Abstract
Background and Aims Polyploidy has important effects on reproductive systems in plants and has been implicated in the evolution of dimorphic sexual systems. In particular, higher ploidy is associated with gender dimorphism across Lycium species (Solanaceae) and across populations within the species Lycium californicum. Previous research on the association of cytotype and sexual system within L. californicum sampled a limited portion of the species range, and did not investigate evolutionary transitions between sexual systems. Lycium californicum occurs in arid regions on offshore islands and mainland regions in the south-western United States and Mexico, motivating a more comprehensive analysis of intraspecific variation in sexual system and cytotype across the full range of this species.
Methods Sexual system (dimorphic vs. cosexual) was determined for 34 populations across the geographical range of L. californicum using field observations of pollen production, and was confirmed using morphological measurements and among-plant correlations of primary sexual traits. Ploidy was inferred using flow cytometry in 28 populations. DNA sequence data from four plastid and two nuclear regions were used to reconstruct relationships among populations and to map transitions in sexual system and ploidy.
Key Results Lycium californicum is monophyletic, ancestrally diploid and cosexual, and the association of gender dimorphism and polyploidy appears to have two evolutionary origins in this species. Compared with cosexual populations, dimorphic populations had bimodal anther size distributions, negative correlations between male and female floral traits, and larger coefficients of variation for primary sexual traits. Flow cytometry confirmed tetraploidy in dimorphic populations, whereas cosexual populations were diploid.
Conclusions Tetraploidy and gender dimorphism are perfectly correlated in L. californicum, and the distribution of tetraploid-dimorphic populations is restricted to populations in Arizona and the Baja California peninsula. The analysis suggests that tetraploidy and dimorphism likely established in Baja California and may have evolved multiple times.
Keywords: DNA content, dioecy, gender dimorphism, Lycium californicum, male sterility, plant mating system, polymorphism, polyploidy, sexual system, Solanaceae
INTRODUCTION
The mechanisms underlying the evolution of gender dimorphism, in which sexual function is deployed into two mating types (i.e. males and females), is an important issue in evolutionary biology. Although only a small fraction of plant species have separate sexes, gender dimorphism has evolved repeatedly among unrelated lineages of flowering plants (Geber et al., 1999), inviting an examination of whether similar processes have acted across species to drive the evolution of separate as opposed to combined sexes. Previously, researchers have noted associations between gender dimorphism and genetic factors such as the breakdown of self-incompatibility (SI) systems (Baker, 1959; Charlesworth, 1985; Miller and Venable, 2002) or whole genome duplication (Miller and Venable, 2000; Ashman et al., 2013). Furthermore, taxonomically broad studies examining the origins and ecological correlates of gender dimorphism in plants have shown associations with a variety of features, including abiotic pollination, inconspicuous floral displays, fleshy animal dispersed fruit and harsh environmental conditions (Renner and Ricklefs, 1995; Sakai and Weller, 1999; Vamosi et al., 2003). Unfortunately, the evolution of many of these traits appears tightly correlated, and distinguishing between the causes and effects of dimorphism at this broad scale has thus been challenging. Attention has therefore shifted to investigations at finer taxonomic scales.
Species with gender polymorphism (i.e. both cosexual and gender dimorphic populations) have been the focus of several studies seeking to reconstruct the direction of sexual system transitions and understand the relevant forces leading to their evolution (e.g. Case and Barrett, 2004a, b; Pannell et al., 2004; Alonso and Herrera, 2011; Yakimowski and Barrett, 2014). For instance, a comparison of environmental stress and pollinator visitation in cosexual and dimorphic populations of Wurmbea dioica ssp. alba suggests that the shift from moist to more arid sites leads to changes in pollinator efficacy and ultimately to gender dimorphism in arid conditions (Case and Barrett, 2004a, b). Alonso and Herrera (2011) use a phylogenetic framework in their study of reproductive system evolution among hermaphroditic and gynodioecious populations of Daphne laureola. Their analyses reveal that the ancestral sexual system in this polymorphic species was gynodioecy, and that reversal to hermaphroditism has occurred at least twice. Such intraspecific comparisons of closely related populations that differ in sexual system allow for investigations into the ecology and evolutionary history of gender dimorphism.
A taxonomic group that has emerged as promising for fine-scale studies of the evolution of sexual systems is the genus Lycium (Solanaceae). In Lycium, most species are cosexual, with hermaphroditic plants and flowers, but gender dimorphism (dioecy and gynodioecy) has been described (Miller and Venable, 2000; Venter and Venter, 2003a, b; Venter, 2007; Levin et al., 2015) and is convergent in the genus (Levin et al., 2007). In two species, polymorphism for sexual expression (cosexuality and dimorphism) occurs among populations within species (Yeung et al., 2005; Blank et al., 2014).
Gender dimorphism in Lycium has evolved despite ancestral SI (Richman, 2000; Savage and Miller, 2006) and in association with polyploidy (Miller and Venable, 2000; Levin et al., 2007, 2015; Blank et al., 2014). Miller and Venable (2000) proposed a pathway in which polyploidy disrupts ancestral SI, which leads to increased selfing and inbreeding depression, and the subsequent establishment of gender dimorphism as a secondary outcrossing mechanism. Comparative data in Lycium are consistent with this pathway. Of the Lycium species for which ploidy and sexual system information is available, the majority are diploid and hermaphroditic (approx. 82 % of species), whereas at least ten species are both polyploid and gender dimorphic. Two additional species are polymorphic for both ploidy and sexual system. One of these, L. carolinianum, serves as the only known exception to the association between polyploidy and dimorphism. Gynodioecy has recently been reported for some diploid populations of L. carolinianum from Hawaii; however, dimorphism in this species also occurs with tetraploidy in the Yucatán (Blank et al., 2014).
In contrast, the strong association of polyploidy and dimorphism is apparently maintained at the intraspecific level within the North American species Lycium californicum. Miller and Venable (2002, 2003) originally described this species as dimorphic and tetraploid based on controlled pollinations and morphological characterization of mating types in a population from Arizona, near the northern edge of the species range. The authors documented that although populations were morphologically gynodioecious (i.e. consisting of females and hermaphrodites), female plants retained vestigial anthers and hermaphrodites were functionally male. A subsequent study by Yeung et al. (2005) documented both diploid and tetraploid populations and determined the sexual system for a subset of these: five populations were cosexual and diploid, whereas three were dimorphic and tetraploid. To date, however, only a limited portion of the range of L. californicum has been sampled, and transitions have not yet been investigated in a phylogenetic context. Furthermore, the discovery of geographical variation in the association between ploidy and dimorphism in L. carolinianum (Blank et al., 2014) motivates more comprehensive sampling of cytotype and sexual system across the range of L. californicum.
Here, we examine the pattern and processes underlying the association between gender dimorphism and ploidy among populations spanning the entire species range (including those along the range margins) of L. californicum. In this study, we (1) characterize the sexual system of populations using field observations and morphological measurements of primary sexual characters to document the full geographical range of gender dimorphism in this species and (2) assess cytotype for individual plants in populations to determine if the association between sexual system and cytotype is uniformly maintained. We also use chloroplast and nuclear sequence data to (3) confirm the monophyly of L. californicum and (4) reconstruct the number of times gender dimorphism and polyploidy have evolved and the locations in which dimorphism likely originated.
