genotypic and phenotypic matrices, the former were used to reduce environmental effects on the estimation of mean effect sizes. We excluded information about hybrids and taxa above the species level. In only three cases was graphical presentation digitalized to obtain data. We also added 36 cases for 21 species to the data set using floral correlation matrices from our own field surveys (Supplementary Data Table S2). In those species with more than one floral morph (monoecious, andromonoecious, heterostylous, dioecious, gynodioecious, androdioecious and sub-dioecious), integration values were obtained for each floral morph and then averaged at the species level.
We take into account only those species with integration values obtained from correlation matrices providing information of morphological traits (size and shape of floral organs), including those on the perianth (calix and corolla) potentially involved in attraction, signalling and morphological fit with pollen vectors, and sexual organs traits (androecium and gynoecium) more probably related to efficiency during pick-up/deposition of pollen (e.g. Rosas-Guerrero et al., 2011) (Fig. 1). The final data set only included those cases with at least two traits for each group of floral traits, discarding 65 cases for 37 species. Our final data set consisted of 35 studies published between 1960 and 2014, including 113 cases of mean correlation values obtained from different floral matrices. This data set corresponds to 64 species belonging to 35 families and 20 orders of angiosperms (Supplementary Data References and Table S1). Phenotypic correlations were obtained for 57 species, while genotypic correlations were available for seven species. The majority of species included in the final database were herbs (61 %), followed by trees (16 %), shrubs (8 %), vines (13 %), and geophytes and aquatics (3 %). Perennials comprised 77 % of the species, while 23 % were annuals. Because our sampling unit was the species, when more than one floral matrix was available for a given species, we averaged the mean correlation values derived from each population (n = 22 cases with 2–12 floral matrices per species). Studies in the final data set included matrices with 4–21 different floral traits (median = 7; Table S1).
Information on floral integration was crossed with published evidence of mating system. Species were categorized as mainly outcrossers (hereafter outcrossing species) from those that either combine both mating strategies (hereafter mixed mating species) or have relatively high levels of selfing (hereafter selfing species). When published evidence provided quantitative estimations of outcrossing rates (t) (n = 18 species), species with values >0·8 were assigned to the category of outcrossing while those with outcrossing rates 0·8 > t > 0·2 were assigned to the mixed mating category, and those with outcrossing rates <0·2 were assigned to the category of selfers (Schemske and Lande, 1985; Goodwillie et al., 2005; Winn et al., 2011). When floral integration and mating information was not reported in the same study, the latter was obtained from other published studies for the same species. To assign an outcrossing rate per species, the available outcrossing rates (unilocus and/or multilocus) were averaged (Supplementary Data References and Table S1). When no quantitative information about the mating system was available, the authors’ suggestion combined with data for the incompatibility system, sexual system and pollination mechanism was also used to assign the species to a mating group. For instance, self-incompatible species were considered outcrossers, as were those self-compatible with specific pollinator species and with sophisticated pollination mechanisms (e.g. Calathea crotalifera and Sprekelia formosissima), or those with polymorphic sexual systems that are known to promote outcrossing (e.g. heterostylous species). Self-compatible species with reported evidence that seed production requires the presence of floral visitors, and no clear evidence of high levels of outcrossing were assigned to the category of mixed mating species, while those with no interaction with floral visitors that are able to produce seeds through autonomous self-fertilization were considered as selfers.
Moderator effects
To test the hypothesis related to mating, effect size of floral integration for both perianth and sexual traits was independently compared among the three mating categories (outcrossing, mixed and selfing species). Because self-incompatibility was used as an indicator of a high level of outcrossing, this was also used as a moderator comparing floral integration between self-incompatible and self-compatible species.
Data analysis
Prior to analyses, the mean correlation value for each species was transformed using the Fisher’s z-transformation. All individual z-scores and their corresponding variances were calculated with the package MAc in R 3.1.1 (http://www.R-project.org/). When more than one species was present in the same published study, each species was considered as an independent value in the final data set. Individual effect sizes and variances were estimated depending on the available data reported by the authors (number of plants, 48 species; number of flowers, ten species; number of genetic families, accessions or recombinant inbred lines, six species). When the numbers both of sampled flowers and of plants were reported, we used the number of plants as the sample size in order to reduce the chance of incurring a Type I error. When both phenotypic and genetic correlation data were available, genetic correlations and number of genetic families were used to estimate effect sizes and their variances.
