Abstract
Background and Aims Intraspecific variation in seed bank dynamics should contribute to local adaptation, but is not well studied. The extent to which genetic and environmental factors affect dormancy cycling and seed mortality was investigated in the annual herb Arabidopsis thaliana by conducting a reciprocal seed burial experiment.
Methods Seeds from two locally adapted populations (from Italy and Sweden) were buried at both of the sites of origin, and seed mortality and germinability were determined during the following 2 years for initially non-dormant glasshouse-matured seeds and dormant field-matured seeds.
Key Results Mean soil temperature was higher at the Italian site compared with the Swedish site throughout the year, and the germination proportions were in general higher for seeds buried in Italy than in Sweden. The rate of secondary dormancy induction of the Italian genotype was faster than that of the Swedish genotype at both sites, while the opposite was true for the rate of dormancy release, at least at the Swedish site. The comparison of non-dormant glasshouse seeds with dormant field seeds demonstrated that A. thaliana seeds can adjust their dormancy levels to current environmental conditions, and suggests that maternal environmental conditions have only minor effects on dormancy cycles. At both sites, locally produced seeds had low germinability in the first year compared with the second year, suggesting that a considerable fraction of the seeds would enter the seed bank. In Italy, but not in Sweden, seed mortality increased rapidly during the second year of burial.
Conclusions This is the first demonstration of intraspecific genetic differentiation in the annual seed dormancy cycle of any species, and the documented difference is likely to contribute to local adaptation. The results suggest that the contribution of a seed bank to seedling recruitment should vary among environments due to differences in the rate of seed mortality.
Keywords: Arabidopsis thaliana, bet-hedging, dormancy cycling, local adaptation, natural variation, physiological dormancy, secondary dormancy, seed bank, seed mortality, seed predation
INTRODUCTION
Many seeds do not germinate directly after dispersal, but end up in the soil where they can accumulate and form a soil seed bank. A reserve of viable seeds in the soil enables dispersal through time and can serve as a bet-hedging strategy against unpredictable fluctuations in survival or reproduction and thus reduce the variance in fitness (Seger and Brockman, 1987). A persistent seed bank, with seeds that stay alive in the soil for >1 year, is common among annual and biennial species in disturbed and variable habitats (Thompson et al., 1998; Saatkamp et al., 2014) and can prevent population extinction due to failure of seed production in a single year (Venable and Brown, 1988; Thompson, 2000; Evans and Dennehy, 2005; Tielbörger et al., 2012). The formation of a persistent seed bank may also allow escape from crowding and kin competition (Venable and Brown, 1988; Thompson, 2000).
The timing of germination strongly affects the probability of seedling survival, and also the conditions for further plant growth and reproduction (Donohue et al., 2010). This should result in strong selection on seeds in the seed bank to respond to seasonal cues and to germinate at the time of the year most favourable for further development and reproduction. Seeds can delay their germination by dormancy, a seed trait that determines the conditions required for germination (Vleeshouwers et al., 1995; Finch-Savage and Leubner-Metzger, 2006). The higher the dormancy level, the narrower the range of conditions under which the seed will germinate.
Most seeds in temperate seed banks are physiologically dormant, meaning that they may increase or decrease their dormancy levels in response to environmental conditions (Finch-Savage and Leubner-Metzger, 2006; Baskin and Baskin, 2014). Freshly matured seeds can have primary dormancy, which is induced during seed development on the mother plant and has the function of delaying germination until the germination season (Hilhorst, 1998; Finch-Savage and Leubner-Metzger, 2006). After dispersal, seeds can acquire secondary dormancy when primary dormancy is lost but favourable germination conditions are not met, e.g. due to light limitation (Hilhorst, 1998; Finch-Savage and Leubner-Metzger, 2006; Baskin and Baskin, 2014). Secondary dormancy can be induced and relieved in the same seed for several cycles, until the required germination conditions appear (Hilhorst, 1998; Baskin and Baskin, 2014).
The genetic basis (Alonso-Blanco et al., 2003; Clerkx et al., 2004; Bentsink et al., 2010) and adaptive significance (Donohue, 2005; Huang et al., 2010; Chiang et al., 2012) of intraspecific variation in primary dormancy has received considerable attention. However, in many species, a substantial portion of seedlings originates from the seed bank (Grime, 2000; Kalamees and Zobel, 2002; Luzuriaga et al., 2005; Pakeman and Small, 2005), suggesting that not only primary dormancy but also secondary dormancy might show adaptive genetic differentiation. Storing seeds in the soil comes at the cost of an increased risk of seed mortality caused by predators, pathogens and ageing (Baskin and Baskin, 2014; Saatkamp et al., 2014). The adaptive value of a seed bank of physiologically dormant seeds therefore depends both on the match between the dormancy cycle and seasonal changes in environmental conditions, and on the rate of seed mortality in the soil. Interspecific variation in these seed bank traits has been well described (Baskin and Baskin, 2014), but little is known about within-species genetic variation and its relationship to climate and soil conditions despite its potential importance for local adaptation.