MATERIALS AND METHODS
Species range and population sampling
Lycium californicum ranges from south-central Arizona through coastal Sonora and into Sinaloa in mainland Mexico, and from southern California to the tip of Baja California Sur, Mexico. In addition, this species is found in a disjunct region of mainland Mexico in the north-central states of Coahuila, Zacatecas, Nuevo León and San Luis Potosí (Fig. 1). The species is distinguished by having a two-seeded drupaceous fruit, which is uncommon in the genus and unique among North American Lycium. Individuals from 34 populations spanning the entire species range were included in the present study (Table 1), including seven populations included in Yeung et al. (2005). Twenty-seven populations were newly sampled, including 11 populations from the length of the Baja California peninsula (to the southern range limit), five from the Channel Islands of California and three from mainland California and Arizona (at the northern and western range limits), six populations in mainland Mexico along the Gulf of California, and two interior populations at the eastern species range limit in Coahuila and Zacatecas (Fig. 1).
Fig. 1.

Populations of Lycium californicum included in the present study (see also Table 1); open circles represent diploid, cosexual populations and closed circles tetraploid, dimorphic populations (see Results). Arrows indicate one cosexual (SMI) and one dimorphic (CDC) population with unknown ploidy. The species range (indicated by dashed lines) was taken from Chiang-Cabrera (1981) and includes southern California (including the Channel Islands), the Baja California peninsula, south-central Arizona, and mainland Mexico along the Gulf of California and in several interior states. Note that this species is found on both the mainland and offshore islands.
Table 1.
Populations, locations, sexual strategy, cytotype and haplotypes for Lycium californicum in the present study (see also Fig. 1). Within sampling regions, populations are listed from northern to southern latitudes. Letters in the haplotype column indicate those haplotypes isolated from individuals in that population. An ‘x’ in the last column indicates that the population was included in phylogenetic analyses
| Population | Latitude (°N) | Longitude (°W) | Sexual strategy | Cytotype |
Haplotype* | Phylogeny† | ||
|---|---|---|---|---|---|---|---|---|
| Inferred ploidy | DNA content (pg) | n | ||||||
| Arizona | ||||||||
| PX | 33·276 | –111·980 | Dimorphic | 4x | 6·29 ± 0·15 | 7 | J, N | x |
| ST | 33·164 | –111·785 | Dimorphic | 4x | 6·46 ± 0·04 | 4 | J, L, N | x |
| HR | 32·780 | –111·630 | Dimorphic | 4x | 5·93 ± 0·06‡ | – | N | x |
| OP | 31·958 | –112·940 | Cosexual | 2x | 3·15 ± 0·07‡ | – | Q, U | x |
| California, Channel Islands | ||||||||
| SCI | 34·034 | –119·520 | Cosexual§ | 2x | 3·23 ± 0·02 | 3 | A | – |
| SMI | 34·020 | –120·323 | Cosexual§ | – | – | – | A | – |
| SBI | 33·476 | –119·034 | Cosexual | 2x | 3·15 ± 0·10 | 20 | A, Q | x |
| CAT | 33·428 | –118·506 | Cosexual | 2x | 3·10 ± 0·09 | 26 | Q | – |
| SNI | 33·224 | –119·441 | Cosexual | 2x | 3·04 ± 0·05 | 12 | A, Q | – |
| California, mainland | ||||||||
| LCB | 34·044 | –118·937 | Cosexual | 2x | 3·09 ± 0·02 | 8 | Q, S | – |
| LAJ | 32·875 | –117·250 | Cosexual | 2x | 3·05 ± 0·02 | 10 | Q | x |
| Mexico, Baja California peninsula | ||||||||
| SSC | 30·879 | –116·093 | Cosexual | 2x | 3·11 ± 0·07 | 3 | – | – |
| VP | 30·297 | –115·805 | Cosexual§ | 2x | 3·04 ± 0·04 | 9 | – | – |
| NCR | 30·198 | –115·791 | Cosexual | 2x | 2·78 ± 0·33‡ | – | Q, B | – |
| ND | 30·086 | –115·647 | Dimorphic | 4x | 6·05 ± 0·01 | 3 | J, M, N, P | x |
| EIR | 30·086 | –115·689 | Cosexual | 2x | 3·00‡ | – | – | – |
| MF | 29·990 | –115·218 | Cosexual | 2x | 3·20 ± 0·20 | 10 | A, Q | – |
| CDM | 29·607 | –114·583 | Dimorphic | 4x | 6·08 ± 0·07 | 15 | N, O, P | – |
| BLA | 29·045 | –113·926 | Cosexual | 2x | 3·02 | 1 | A, Q, B | x |
| ROS | 28·554 | –114·059 | Dimorphic | 4x | 6·03 ± 0·01 | 3 | N, P | x |
| DU | 28·452 | –114·037 | Cosexual | 2x | 3·15 ± 0·15 | 7 | – | – |
| GN | 27·968 | –114·073 | Cosexual | 2x | 3·71 ± 0·11¶ | 5 | A, Q, B | x |
| CI | 25·216 | –111·760 | Dimorphic | 4x | 7·42 ± 0·13¶ | 6 | Q, M | x |
| CDC | 25·045 | –111·713 | Dimorphic§ | – | – | – | A, J, K | x |
| PB | 24·312 | –110·314 | Dimorphic | 4x | 7·74 ± 0·13¶ | 3 | J, M | – |
| IHC | 23·402 | –110·198 | Dimorphic | 4x | 6·89 ± 0·57¶ | 3 | J | x |
| Mexico, mainland | ||||||||
| PL | 29·881 | –112·642 | Cosexual§ | 2x | 3·24 ± 0·14 | 5 | Q, B, T | x |
| KB | 28·831 | –111·778 | Cosexual§ | 2x | 3·12 ± 0·08 | 9 | Q, R | x |
| GY | 27·951 | –110·802 | Cosexual | 2x | 3·14 ± 0·09 | 11 | Q | – |
| GS | 27·907 | –110·573 | Cosexual§ | 2x | 3·06 ± 0·06 | 6 | – | – |
| LL | 27·284 | –102·887 | Cosexual | 2x | 3·18 ± 0·11 | 11 | C, H, I | x |
| LB | 26·713 | –109·290 | Cosexual§ | 2x | 3·15 ± 0·06 | 12 | Q | x |
| SB | 26·653 | –109·225 | Cosexual | 2x | 3·21 ± 0·14 | 10 | – | – |
| PR | 24·700 | –101·250 | Cosexual | 2x | 3·02 ± 0·12 | 8 | D, E, F, G | x |
*An average of 5·1 (range 3–6) individuals per population were included in the haplotype network.
†One to three individuals per population were included in the phylogenetic study.
‡DNA content taken from Yeung et al. (2005).
§Sexual strategy determined in field only.
¶DNA content estimated using silica-dried leaf material.