Flower size (Armbruster et al., 2002; Igic et al., 2006; Karron et al., 2012) and number of traits (Ordano et al., 2008, Pavlicev et al., 2009; Haber, 2011) might affect the correlation between traits and the observed level of floral integration. Thus, to avoid the presence of potential biases in our results, a phylogenetic regression model including flower size (log transformed), number of traits and their interaction was performed to test for an effect on phenotypic integration. Phylogenetic general least square analysis (PGLS) was implemented following Revell (2010). This analysis assumes that the residual correlated structure (‘corPagel’ of package ape; Paradis et al., 2004) corresponds to the phylogenetic relationships among species in our meta-analysis. Pagel’s λ was estimated according to a Brownian motion model of evolution where λ = 0 indicates no phylogenetic signal, and λ = 1 indicates a high phylogenetic signal. Additionally, Blomberg’s K statistic was also estimated with the package phytools (http://www.R-project.org/; Blomberg et al., 2003). Results from PGLS indicated no significant effect of flower size or number of traits on floral integration (–0·566 < t < 0·99, 0·325 < P <0·779; Supplementary Data Table S2). Pagel’s λ values for floral integration and flower size were λ = 0·949 (P < 0·0117) and λ = 1·04 (P < 0·0001), respectively. Blomberg’s K values were KFloral integration = 0·379 (P = 0·0077) and KFlower size = 0·736 (P = 0·0001), respectively. These results indicated that both flower size and floral integration expressed phylogenetic inertia, but this effect was stronger for flower size than for floral integration. Overall, the analyses validated the use of a phylogenetically controlled meta-analysis.
The phylogenetically controlled meta-analysis was performed following the method proposed by Lajeunesse (2009), implemented in the program PHYLOMETA 1.3 (Lajeunesse, 2009), coded in R by Scott Chamberlain and Joanna Rifkin. Before each contrast, phylogenetic relationships among species (Supplementary Data Fig. S1) were obtained using the software PHYLOMATIC (Webb and Donoghue, 2005) based on the megatree R20120829 (Stevens, 2001; Webb and Donoghue, 2005; Chase and Reveal, 2009). Adjustments for branch lengths were carried out in Phylocom v. 4.2 (Webb et al., 2008).
Following Gurevitch and Hedges (1999), results from the phylogenetically informed meta-analyses using random effect models for all contrasts are presented. Pooled effect sizes were considered statistically significant if 95 % confidence intervals (CIs) did not overlap with zero (Lajeunesse, 2009). Between-groups χ2 test Qb was used to determine whether explanatory factors (moderators) accounted for a significant amount of heterogeneity among effect sizes (Hedges and Olkin, 1985; Koricheva et al., 2013). Graphics for meta-analyses were made using the metafor package in R (http://www.R-project.org/).
Publication bias
Possible publication biases were examined through the visual inspection of the asymmetry in a funnel plot between effects sizes and sample sizes across studies and by a rank correlation of these two variables (Koricheva et al., 2013). The presence of a significant correlation, reflecting a funnel-shaped distribution of effect sizes, would indicate that studies with small or null effect sizes are less represented or non-existent in the sample of retrieved studies. A Spearman’s rank correlation between effect size and sample size indicated low probability of publication bias on the magnitude of floral integration (r = 0·194, P = 0·125; Supplementary Data Fig. S2). Also the fail-safe number obtained with the metafor package in R (http://www.R-project.org/) indicated that at least >21 734 species with a mean correlation value of zero would need to be added to the data set to nullify the overall effect size. Because the fail-safe number was larger than 5n + 10, where n is the number of studies, publication bias can be safely ignored (Koricheva et al., 2013).
RESULTS
As expected, mean floral integration (z-transformed values) for the whole sample (n = 64 species) differed significantly from zero (mean effect size = 0·448, 95 % CI 0·377–0·518). A higher level of floral integration was detected for selfing than outcrossing species (Qb = 5·39, P = 0·0203), while mixed mating species had an intermediate effect size (Fig. 2A). Comparison of sexual organ integration values revealed significantly higher phenotypic integration among mixed and selfing species than outcrossing species (Qb = 4·34, P = 0·0373) (Fig. 2B). The same contrast for the integration of perianth traits detected no differences between mating categories (Qb = 0·10, P = 0·7573) (Fig. 2C). Despite a small difference on mean effect sizes, levels of floral integration among self-incompatible species were higher (mean = 0·380, 95 % CI 0·279–0·480) than among self-compatible species (mean =0·369, 95 % CI 0·286–0·452) (Qb = 4·97, P = 0·0258).
Fig. 2.
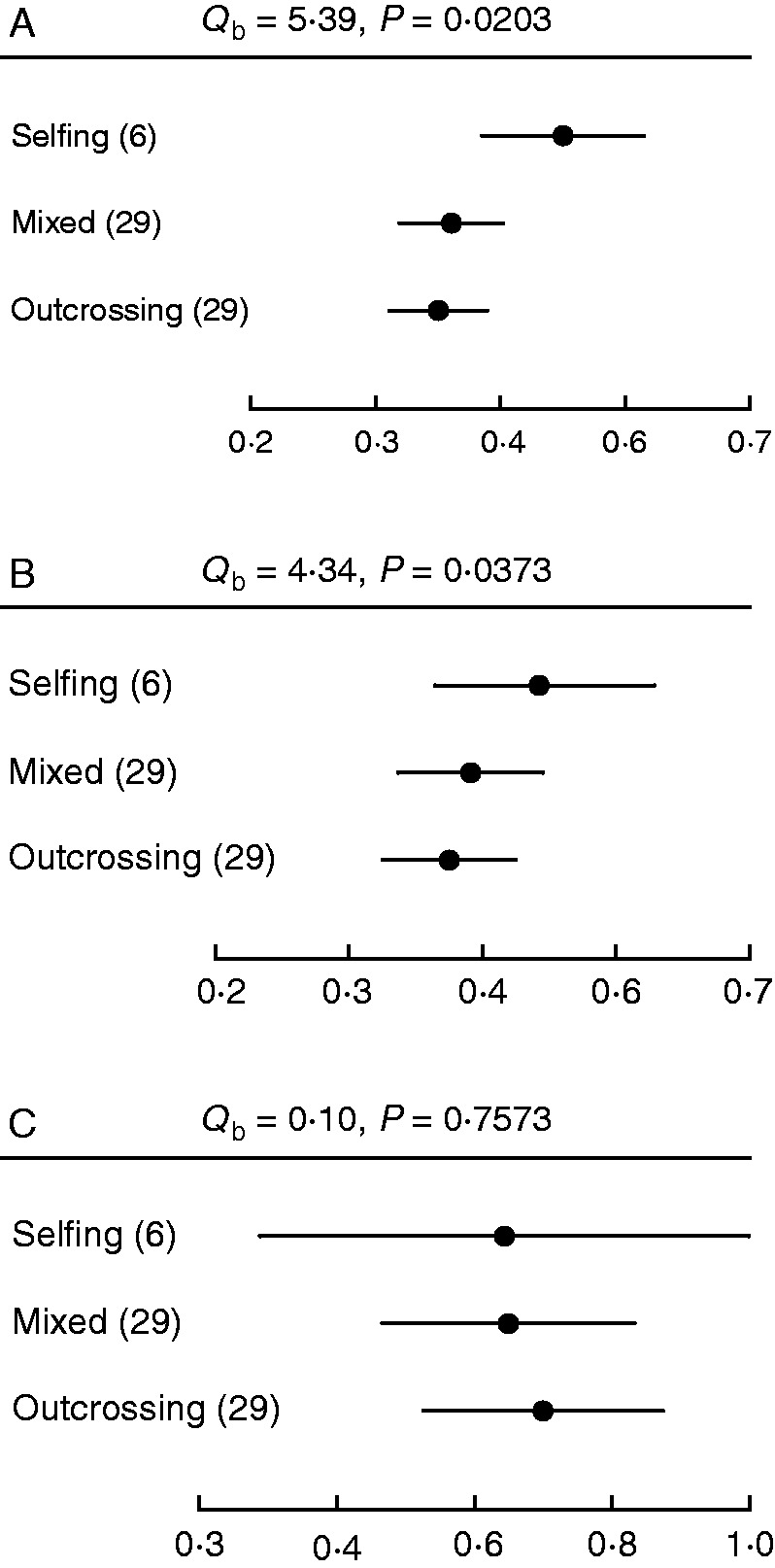
Mean effect sizes of floral integration for selfing, mixed and outcrossing species for the whole set of species surveyed. Bars around the mean denote standard deviations All mean effect sizes were significant since their confidence intervals did not overlap with zero. Numbers in parentheses indicate the sample size for each mating category. (A) Phenotypic integration of the whole set of morphometric floral traits. (B) Phenotypic integration of sexual organs (androecium and gynoecium). (C) Phenotypic integration of perianth traits (calix and corolla).