Ploidy level assessment by flow cytometry
In total, 230 individuals from 28 populations (mean 8·2, range 1–26 individuals) were assessed for cytotype using flow cytometry (Table 1). Individuals from three populations (LAJ, SSC and ND) that were previously included in Yeung et al. (2005) were re-sampled and ploidy re-estimated to compare DNA content estimates between studies. For most samples, young leaf material was collected and kept on ice until processed. However, fresh leaves were not available for four populations (GN, CI, PB and IHC) and silica-dried material was substituted. DNA content was estimated on a BD FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Nuclei from individual Lycium plants and from an internal standard (either Sorghum bicolor ‘Pioneer 8695’ or Zea mays ‘CE-777’) were isolated from leaves (five Lycium leaves, 0·5–1 cm in length) by co-chopping with a fresh razor blade in 0·7 mL of modified De Laat buffer (including 0·25 mmol L−1 PVP-40; De Laat and Blaas, 1984; Bino et al., 1992) with 100 µg mL–1 of the DNA-selective dye propidium iodide, and 50 µg RNase. After staining for 20–45 min, samples were run for 3–8 min on the flow cytometer at low speed so as to acquire >1000 nuclei for G1 peaks of Lycium and the standard. Integrated fluorescence (fluorescence area) of nuclei was measured with a 585/42 nm photodetector, using Cell Quest Pro 4.0 software (1996, BD Biosciences). Peak means, coefficients of variation (CV) and nuclei numbers were estimated using ModFit Lt software (Verity Software House, Topsham, ME, USA). DNA content of each sample was measured as the ratio of the Lycium 2C mean fluorescence value to the 2C fluorescence value of the standard multiplied by the known DNA content of the standard [S. bicolor ‘Pioneer 8695’, 2C = 1·74 pg (Johnston et al., 1999); Zea mays ‘CE-777’, 2C = 5·433 pg (Lysak and Dolezel, 1998)]. Information on chromosome counts for L. californicum from Chiang-Cabrera (1981) and comparison with Yeung et al. (2005) and Blank et al. (2014) was used to infer ploidy from DNA content.
Sexual system and mating type variation
We assessed the sexual system of populations (cosexual or dimorphic) and the sexual mating type of individual plants (hermaphroditic in cosexual populations; female or male in dimorphic populations) using field observations of pollen production in plants. For the majority of populations, we then compared floral morphological measurements among plants of different mating types (see below). We collected flowers during January–March in 2004–2005 and 2009–2010 from populations in mainland Mexico (GY, LL, SB, PR), Arizona (PX, ST, HR, OP), California (including the Channel Islands; LCB, LAJ, SBI, CAT, SNI) and the Baja California peninsula (SSC, NCR, ND, EIR, MF, CDM, BLA, ROS, DU, GN, CI, PB, IHC). Two populations (OP and EIR, Table 1) included here were estimated as diploid by Yeung et al. (2005) but were not characterized for sexual system in that study. In addition, five populations (three cosexual: NCR, LAJ, SSC; two dimorphic: HR, ND) were reassessed in the present study to confirm the sexual system classification in Yeung et al. (2005). We measured five floral traits (anther length, anther width, style length, stigma width, ovary length) using a Nikon SMZ800 stereoscopic microscope equipped with an ocular micrometer. In total, 2240 flowers from 463 plants in 26 populations were measured (mean 4·8 flowers per plant, mean 13·3 plants per population).
Previous studies of four species of Lycium with gender dimorphic populations (including L. californicum) documented significantly smaller anthers that lacked pollen in flowers on female plants compared with those on either hermaphrodites or functional males (Miller and Venable, 2003; Yeung et al., 2005; Levin et al., 2015). Thus, we examined anther size (length and width) using frequency distributions and general linear mixed models using the restricted maximum likelihood (REML) method as implemented in JMP version 10 (SAS Institute, Cary, NC, USA). Models included the fixed effects of sexual system (cosexual or dimorphic) and mating type (hermaphrodite, female or male) nested within sexual system, and the random effect of population nested within sexual system. For traits with a significant effect of mating type nested within sexual system, we used Tukey’s honestly significant difference (HSD) tests to assess mating type differences between females and males in dimorphic populations. Models were similarly conducted for the remaining three floral traits (stigma width, style length and ovary length).
A second approach tested the hypothesis that dimorphic populations would have significantly negative among-plant correlations between male (anther length and anther width) and female (stigma width, style length, and ovary length) floral traits, as shown by Yeung et al. (2005). Trait measurements for individual plants were averaged (mean 4·8 flowers per plant), and among-plant pairwise correlations for floral traits were determined in JMP (n = 227 and 236 for plants in cosexual and dimorphic populations, respectively). Finally, for individual traits, we calculated the CV for populations and used non-parametric Mann–Whitney U tests to determine whether dimorphic populations had greater CVs compared with cosexual populations, as would be predicted with sexual dimorphism.
Haplotype network and diversity
Four plastid regions (trnH-psbA, trnDGUC–trnTGGU, rpl32–trnLUAG and ndhF–rpl32) were amplified and sequenced in 142 individuals from 28 populations (18 cosexual, ten dimorphic) following protocols in Miller et al. (2009); see Supplementary Data Table S1 for GenBank accession numbers. Sequences were edited and assembled in Sequencher version 4.8 (Gene Codes Corp., Ann Arbor, MI, USA, 1991–2007) and aligned manually using SeAl version 2.0a11 (Rambaut, 1996–2002). We used the statistical parsimony method implemented in TCS version 1.21 (Clement et al., 2000) to estimate haplotypes and haplotype outgroup probabilities, and to infer a haplotype network. Mutational steps with 95 % confidence limits connected haplotypes and the option for gaps was set to missing. Insertion/deletion events greater than a single base pair in length (12 indels; range 5–23 bp) were coded separately as additional characters. The number and distribution of haplotypes and haplotype diversity (Hd) were calculated using DNAsp version 5.10.01 (Rozas and Rozas, 1995) and an analysis of molecular variance (AMOVA, as implemented in Arlequin version 3.5.1.2; Excoffier and Lischer, 2010) that partitioned haplotype variation at several hierarchical levels: between cosexual and dimorphic populations, among populations within sexual systems and within populations.
Phylogenetic analysis and ancestral state reconstruction
For a subset of individuals (25 individuals from ten cosexual and eight dimorphic populations, see Table 1) included in the plastid-only analyses, two nuclear regions (COS16 = C2_At1g78690 and COS30 = C2_At3g21610; Wu et al., 2006) were amplified and cloned following Levin et al. (2009). Cleaned amplification products were sequenced at Retrogen (San Diego, CA, USA) using vector primers (U19 and R20). Sequences were edited and assembled in Sequencher version 4.8 (Gene Codes, 1991–2007) and aligned manually across species and populations using SeAl version 2.0a11 (Rambaut, 1996–2002). In total, 158 clones for COS16 and 235 clones for COS30 were sequenced, with an average of 8·2 colonies per individual. A mean of 1·7 alleles was retrieved for diploid individuals compared with a mean of 2·3 alleles for tetraploids. Due to computational constraints, for each individual, up to two (in one case, three) alleles that maximized allelic diversity were included in phylogenetic analyses (Supplementary Data Table S1).