DISCUSSION
Contrasts among mating categories supported the observation of lower floral integration among outcrossing than among selfing species. This pattern was caused by a significantly lower floral integration among traits of sexual organs in outcrossing than in selfing species, whereas mixed mating species show an intermediate pattern. In contrast, no differences were detected for perianth traits. Our results are consistent with the low phylogenetic signal of floral integration detected in previous studies (Pérez et al., 2007; Rosas-Guerrero et al., 2011; Alcantara et al., 2013) and suggest that intra-floral integration among sexual organs represents an additional complex trait associated with the selfing syndrome.
Independent evidence recorded variation among populations in floral integration and mating (e.g. Pérez-Barrales et al., 2007, 2014; Sosenski et al., 2010; González et al., 2015), but the occurrence of correlated variation remains largely unexplored. Variation among populations in mating system would have increased the variance on the mean effect sizes in our meta-analysis, reducing the power to detect strong differences among mating categories. Although the results of this and previous studies support the presence of an association between floral integration and selfing (Pérez et al., 2007; Ferrero et al., 2010; Rosas-Guerrero et al., 2011), we are still far from understanding the causal mechanism responsible for this pattern. To our knowledge, there are at least three possible explanations (Pérez et al., 2007), a non-functional one proposing that the observed pattern is a by-product of the genetic consequences of the evolution of selfing, and two mutually exclusive functional hypotheses.
Recurrent selfing and linkage disequilibrium increase genetic correlations and floral integration
It has been suggested that selfing species suffer increased levels of linkage disequilibrium among genes (traits), thus producing higher genetic correlations that eventually lead to relatively high values of phenotypic integration (Anderson and Busch, 2006; Pérez et al., 2007). Under this interpretation, the pattern uncovered in the present study would be a non-functional by-product of the increased rates of selfing. Such a mechanism has been proposed to explain the higher levels of floral integration found among self-compatible vs. self-incompatible taxa within the genus Ipomoea (Rosas-Guerrero et al., 2011). In contrast, Anderson and Bush (2006) found that self-compatible Leavenworthia species (presumably exposed to higher levels of selfing) exhibited lower integration in traits associated with pollen export/import (those involved in flower–pollinator interaction) than self-incompatible species. Given that linkage disequilibrium should affect the whole individual’s set of attributes (genes), a potential test of this hypothesis should demonstrate that selfing species express relatively high and equivalent levels of integration within and between floral and vegetative traits. Moreover, if this genetic mechanism accounts for the observed association among selfing and whole-flower integration, a positive relationship should be found between levels of linkage disequilibrium, genetic correlations and floral/vegetative integration. Our results, however, indicated that only a sub-set of traits within the flower expressed an increased level of integration among selfing species. In addition, self-incompatible species had higher levels of floral integration than self-compatible species, providing no support for this hypothesis.
Pollinator-mediated selection decouples functional from non-functional floral traits, reducing whole-flower integration
Based on the original hypothesis of Berg, Pérez et al. (2007) proposed that pollinator-mediated selection should decouple functional and non-functional traits within the flower, thus reducing the whole-flower integration. Their hypothesis assumes that high floral integration, determined by developmental/genetic factors, was the ancestral condition among flowering plants. Then, species experiencing pollinator-mediated selection should express relatively high levels of intra-floral integration (the evolution of floral modularity) and a relatively low floral integration (e.g. Baranzelli et al., 2014). In accordance with this perspective, Ordano et al. (2008) found that intra-floral but not whole-flower integration was the target of selection in Prunus mahaleb and Sorbus torminalis. These authors also proposed that this finding might explain the moderate level of floral integration observed among flowering plants (Ashman and Majetic, 2006). A more recent study on Morrenia brachystephana also indicated that selection promoting intra-floral integration and a decoupling of developmentally related traits accounted for low levels of whole-flower integration (Baranzelli et al., 2014). Hence, the functional hypothesis of Pérez et al. (2007) is based on Berg’s assumption that pollinator-mediated pollen transfer among plants is the critical selective pressure responsible for the evolution of floral integration. Nonetheless, unlike Berg’s perspective, they proposed that pollinator-mediated selection results in lower floral integration. Our results suggest that intra-floral integration was promoted by the evolution of selfing increasing the integration of sexual organs, rather than that of perianth traits. If pollinator-mediated selection reduced whole-flower integration this was not related to a decoupling of perianth traits that are expected to promote morphological matching with pollinators (e.g. Benitez-Vieyra et al., 2009). Hence a definitive test of this second hypothesis will require a direct demonstration that pollinator-mediated selection is promoting decoupling among floral traits reducing floral integration.
Selfing promotes an increment in floral integration because of its fitness benefits
Many studies have emphasized the role of inbreeding depression on the diversification of plant reproductive systems (Lloyd, 1979; Schemske and Lande, 1985; Charlesworth and Charlesworth, 1990; Barrett, 2003). In contrast, few studies have explored the evolutionary consequences of the adaptive advantages of selfing on floral evolution (Baker, 1967; Goodwillie et al., 2005; Karron et al., 2012; de Vos et al., 2014), and no studies have considered the implication for floral integration. Self-fertilization is a derived condition within the Angiosperms (Igic et al., 2006) and has been recognized as an important process influencing the evolution of plant reproduction (Lloyd and Yates, 1982; Lloyd and Webb, 1986; Barrett, 2003; Sicard and Lenhard, 2011; Karron et al., 2012; Busch and Delph, 2012). The evolutionary transition from outcrossing toward selfing is characterized by correlated reductions in herkogamy, flower size and sexual allocation (de Vos et al., 2014). These changes have been interpreted as adaptive adjustments to save resources while increasing self-fertilization (‘selfing syndrome’) (reviewed in Sicard and Lenhard, 2011). Although the position of sexual organs within a flower and their relationship with other floral attributes are known to play a critical role for successful cross-pollination (Ganders, 1979; Webb and Lloyd, 1986; Armbruster et al., 2009), they are also relevant to ensure fertilization when selfing provides fitness benefits (de Vos et al., 2014). A study in the genus Collinsia showed a strong covariance between flower size and time of anther–stigma contact (Armbruster et al., 2002), increasing precision in anther position at the end of flower elongation. This pattern was consistent with the occurrence of delayed self-fertilization, high levels of early herkogamy and outcrossing in species with larger flowers, suggesting that integration of sexual organs can provide fitness benefits through outcrossing and selfing in mixed mating species. Our results suggest that although integration of sexual organs could represent an additional component of the selfing syndrome, ontogenetic changes in integration during the flower life span can improve both outcrossing and selfing.
Overall, our findings add to the traditional view that highly integrated flowers are the consequence of a tight morphological adjustment between floral and pollinator traits to ensure not only outcrossing, but also selfing. Whereas Berg’s hypothesis highlights the functional value of floral integration to increase the efficiency of pollen transfer among plants, both the results from this study and the scarce available evidence suggest that this complex attribute could also be associated with pollen movement within the flower and the evolution of selfing. Future studies should explore the quantitative relationship between (intra)floral integration and outcrossing/selfing rates. Finally, whether high levels of intra-floral integration promoting modularity provide fitness benefits through outcrossing, selfing or both remains to be empirically demonstrated.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: database for meta-analysis. Table S2: unpublished correlation values among floral traits for 21 species. Figure S1: hypothetical phylogenetic relationships among 64 plant species used in the phylogenetically informed meta-analyses. Figure S2: Spearman rank correlation between effect sizes and sample sizes. The absence of a significant correlation (r = 0·194, P = 0·125) indicates low chances of publication bias. References: references cited in the meta-analysis database (Table S1).
ACKNOWLEDGEMENTS
The authors wish to thank Ramiro Aguilar, Lorena Ashworth, Kasey Barton, Andrea Cocucci, Santiago Benítez-Vieyra, Annat Haber, Julia Koricheva, Marc Lajeunesse, Michaela Pavlicev, Fernanda Pérez, Jeffrey Karron, Scott Armbruster and two anonymous reviewers for the criticisms and suggestions that greatly improved the manuscript. The following colleagues also shared unpublished data of their study systems: Matías Baranzelli, Rodrigo Barba González, Facundo Bernacki, Santiago Benitez-Vieyra, Andrea Cosacov, Xochitl Damián, Hiroshi Kudoh, Adriana López-Villalobos, Andrea Panseri, Alicia Sérsic, Paula Sosenski, Mariana Valoy, Petra Wester and Ana Pía Weimer. We thank Salvador Marino for the design and elaboration of Fig. 1. J.F. thanks DGAPA for the scholarship for a sabbatical stay at the Instituto Multidisciplinario de Biología Vegetal (IMBIV) – CONICET – Universidad Nacional de Córdoba, Argentina. This research was supported by grants from the Consejo Nacional de Ciencia y Tecnología (CONACYT 132404) to J.F., C.A.D. and K.B., and (MINCYT-CONACYT MEX 0713) to J.F., and Universidad Nacional Autónoma de México (PAPIIT 221310) to J.F. and (PAPIIT 221210) to C.A.D.