The two nuclear datasets plus a subset of the plastid data were analysed simultaneously using Bayesian Estimation of Species Trees (BEST version 2.2; Liu, 2008), which estimates a posterior distribution of species trees based on distributions of gene trees. In addition to 25 L. californicum individuals, five additional species of Lycium were included (including all species allied to L. californicum in previous studies) plus the outgroup species Nolana werdermannii (Levin and Miller, 2005; Levin et al., 2007, 2009). See Table S1 for complete taxon sampling and GenBank accession numbers. For the BEST analysis, each genomic region had its own substitution model [Nst = 6 for all regions, rates = gamma for COS16 and plastid, rates = equal for COS30; estimated using the Akaike Information Criterion in Modeltest version 3.7 (Posada and Crandall, 1998)]. The analysis included two independent runs, each with four Markov chains, 80 million generations with trees sampled every 1000 generations, and a temperature of 0·15. As both sexual system and cytotype are treated as population-level traits (cytotype did not vary within populations, see Results), this analysis was constrained such that individuals belonging to the same population were monophyletic. A consensus of the estimated distribution of species trees was constructed in BEST using the sumt command and a burn-in of 12·5 % of the trees from each run.
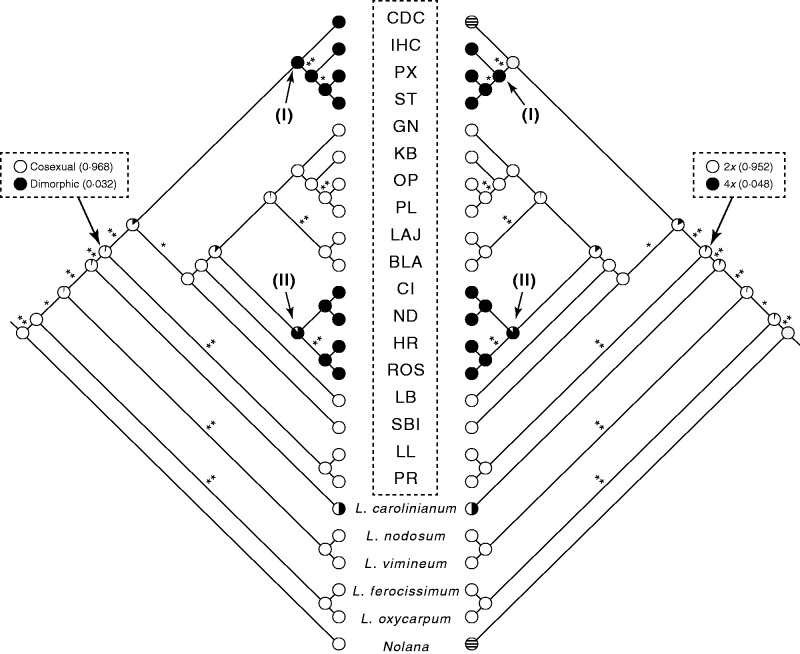
The resulting topology was used to (1) assess the monophyly of L. californicum, (2) reconstruct the likely ancestral character states for sexual system and cytotype, and (3) assess the association of sexual system and cytotype. Maximum-likelihood ancestral state reconstructions were conducted for sexual system and cytotype in Mesquite version 3.01 (Maddison and Maddison, 2014). Both traits were coded as binary characters (sexual system: cosexual, 0 and dimorphic, 1; cytotype: diploid, 0 and tetraploid, 1) and reconstructed using the Markov k-state one parameter (Mk1) stochastic model; transitions between states were assumed to be equally likely.
RESULTS
Ploidy level assessment by flow cytometry
2C DNA content values estimated in the present study (230 individuals from 28 populations) ranged from 2·80 to 8·01 pg and were distributed bimodally (i.e. there were no estimates between 3·86 and 5·95 pg). The first cluster included 186 individuals in 20 populations with an average DNA content of 3·14 ± 0·15 pg (mean ± s.d.) and is consistent with previous DNA content estimates for diploid species (3·06 pg in L. andersonii, Yeung et al., 2005) or diploid populations (3·23–3·33 pg in L. carolinianum, Blank et al., 2014); thus, these samples are inferred to be diploid. By contrast, the second group contained 44 individuals from eight populations and the average 2C DNA content was roughly twice that of the former group (6·49 ± 0·60 pg); these samples were inferred to be tetraploid. Within clusters, DNA content estimates using silica-dried leaves were approx. 17 % higher than those using fresh leaf tissue, but this difference did not affect ploidy inference.
There was no variation in ploidy detected among individuals within populations. Both diploid and tetraploid populations occur in Arizona and in the Baja California peninsula, whereas only diploid populations are known from California (including the Channel Islands), and mainland Mexico (Table 1; Fig. 1). DNA content measures and inferences of ploidy in three populations (LAJ, SSC, ND) analysed independently by Yeung et al. (2005) and repeated in this study are completely consistent.
Sexual system and mating type variation
Mating type explained a significant amount of variation for all floral traits (F ≥ 21·0, P < 0·0001; Table 2A) and Tukey’s HSD tests revealed significant sexual dimorphism between males and females for all traits (Table 2B). In dimorphic populations (n = 9), anthers were nearly twice as large in flowers from male as compared with female plants [anther length: 1·13 ± 0·03 mm (mean ± s.e.) vs. 0·58 ± 0·04 mm, see Supplementary Data Fig. S1; anther width: 0·50 ± 0·02 vs. 0·27 ± 0·02 mm; Fig. 2A]. By contrast, flowers on female plants had significantly wider stigmas and longer styles compared with males (stigma width: 0·53 ± 0·03 vs. 0·38 ± 0·03 mm; style length: 3·65 ± 0·18 vs. 3·44 ± 0·18 mm). In cosexual populations (n = 17), anther length and width were 0·94 ± 0·03 and 0·47 ± 0·01 mm, respectively, and these measures of anther size in hermaphroditic flowers were significantly larger as compared to females (Table 2B). For stigma width and style length, the pattern was reversed; these female traits on male plants were significantly smaller than those on hermaphrodites (Table 2B).
Table 2.
(A) Results of general linear mixed models including the fixed effects of sexual system (cosexual or dimorphic) and mating type nested within sexual system (female, male or hermaphrodite), and the random effect of population nested within sexual system; values for F, P, and the numerator, denominator degrees of freedom are given for fixed effects, and the per cent of total variation explained by random effects is also reported
| Dependent | Source of variation |
Population % variation | |
|---|---|---|---|
| Sexual system | Mating type | ||
| Anther length | F1,23·6 = 3·61, P = 0·0697 | F1,1981·2 = 2754·2, P < 0·0001 | 29·8 |
| Anther width | F1,22·0 = 13·04, P = 0·0015 | F1,1874·8 = 1550·5, P < 0·0001 | 27·9 |
| Stigma width | F1,23·5 = 0·65, P = 0·4295 | F1,2190·6 = 491·2, P < 0·0001 | 35·2 |
| Style length | F1,23·3 = 8·05, P = 0·0093 | F1,2174·5 = 40·5, P < 0·0001 | 50·2 |
| Ovary length | F1,23·8 = 2·90, P = 0·1017 | F1,2195·8 = 21·0, P < 0·0001 | 28·5 |
| (B) Values are LS means for mating types and different letters indicate statistically significant differences following Tukey’s HSD post hoc comparisons; units are millimetres | |||
|---|---|---|---|
| Dependent | Female | Male | Hermaphrodite |
| Anther length | 0·58C | 1·13A | 0·94B |
| Anther width | 0·27B | 0·50A | 0·47A |
| Stigma width | 0·53A | 0·38B | 0·48A |
| Style length | 3·65A | 3·44B | 4·18A |
| Ovary length | 1·07B | 1·11A | 1·02AB |
Fig. 2.