LITERATURE CITED
- Alcantara S, Oliveira FB de, Lohmann LG. 2013. Phenotypic integration in flowers of neotropical lianas: diversification of form with stasis of underlying patterns. Journal of Evolutionary Biology 26: 2283–2296. [DOI] [PubMed] [Google Scholar]
- Anderson I, Busch J. 2006. Relaxed pollinator-mediated selection weakens floral integration in self-compatible taxa of Leavenworthia (Brassicaceae). American Journal of Botany 93: 860–867. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Di Stilio VS, Tuxill JD, Flores TC, Runk JLV. 1999. Covariance and decoupling of floral and vegetative traits in nine neotropical plants: a re-evaluation of Berg’s correlation-pleiades concept. American Journal of Botany 86: 39–55. [PubMed] [Google Scholar]
- Armbruster WS, Mulder CP, Baldwin BG, Kalisz S, Wessa B, Nute H. 2002. Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae sl). American Journal of Botany 89: 37–49. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Pélabon C, Hansen TF, Mulder CH. 2004. Floral integration, modularity, and accuracy: distinguishing complex adaptations from genetic constraints. In: Pigliucci M, Preston K. eds. Phenotypic integration: studying the ecology and evolution of complex phenotypes. New York: Oxford University Press, 23–79. [Google Scholar]
- Armbruster WS, Hansen TF, Pélabon C, Bolstad GH. 2009. Macroevolutionary patterns of pollination accuracy: a comparison of three genera. New Phytologist 183: 600–617. [DOI] [PubMed] [Google Scholar]
- Armbruster WS, Pélabon C, Bolstad GH, Hansen TF. 2014. Integrated phenotypes: understanding trait covariation in plants and animals. Philosophical Transactions of the Royal Society B: Biological Sciences 369: 20130245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman T-L, Majetic CJ. 2006. Genetic constraints on floral evolution: a review and evaluation of patterns. Heredity 96: 343–352. [DOI] [PubMed] [Google Scholar]
- Baker HG. 1967. Support for Baker’s law as a rule. Evolution 21: 853–856. [DOI] [PubMed] [Google Scholar]
- Baranzelli MC, Sérsic AN, Cocucci AA. 2014. The search for Pleiades in traits constellations: functional integration and phenotypic selection in the complex flowers of Morrenia brachystephana (Apocynaceae). Journal of Evolutionary Biology 27: 724–736. [DOI] [PubMed] [Google Scholar]
- Barrett SCH. 2003. Mating strategies in flowering plants: the outcrossing–selfing paradigm and beyond. Philosophical Transactions of the Royal Society B: Biological Sciences 358: 991–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. 1985. On the function of flowers. Proceedings of the Royal Society B: Biolgical Sciences 224: 223–265. [Google Scholar]
- Benitez-Vieyra S, Medina AM, Cocucci AA. 2009. Variable selection patterns on the labellum shape of Goeblasta pennicilatta, a sexually deceptive orchid. Journal of Evolutionary Biology 22: 2354–2362. [DOI] [PubMed] [Google Scholar]
- Berg RL. 1960. The ecological significance of correlation pleiades. Evolution 14: 171–180. [Google Scholar]
- Bissell EK, Diggle PK. 2010. Modular genetic architecture of floral morphology in Nicotiana: comparative phenotypic and quantitative genetic approaches to floral integration. Journal of Evolutionary Biology 23: 1744–1758. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland TJr, Ives AR. 2003. Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57: 717–745. [DOI] [PubMed] [Google Scholar]
- Busch JW, Delph LF. 2012. The relative importance of reproductive assurance and automatic selection as hypotheses for the evolution of self-fertilization. Annals of Botany 109: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MW, Reveal JL. 2009. A phylogenetic classification of the land plants to accompany APG III. Botanical Journal of the Linnean Society 161: 122–127. [Google Scholar]
- Charlesworth D, Charlesworth B. 1990. Inbreeding depression with heterozygote advantage and its effect on selection for modifiers changing the outcrossing rate. Evolution 44: 870–888. [DOI] [PubMed] [Google Scholar]
- Conner JK, Lande R. 2014. Raissa L. Berg’s contributions to the study of phenotypic integration with a professional biographical sketch. Philosophical Transactions of the Royal Society B: Biological Sciences 369: 20130250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner JK, Sterling A. 1995. Testing hypotheses of functional relationships: a comparative survey of correlation patterns among floral traits in five insect-pollinated plants. American Journal of Botany 82: 1399–1406. [Google Scholar]
- Devaux C, Lepers C, Porcher E. 2014. Constraints imposed by pollinator behaviour on the ecology and evolution of plant mating systems. Journal of Evolutionary Biology 27: 1413–1430. [DOI] [PubMed] [Google Scholar]
- Faegri K, van der Pijl L. 1966. The principles of pollination ecology. Oxford: Pergamon. [Google Scholar]
- Fenster CB, Armbruster WS, Wilson P, Dudash MR, Thomson JD. 2004. Pollination syndromes and floral specialization. Annual Review of Ecology, Evolution and Systematics 35: 375–403. [Google Scholar]
- Ferrero V, Arroyo J, Vargas P, Thompson JD, Navarro L. 2010. Evolutionary transitions of style polymorphisms in Lithodora (Boraginaceae). Perspectives in Plant Ecology, Evolution and Systematics 11: 111–125. [Google Scholar]
- Fornoni J, Ordano M, Boege K, Domínguez CA. 2009. Phenotypic integration: between zero and how much is too much. New Phytologist 183: 248–250. [DOI] [PubMed] [Google Scholar]
- Ganders FR. 1979. The biology of heterostyly. New Zealand Journal of Botany 17: 607–635. [Google Scholar]
- Gómez JM, Perfectti F, Klingenberg CP. 2014. The role of pollinator diversity in the evolution of corolla-shape integration in a pollination-generalist plant clade. Philosophical Transactions of the Royal Society B: Biological Sciences 369: 20130257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González AV, Murúa MM, Pérez F. 2015. Floral integration and pollinator diversity in the generalized plant–pollinator system of Alstroemeria ligtu (Alstroemeriaceae). Evolutionary Ecology 29: 63–75. [Google Scholar]
- Goodwillie C, Kalisz S, Eckert CG. 2005. The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annual Review of Ecology, Evolution, and Systematics 36: 47–79. [Google Scholar]
- Gurevitch J, Hedges LV. 1999. Statistical issues in ecological meta-analyses. Ecology 80: 1142–1149. [Google Scholar]
- Haber A. 2011. A comparative analysis of integration indices. Evolutionary Biology 38: 476–488. [Google Scholar]
- Harder LD. 2009. Questions about floral (dis)integration. New Phytologist 183: 247–248. [DOI] [PubMed] [Google Scholar]
- Hedges LV, Olkin I. 1985. Statistical methods for meta-analysis. Orlando, FL: Academic Press. [Google Scholar]
- Herrera CM, Cerdá X, García MB, et al. 2002. Floral integration, phenotypic covariance structure and pollinator variation in bumblebee-pollinated Helleborus foetidus. Journal of Evolutionary Biology 15: 108–121. [Google Scholar]
- Igic B, Bohs JR, Kohn JR. 2006. Ancient polymorphim reveals unidirectional breeding system shifts. Proceedings of the National Academy of Sciences, USA 103: 1359–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karron JD, Ivey CT, Mitchell RJ, Whitehead MR, Peakall R, Case AL. 2012. New perspectives on the evolution of plant mating systems. Annals of Botany 109: 493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg CP. 2008. Morphological integration and developmental modularity. Annual Review of Ecology, Evolution and Systematics 39: 115–132. [Google Scholar]
- Koricheva J, Gurevitch J, Mengersen K. 2013. Handbook of meta-analysis in ecology and evolution. Princeton, NJ: Princeton University Press. [Google Scholar]
- Lajeunesse MJ. 2009. Meta-analysis and the comparative phylogenetic method. American Naturalist 174: 369–381. [DOI] [PubMed] [Google Scholar]
- Lloyd DG. 1979. Some reproductive factors affecting the selection of self-fertilization in plants. American Naturalist 113: 67–79. [Google Scholar]
- Lloyd DG, Webb CJ. 1986. The avoidance of interference between the presentation of pollen and stigmas in Angiosperms. 1. Dichogamy. New Zealand Journal of Botany 24: 135–162. [Google Scholar]