(A) Frequency distribution of anther length for hermaphroditic plants in cosexual populations (open bars) and males and females in dimorphic populations (shaded bars) in Lycium californicum. (B) Among-plant pairwise correlations for floral traits in cosexual (open bars) and dimorphic (shaded bars) populations. Significance is shown for three levels: ***P < 0·0001, **P < 0·005 and *P < 0·05. Numbers of populations, individuals and flowers are, respectively, 17, 227 and 1094 in cosexual populations and nine, 236 and 1146 in dimorphic populations.
Cosexual populations exhibited significantly positive correlations between male and female traits, whereas dimorphic populations showed significantly negative correlations for stigma width vs. anther length and stigma width vs. anther width (Fig. 2B). As predicted, the CV was significantly higher in dimorphic populations as compared with cosexual populations for anther length (Z = 4·1, n1 = 17, n2 = 9, P < 0·0001), anther width (Z = 3·9, n1 = 17, n2 = 8, P < 0·0001), style length (Z = 2·6, n1 = 17, n2 = 9, P = 0·0083) and stigma width (Z = 3·6, n1 = 17, n2 = 9, P = 0·0004), consistent with the greater range of variation (i.e. sexual dimorphism) in dimorphic populations.
Haplotype network and diversity
The TCS analysis of plastid variation identified 21 unique haplotypes (Fig. 3; Supplementary Data Fig. S2). Subsequent analyses found differences in haplotype number, frequency and variation between cosexual and dimorphic populations. There were 14 haplotypes sampled in cosexual populations, but the majority of these (75·8 % of 91 cosexual accessions) were either haplotype Q or A. In contrast, 92·2 % of individuals sampled from dimorphic populations (51 accessions in total) were included in seven unique haplotypes (J–P), and the remaining four individuals were included in haplotypes Q and A. The haplotype with the largest outgroup probability was haplotype A, which was recovered frequently in populations in California and the Baja California peninsula, but never in Arizona or mainland Mexico. Haplotypes C–I were restricted to the disjunct, eastern-most populations in mainland Mexico (note longitude and haplotypes for LL and PR in Table 1). Haplotype diversity was 0·686 ± 0·04 (mean ± s.d.) in cosexual and 0·813 ± 0·03 in dimorphic populations. Sexual system and populations within sexual system explained 28·6 and 29·9 % of the variation, respectively, whereas 41·5 % of the variation was found within populations (Table 3).
Fig. 3.
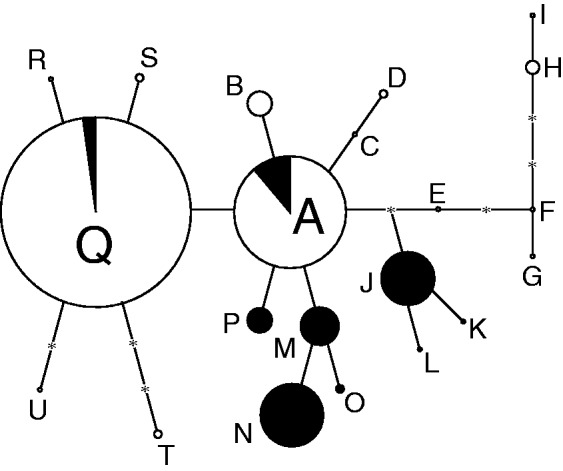
Haplotype network based on four plastid regions including 142 accessions of Lycium californicum sampled from 28 populations. Haplotypes are represented by letters and the size of the circle represents the haplotype frequency in the analysis. Shading indicates the recovery of a haplotype in either cosexual (white) or dimorphic (black) populations. The haplotype with the highest outgroup probability, as determined by TCS, was haplotype A. Asterisks represent missing haplotypes.
Table 3.
AMOVA for 18 cosexual and ten dimorphic populations of Lycium californicum; all fixation indices were significant at P < 0·0001
| Source of variation |
d.f. | Sum of squares | Variance components | % of variation |
|---|---|---|---|---|
| Among sexual systems | 1 | 26·437 | 0·36620 | 28·62 |
| Among populations within sexual system | 26 | 64·194 | 0·38259 | 29·90 |
| Within populations | 114 | 60·517 | 0·53085 | 41·48 |
| Total | 141 | 151·148 | 1·27964 |
FSC = 0·41885, FST = 0·58516, FCT = 0·28617.
Phylogenetic analysis and ancestral state reconstruction
The combined nuclear and plastid analysis supports the monophyly of L. californicum (posterior probability = 0·86; Fig. 4). Cosexuality and diploidy are the most likely ancestral character states (proportional likelihood = 0·968 for cosexuality, 0·952 for diploidy; Fig. 4). Two transitions to tetraploidy and dimorphism (nodes I and II in Fig. 4) are reconstructed and the two traits are clearly associated. One of the tetraploid-dimorphic clades includes individuals that span the full geographical range of dimorphism (HR from Arizona, ND and ROS from Baja California, and CI from Baja California Sur), whereas the second clade contains populations from Arizona (PX and ST) and Baja California Sur (CDC and IHC).
Fig. 4.
Maximum-likelihood ancestral state reconstruction of sexual strategy (left) and cytotype (right) on the tree from a combined analysis of plastid and nuclear data. Posterior probabilities are illustrated with asterisks (*≥50 %, **≥70 %). Closed circles represent proportional likelihood support for dimorphic or 4x character states, and open circles for cosexual or 2x states. Circles with horizontal lines represent taxa for which character state data are unknown. Lycium carolinianum shows variation in sexual system and cytotype and was coded as polymorphic. Populations of L. californicum are labelled as in Table 1 and are included in the dashed box.
DISCUSSION
Association of gender dimorphism and tetraploidy
There is a clear association between the presence of gender dimorphism and tetraploidy in L. californicum. Extensive sampling across its geographical range reveals that gender dimorphism and tetraploidy in L. californicum are restricted to Arizona and the Baja California peninsula (Table 1, Fig. 1). Gender-dimorphic populations are characterized by having pollen-sterile individuals (females) with reduced anther size. Although pollen-fertile (male) plants retain female sexual structures to varying degrees (i.e. they are morphological hermaphrodites), previous work has shown that these plants have virtually no female function (Miller and Venable, 2002). In contrast, plants in cosexual populations produce pollen and set fruit as expected for hermaphrodites. To date, 40 populations have been sampled, and gender dimorphism is associated with tetraploidy (n = 10 populations) and cosexuality with diploidy (n = 26 populations) in all populations for which both traits are known. For those populations re-sampled in the present study for either sexual expression (five populations) or ploidy inference (three populations), there was no evidence that either trait changed over time.
We were unable to collect fresh material for flow cytometric analysis of four populations and substituted silica-dried material. Consistent with Bainard et al. (2011), mean DNA content of tetraploid populations was 17·8 % higher using silica-dried material (three populations, 12 individuals) than fresh leaves (five populations, 32 individuals). Likewise, the single diploid population for which silica-dried tissue was used had a mean DNA content 17·3 % higher than the average for diploid populations examined using fresh tissue (19 populations, 181 individuals). Despite this variation, the difference between ploidy levels (roughly twice the DNA content in tetraploids) was much greater than the variation as a result of tissue preparation; thus, ploidy inference in Lycium is possible using silica-dried leaf tissue, and has enhanced our ability to study ploidy variation broadly across the genus (e.g. Levin et al., 2015).
Previous chromosome counts for L. californicum are largely consistent with our ploidy inferences. Chiang-Cabrera (1981) documented diploidy (haploid chromosome count, n = 12) from a specimen collected in Coahuila, Mexico, that is approx. 30 km from the PR population, which was found to be diploid in this study. He also reported tetraploid counts (n = 24) for a southern Baja sample (approx. 6 km from the IHC population) and at the northern species limit in Arizona (approx. 20 or approx. 30 km from PX and ST, respectively); all of these populations were inferred to be tetraploid in our study (Table 1). Chiang-Cabrera (1981) also reported a tetraploid count in Baja California approx. 30 km south of the US–Mexico border and another specimen from the same population with sterile anthers. The presence of tetraploidy and sterile anthers in some specimens from this area implies gender dimorphism; to date, however, no dimorphic populations have been documented this far north in Baja California.
Intraspecific polymorphism and trait evolution
Despite polymorphism in both ploidy and sexual system, L. californicum is supported as being monophyletic using plastid and nuclear DNA sequence data. Ancestral state reconstruction suggests that cosexuality and diploidy are ancestral in L. californicum, which is also likely for the genus as a whole. However, our analyses also suggest that lability exists with regard to both traits; in particular, the tetraploid-dimorphic populations are not monophyletic, as would be expected with single origins of tetraploidy and dimorphism. Rather, our data suggest that dimorphism and tetraploidy may have evolved twice within L. californicum. Observed levels of plastid haplotype variation are consistent with the possibility of two intraspecific origins of dimorphism. If gender dimorphism was recently and singly derived, then the expectation would be that dimorphic populations would contain a subset of haplotypes present in cosexual populations and lower haplotype diversity overall (Dorken and Barrett, 2004; see also Hodgins and Barrett, 2007). Although dimorphic populations had fewer total haplotypes (nine vs. 14), haplotype diversity was higher in dimorphic than in cosexual populations (0·813 vs. 0·686), likely reflecting the more even distribution of haplotypes in dimorphic populations. In addition, the majority of haplotypes were exclusive to either cosexual or dimorphic populations, a pattern consistent with an AMOVA that detected as much variation between sexual systems (28·6 %) as among populations within sexual system (29·9 %, Table 3). We note that the private haplotypes in dimorphic populations (P, M–N–O, and J–K–L) branch only from the ancestral haplotype A (Fig. 3). Given the absence of haplotype A from mainland Mexico and Arizona, it seems likely that the origin of dimorphism, regardless of the number of times it has arisen, occurred in Baja California. Indeed, dimorphic populations are sympatric with cosexual populations in Baja California and contain greater haplotype variation than dimorphic populations in Arizona.
Why the association between polyploidy and dimorphism?
Miller and Venable (2000) suggested that a transition from SI to self-compatibility preceded the evolution of gender dimorphism in Lycium and possibly other genera. They hypothesized that polyploidy disrupted SI, leading to the subsequent evolution of gender dimorphism, which arises as an alternative outcrossing mechanism. Considerable support for the breakdown of gametophytically controlled self-incompatibility (GSI) with polyploidy exists both within Solanaceae and within other families with S-RNase-based GSI (Miller and Venable, 2000, 2002; Stone, 2002; see references in Mable, 2004).
Consistent with this idea, Yeung et al. (2005) reported strong SI in a diploid, cosexual L. californicum population from California. However, establishing the disruption of SI in dimorphic populations of L. californicum has been complicated by the lack of female function in the polliniferous mating type in dimorphic populations (i.e. hermaphroditic plants are best described as males; Miller and Venable, 2002). Furthermore, this species is difficult to cultivate, which prevents biparental tests of incompatibility using crosses among known GSI genotypes. S-RNase allelic polymorphism is expected to be lost in taxa that have evolved selfing (Igić et al., 2008); thus, comparison of S-RNase allelic diversity in cosexual and dimorphic populations may prove useful as a proxy for mating system (J. S. Miller, unpubl. res.). More recent studies of the pollen determinant gene in S-RNase-based GSI (Tsukamoto et al., 2005; Wang and Kao, 2011) indicate that duplications of the SLF gene(s), which are likely to occur with polyploidy, can indeed result in the breakdown of SI.
Alternative explanations for the perfect association between tetraploidy and dimorphism should also be considered. For example, hybridization and allopolyploidy may lead directly to gender dimorphism. Obbard et al. (2006) suggest that gender dimorphism (i.e. androdioecy) in hexaploid Mercurialis annua has arisen via hybridization between a tetraploid, monoecious lineage of M. annua and a distinct diploid, dioecious species. Indeed, M. annua provides a compelling example of the joint evolution of sexual system with higher ploidy levels. Similarly, Barr and Fishman (2010) have demonstrated that cytonuclear incompatibilities between hybridizing Mimulus species may immediately expose otherwise hidden male sterility in the parental lineages. If such hybridization events were also coupled with increases in ploidy, this might also explain the association of dimorphism and tetraploidy in L. californicum.
Several studies have documented an association between gender dimorphism and harsh environmental conditions (Hart, 1985; Barrett, 1992; Costich, 1995; Weller et al., 1998; Case and Barrett, 2004a), and preliminary observations suggest that dimorphic populations of L. californicum may be present in areas with lower precipitation (and possibly higher temperatures; J. S. Miller et al., unpubl. data). However, ecological and climatic differences between ploidy levels have also been documented. For example, Thompson et al. (2014) report that tetraploids of Chamerion angustifolium occur in warmer and drier locations compared with diploids, and their observations are consistent with previous physiological studies demonstrating increased tolerance to water stress in tetraploids (Maherali et al., 2009). Given the perfect correlation between dimorphism and tetraploidy in L. californicum, it may be difficult to differentiate the relative contributions of dimorphism and ploidy to its present geographical distribution. Tetraploid-dimorphic populations occur at both the north-eastern (AZ) and the southern (Baja California Sur) range margins of L. californicum, consistent with the idea that polyploidy can facilitate range expansion (Otto and Whitton, 2000; Treier et al., 2009). However, in central Baja, diploid-cosexual and tetraploid-dimorphic populations are in remarkable proximity, in some cases occurring only approx. 5 km apart. Such proximity invites more localized ecological studies of paired populations differing in sexual system, as well as studies of the physiological tolerances of individuals of different ploidy levels.