- Lloyd DG, Yates MA. 1982. Intrasexual selection and the segregation of pollen and stigmas in hermaphrodite plants, exemplified by Wahlenbergia albomarginata (Campanulaceae). Evolution 36: 903–913. [DOI] [PubMed] [Google Scholar]
- Meng J-L, Zhou X-H, Zhao Z-G, Du G-Z. 2008. Covariance of floral and vegetative traits in four species of Ranunculaceae: a comparison between specialized and generalized pollination systems. Journal of International Plant Biology 50: 1161–1170. [DOI] [PubMed] [Google Scholar]
- Murren CJ. 2002. Phenotypic integration in plants. Plant Species Biology 17: 89–99. [Google Scholar]
- Nattero J, Sérsic A, Cocucci AA. 2010. Patterns of contemporary phenotypic selection and flower integration in the hummingbird-pollinated Nicotiana glauca between populations with different flower–pollinator combinations. Oikos 119: 852–863. [Google Scholar]
- Ordano M, Fornoni J, Boege K, Dominguez CA. 2008. The adaptive value of phenotypic floral integration. New Phytologist 179: 1183–1192. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. 2004. APE: analyses of phylogenetic and evolution in R language. Bioinformatics Applications Note 29: 289–290. [DOI] [PubMed] [Google Scholar]
- Pavlicev M, Cheverud J, Wagner GÃ. 2009. Measuring morphological integration using eigenvalue variance. Evolutionary Biology 36: 157–170. [Google Scholar]
- Pérez F, Arroyo MTK, Medel R. 2007. Phylogenetic analysis of floral integration in Schizanthus (Solanaceae): does pollination truly integrate corolla traits? Journal of Evolutionary Biology 20: 1730–1738. [DOI] [PubMed] [Google Scholar]
- Pérez-Barrales R, Arroyo J, Armbruster WS. 2007. Differences in pollinator faunas may generate geographic differences in floral morphology and integration in Narcissus papyraceus (Amaryllidaceae). Oikos 116: 1904–1918. [Google Scholar]
- Pérez-Barrales R, Simón-Porcar VI, Santos-Gally R, Arroyo J. 2014. Phenotypic integration in style dimorphic daffodils (Narcissus, Amaryllidaceae) with different pollinators. Philosophical Transactions of the Royal Society B: Biological Sciences 369: 20130258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell LJ. 2010. Phylogenetic signal and linear regression on species data. Methods in Ecology and Evolution 1: 319–329. [Google Scholar]
- Rosas-Guerrero V, Quesada M, Armbruster WS, Pérez-Barrales R, Smith SD. 2011. Influence of pollination specialization and breeding system on floral integration and phenotypic variation in Ipomoea. Evolution 65: 350–364. [DOI] [PubMed] [Google Scholar]
- Schemske DW, Lande R. 1985. The evolution of self-fertilization and inbreeding depression in plants. II. Empirical observations. Evolution 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Sicard A, Lenhard M. 2011. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany 107: 1433–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosenski P, Fornoni J, Molina-Freaner FE, Weller SG, Domínguez CA. 2010. Changes in sexual organ reciprocity and phenotypic floral integration during the tristily–distyly transition in Oxalis alpina. New Phytologist 185: 829–840. [DOI] [PubMed] [Google Scholar]
- Stevens PF. 2001. Angiosperm Phylogeny Website. URL http://www.mobot.org/MOBOT/research/APweb/ [Google Scholar]
- de Vos JM, Wuest RO, Conti E. 2014. Small and ugly? Phylogenetic analyses of the ‘selfing syndrome’ reveal complex evolutionary fates of monomorphic primrose flowers. Evolution 68: 1042–1057. [DOI] [PubMed] [Google Scholar]
- Webb CJ, Lloyd DG. 1986. The avoidance of interference between the presentation of pollen and stigmas in Angiosperms. 2. Herkogamy. New Zealand Journal of Botany 24: 163–178. [Google Scholar]
- Webb CO, Donoghue MJ. 2005. Phylomatic: tree assembly for applied phylogenetics. Molecular Ecology Notes 5: 181–183. [Google Scholar]
- Webb CO, Ackerly DD, Kembel SW. 2008. Phylocom: software for the analysis of phylogenetic community structure and character evolution. Bioinformatics 24: 2098–2100. [DOI] [PubMed] [Google Scholar]
- Winn AA, Elle E, Kalisz S, et al. 2011. Analysis of inbreeding depression in mixed-mating plants provides evidence for selective interference and stable mating. Evolution 65: 3339–3359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.