CONCLUSIONS
Lycium californicum joins a growing number of species with documented polymorphism in sexual system (Wurmbea dioica, Case and Barrett, 2004a, b; Sagittaria latifolia, Dorken and Barrett, 2004; Yakimowski and Barrett, 2014; Mercurialis annua, Pannell et al., 2004; Ecballium elaterium, Costich, 1995; Daphne laureola, Alonso and Herrera, 2011). In Lycium, there is also a compelling association of gender dimorphism with higher ploidy levels that holds both across species and within L. californicum. The only exception is L. carolinianum; although individuals of this species from the Yucatán peninsula are both dimorphic and tetraploid, gender dimorphism is also present in several diploid populations in Hawaii (Blank et al., 2014). Further investigation of Lycium, and studies in these two polymorphic species in particular, will contribute to our understanding of sexual system evolution and its relationship to ploidy variation and ecological conditions.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Table S1: taxa, population localities, voucher information and GenBank accession numbers for all sequences included in the study. Figure S1: frequency histograms for anther length in 26 populations of Lycium californicum. Figure S2: plastid haplotype frequencies in populations of Lycium californicum.
ACKNOWLEDGMENTS
We thank P. Kron for assistance with flow cytometry measurements; S. Ratay of the Catalina Island Conservancy, G. Smith of the US Navy, I. Kay of the University of California Natural Reserve System, and S. Rutman, D. Rodriguez and I. Williams at the National Park Service for access to field sites. M. Fishbein provided field assistance, and L. A. McDade supported CA fieldwork. J. L. León de la Luz facilitated collections on the Baja California peninsula. This work was supported by the National Science Foundation (DEB-0843364 to J.S.M. and R.A.L.) and awards from the Department of Biology and Schupf Scholars Program at Amherst College (to A.K.).
LITERATURE CITED
- Alonso C, Herrera CM. 2011. Back-and-forth hermaphroditism: phylogenetic context of reproductive system evolution in subdioecious Daphne laureola. Evolution 65: 1680–1692. [DOI] [PubMed] [Google Scholar]
- Ashman TL, Kwok A, Husband BC. 2013. Revisiting the dioecy–polyploidy association: alternate pathways and research opportunities. Cytogenetic and Genome Research 140: 241–255. [DOI] [PubMed] [Google Scholar]
- Bainard JD, Husband BC, Baldwin SJ, et al. 2011. The effects of rapid desiccation on estimates of plant genome size. Chromosome Research 19: 825–842. [DOI] [PubMed] [Google Scholar]
- Baker HG. 1959. Reproductive methods as factors in speciation in flowering plants. Cold Spring Harbor Symposium in Quantitative Biology 24: 177–191. [DOI] [PubMed] [Google Scholar]
- Barr CM, Fishman L. 2010. The nuclear component of a cytonuclear hybrid incompatibility in Mimulus maps to a cluster of pentatricopeptide repeat genes. Genetics 184: 455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SCH. 1992. Gender variation and the evolution of dioecy in Wurmbea dioica (Liliaceae). Journal of Evolutionary Biology 5: 423–444. [Google Scholar]
- Bino RJ, De Vries JN, Kraak HL, Van Pijlen JG. 1992. Flow cytometric determination of nuclear replication stages in tomato seeds during priming and germination. Annals of Botany 69: 231–236. [Google Scholar]
- Blank CM, Levin RA, Miller JS. 2014. Intraspecific variation in gender strategies in Lycium (Solanaceae): associations with ploidy and changes in floral form following the evolution of gender dimorphism. American Journal of Botany 101: 2160–2168. [DOI] [PubMed] [Google Scholar]
- Case AL, Barrett SCH. 2004a. Environmental stress and the evolution of dioecy: Wurmbea dioica (Colchicaceae) in Western Australia. Evolutionary Ecology 18: 145–164. [Google Scholar]
- Case AL, Barrett SCH. 2004b. Floral biology of gender monomorphism and dimorphism in Wurmbea dioica (Colchicaceae) in Western Australia. International Journal of Plant Sciences 165: 289–301. [Google Scholar]
- Charlesworth D. 1985. Distribution of dioecy and self-incompatibility in angiosperms. In Greenwood PJ, Slatkin M, eds. Evolution: essays in honour of John Maynard Smith. Cambridge, UK: Cambridge University Press, 237–268. [Google Scholar]
- Chiang-Cabrera F. 1981. A taxonomic study of the North American species of Lycium (Solanaceae). PhD dissertation, University of Texas Austin. [Google Scholar]
- Clement M, Posada D, Crandall KA. 2000. TCS: a computer program to estimate gene genealogies. Molecular Ecology 9: 1657–1660. [DOI] [PubMed] [Google Scholar]
- Costich DE. 1995. Gender specialization across a climatic gradient: experimental comparison of monoecious and dioecious Ecballium. Ecology 76: 1036–1050. [Google Scholar]
- De Laat AMM, Blaas J. 1984. Flow-cytometric characterization and sorting of plant chromosomes. Theoretical and Applied Genetics 67: 463–467. [DOI] [PubMed] [Google Scholar]
- Dorken ME, Barrett SCH. 2004. Chloroplast haplotype variation among monoecious and dioecious populations of Sagittaria latifolia (Alismataceae) in eastern North America. Molecular Ecology 13: 2699–2707. [DOI] [PubMed] [Google Scholar]
- Excoffier L, Lischer HEL. 2010. Arlequin suite ver 3·5: a new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources 10: 564–567. [DOI] [PubMed] [Google Scholar]
- Geber MA, Dawson TE, Delph LF, eds 1999. Gender and sexual dimorphism in flowering plants. Berlin: Springer. [Google Scholar]
- Hart JA. 1985. Evolution of dioecism in Lepechinia Willd. sect. Parvifiorae (Lamiaceae). Systematic Botany 10: 147–154. [Google Scholar]
- Hodgins KA, Barrett SCH. 2007. Population structure and genetic diversity in tristylous Narcissus triandrus: insights from microsatellite and chloroplast DNA variation. Molecular Ecology 16: 2317–2332. [DOI] [PubMed] [Google Scholar]
- Igić B, Lande R, Kohn JR. 2008. Loss of self-incompatibility and its evolutionary consequences. International Journal of Plant Sciences 169: 93–104. [Google Scholar]
- Mable BK. 2004. Polyploidy and self-compatibility: is there an association? New Phytologist 162: 803–811. [DOI] [PubMed] [Google Scholar]
- Johnston S, Bennett MD, Rayburn AL, Galbraith DW, Price HJ. 1999. Reference standards for determination of DNA content of plant nuclei. American Journal of Botany 86: 609–613. [PubMed] [Google Scholar]
- Levin RA, Miller JS. 2005. Relationships within tribe Lycieae (Solanaceae): paraphyly of Lycium and multiple origins of gender dimorphism. American Journal of Botany 92: 2044–2053. [DOI] [PubMed] [Google Scholar]
- Levin RA, Bernadello G, Venter AM, Shak JR, Miller JS. 2007. Evolutionary relationships in tribe Lycieae (Solanaceae). Acta Horticulturae 745: 225–239. [Google Scholar]
- Levin RA, Whelan A, Miller JS. 2009. The utility of nuclear conserved ortholog set II (COSII) genomic regions for species-level phylogenetic inference in Lycium (Solanaceae). Molecular Phylogenetics and Evolution 53: 881–890. [DOI] [PubMed] [Google Scholar]
- Levin RA, Keyes EM, Miller JS. 2015. Evolutionary relationships, gynodioecy, and polyploidy in the Galápagos endemic Lycium minimum (Solanaceae). International Journal of Plant Sciences 176 [DOI:10·1086/679492]. [Google Scholar]
- Liu L. 2008. BEST: Bayesian estimation of species trees under the coalescent model. Bioinformatics 24: 2542–2543. [DOI] [PubMed] [Google Scholar]
- Lysak MA, Dolezel J. 1998. Estimation of nuclear DNA content in Sesleria (Poaceae). Caryologia 51: 123–132. [Google Scholar]
- Maddison WP, Maddison DR. 2014. Mesquite: a modular system for evolutionary analysis, version 3.01. http://mesquiteproject.org. [Google Scholar]
- Maherali H, Walden AE, Husband BC. 2009. Genome duplication and the evolution of physiological responses to water stress. New Phytologist 184: 721–731. [DOI] [PubMed] [Google Scholar]
- Miller JS, Venable DL. 2000. Polyploidy and the evolution of gender dimorphism in plants. Science 289: 2335–2338. [DOI] [PubMed] [Google Scholar]
- Miller JS, Venable DL. 2002. The transition to gender dimorphism on an evolutionary background of self-incompatibility: an example from Lycium (Solanaceae). American Journal of Botany 89: 1907–1915. [DOI] [PubMed] [Google Scholar]
- Miller JS, Venable DL. 2003. Floral morphometrics and the evolution of sexual dimorphism in Lycium (Solanaceae). Evolution 57: 74–86. [DOI] [PubMed] [Google Scholar]
- Miller JS, Kamath A, Levin RA. 2009. Do multiple tortoises equal a hare? The utility of nine noncoding plastid regions for species-level phylogenetics in tribe Lycieae (Solanaceae). Systematic Botany 34: 796–804. [Google Scholar]
- Obbard DJ, Harris SA, Buggs RA, Pannell JR. 2006. Hybridization, polyploidy, and the evolution of sexual systems in Mercurialis (Euphorbiaceae). Evolution 60: 1801–1815. [PubMed] [Google Scholar]
- Otto SP, Whitton J. 2000. Polyploid incidence and evolution. Annual Review of Genetics 34: 401–437. [DOI] [PubMed] [Google Scholar]
- Pannell JR, Obbard DJ, Buggs RA. 2004. Polyploidy and the sexual system: what can we learn from Mercurialis annua? Biological Journal of the Linnean Society 82: 547–560. [Google Scholar]
- Posada D, Crandall KA. 1998. ModelTest: testing the model of DNA substitution. Bioinformatics 14: 817–818. [DOI] [PubMed] [Google Scholar]
- Rambaut A. 1996–2002. SeAl: sequence alignment editor, Version 2·0a11. Available from: tree.bio.ed.ac.uk/software/seal/. [Google Scholar]
- Renner SS, Ricklefs RE. 1995. Dioecy and its correlates in the flowering plants. American Journal of Botany 82: 596–606. [Google Scholar]
- Richman AD. 2000. S-allele diversity in Lycium andersonii: implications for the evolution of S-allele age in the Solanaceae. Annals of Botany 85: 241–245. [Google Scholar]
- Rozas J, Rozas R. 1995. DnaSP, DNA sequence polymorphism: an interactive program for estimating population genetics parameters from DNA sequence data. Computer Applications in the Biosciences 11: 621–625. [DOI] [PubMed] [Google Scholar]
- Sakai AK, Weller SG. 1999. Gender and sexual dimorphism in flowering plants: a review of terminology, biogeographic patterns, ecological correlates, and phylogenetic approaches. In Geber MA, Dawson TE, Delph LF, eds. Gender and sexual dimorphism in Plants. Berlin: Springer, 1–32. [Google Scholar]
- Savage AE, Miller JS. 2006. Gametophytic self-incompatibility in Lycium parishii (Solanaceae): allelic diversity, genealogical structure, and patterns of molecular evolution at the S-RNase locus. Heredity 96: 434–444. [DOI] [PubMed] [Google Scholar]
- Stone JL. 2002. Molecular mechanisms underlying the breakdown of gametophytic self-incompatibility. Quarterly Review of Biology 77: 17–32. [DOI] [PubMed] [Google Scholar]
- Thompson KA, Husband BC, Maherali H. 2014. Climatic niche differences between diploid and tetraploid cytotypes of Chamerion angustifolium (Onagraceae). American Journal of Botany 101: 1868–1875. [DOI] [PubMed] [Google Scholar]
- Treier UA, Broennimann O, Normand S, et al. 2009. Shift in cytotype frequency and niche space in the invasive plant Centaurea maculosa. Ecology 90: 1366–1377. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Ando T, Watanabe H, Marchesi E, Kao T.-H. 2005. Duplication of the S-locus F-box gene is associated with breakdown of pollen function in an S-haplotype identified in a natural population of self-incompatible Petunia axillaris. Plant Molecular Biology 57: 141–153. [DOI] [PubMed] [Google Scholar]
- Vamosi JC, Otto SP, Barrett SCH. 2003. Phylogenetic analysis of the ecological correlates of dioecy in angiosperms. Journal of Evolutionary Biology 16: 1006–1018. [DOI] [PubMed] [Google Scholar]
- Venter AM. 2007. Lycium hantamense (Solanaceae), a new species from the Hantam- Roggeveld centre of plant endemism, South Africa. South African Journal of Botany 73: 214–217. [Google Scholar]
- Venter AM, Venter HJT. 2003a. Lycium gariepense (Solanaceae), a new species from South Africa and Namibia. South African Journal of Botany 69: 161–164. [Google Scholar]
- Venter AM, Venter HJT. 2003b. Lycium strandveldense (Solanaceae), a new species from the western coast of South Africa. South African Journal of Botany 69: 476–479. [Google Scholar]
- Wang N, Kao T-H. 2011. Self-incompatibility in Petunia: a self/nonself-recognition mechanism employing S-locus F-box proteins and S-RNase to prevent inbreeding. Wiley Interdisciplinary Reviews: Developmental Biology 1: 267–275. [DOI] [PubMed] [Google Scholar]
- Weller SG, Sakai AK, Rankin AE, Golonka A, Kutcher B, Ashby KE. 1998. Dioecy and the evolution of pollination systems in Schiedea and Alsinidendron (Caryophyllaceae: Alsinideae) in the Hawaiian islands. American Journal of Botany 85: 1377–1388. [PubMed] [Google Scholar]
- Wu F, Mueller LA, Crouzillat D, Pétiard V, Tanksley SD. 2006. Combining bioinformatics and phylogenetics to identify large sets of single-copy orthologous genes (COSII) for comparative, evolutionary and systematic studies: a test case in the euasterid plant clade. Genetics 174: 1407–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimowski SB, Barrett SCH. 2014. Variation and evolution of sex ratios at the northern range limit of a sexually polymorphic plant. Journal of Evolutionary Biology 27: 1454–1466. [DOI] [PubMed] [Google Scholar]
- Yeung K, Miller JS, Savage AE, Husband BC, Igić B, Kohn JR. 2005. Association of ploidy and sexual system in Lycium californicum (Solanaceae). Evolution 59: 2048–2055. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.