We examined the extent to which genetic and environmental factors affect dormancy cycling and seed mortality by conducting a reciprocal seed burial experiment using the annual plant Arabidopsis thaliana. Natural populations of A. thaliana possess a persistent seed bank (Lundemo et al., 2009; Falahati-Anbaran et al., 2014), and seeds exhibit annual dormancy cycles (Baskin and Baskin, 1983, 1985; Derkx and Karssen, 1994). We used two natural populations, one originating from Italy and one from Sweden, for which reciprocal transplants with seeds and seedlings carried out in 6 years have demonstrated that the populations are locally adapted and show genetic differentiation for key life-history traits (Ågren and Schemske, 2012; Ågren et al., 2013). Both populations are winter annuals: seeds germinate in the autumn, overwinter in the rosette stage, and flower, set seeds and die in the following spring and early summer. Despite the shared life history, primary dormancy of seeds differs markedly between the two populations, and nine quantitative trait loci contributing to this difference have been identified (Postma and Ågren, 2015).
In the present study, experimental seeds of both populations were buried at the sites of the source populations. We used freshly matured seeds produced at the respective burial sites, because the level of primary dormancy in seeds is not only genetically determined but is also affected by the environment of the mother plant (Donohue, 2009; Postma and Ågren, 2015). To investigate whether seeds are able to adjust their dormancy levels in response to environmental cues, we also buried non-dormant seeds produced in a glasshouse, which had lost their primary dormancy by after-ripening.
With the reciprocal seed burial experiment including dormant and non-dormant seeds of two different genotypes, we address the following questions. (a) Do the two populations show genetic differences in their dormancy cycling behaviour (primary dormancy loss, secondary dormancy induction and secondary dormancy loss)? (b) Does the dormancy cycle of the local genotype match the natural germination period? (c) Do field conditions induce secondary dormancy in non-dormant seeds buried at the time of natural seed dispersal? (d) Are there differences in seed mortality between sites, and is the mortality rate affected by genotype and/or maternal environment of the seeds? Our results demonstrate that both dormancy cycling and seed mortality are affected by a combination of genetically and environmentally determined seed properties and by soil environment. Further, they suggest that dormancy cycling has diverged adaptively in response to differences in seasonality of soil conditions.
MATERIALS AND METHODS
Plant material and study sites
We used lines originating from two natural populations of Arabidopsis thaliana (L.) Heynh. taken from the northern and southern end of the species’ distribution range in Europe: Rödåsen in north-central Sweden (62°48'N, 18°12'E) and Castelnuovo in central Italy (42°07'N, 12°29'E) (Ågren and Schemske, 2012). We buried the seeds in experimental gardens located at the sites of the source populations. The soil at the Italian site is of volcanic origin (sandy loam) and the Swedish site has a morainic soil (loamy sand). We recorded soil temperature every hour with HOBO Temperature Data Loggers (HOBO Pro Data Logger Serie H08-031-08, Onset Computer Corporation, Bourne, MA, USA) with temperature sensors placed approx. 1 cm below the soil surface (two sensors at each site). Soil moisture was recorded hourly as water potential with sensors located approx. 1 cm below the soil surface (four sensors per site; Decagon Devices, Pullman, WA, USA).
Reciprocal seed burial
We produced experimental seeds for the burial experiment of both the Italian and Swedish genotype in three maternal environments: in the glasshouse and at the sites of the two source populations. The maternal lines used to produce the experimental seeds had gone through nine generations of selfing in the glasshouse and were descendants of two plants that were the founders of a population of recombinant inbred lines used in other studies exploring the genetic basis of differences in fitness and putative adaptive traits between the two populations (Ågren et al., 2013; Dittmar et al., 2014; Oakley et al., 2014; Postma and Ågren, 2015). The maternal plants were transplanted as 10- to 14-day-old seedlings to their respective environment. Seeds produced in the glasshouse environment were matured under standard glasshouse conditions (20 °C 16 h light and 16 °C 8 h dark) and ripe seeds were collected from 20 replicate plants per genotype on 31 January 2011. At the field sites, the seeds matured under the local environmental conditions, and were harvested from 28 replicate plants per genotype on 17–20 April 2011 (Italian field) and 18–21 June 2011 (Swedish field). Seed viability was determined for all population × maternal environment combinations with a germination test on fertilized agar after 2 weeks of cold stratification (Postma and Ågren, 2015), demonstrating that seeds of both genotypes and from all three maternal environments were 95–100 % viable after >1 year of storage at room temperature.
At each field site, we buried seeds of both genotypes that had been produced at that site and in the glasshouse. Burial occurred as soon as possible after seed harvest at the field site (28 May 2011 in Italy, and 2 July 2011 in Sweden) to mimic the natural seed dispersal period. This resulted in 17 (Italian site) and 22 weeks (Swedish site) of after-ripening at room temperature for the glasshouse-produced seeds, promoting the loss of primary dormancy. Due to poor seed production of the Swedish genotype at the Italian field site, the burial experiment in Italy included glasshouse-produced seeds of both genotypes, but field-produced seeds of only the Italian genotype.
The seeds were buried in batches of approx. 100 healthy, filled seeds of a given genotype × maternal environment combination. Each batch was placed in a fine-meshed (mesh size 0·3–0·4 mm) polyester bag, ensuring that the seeds would be exposed to soil particles, water and soil organisms during the burial period. We placed 60 replicate bags per genotype × maternal environment combination in a randomized design approx. 3 cm under the soil surface.
Seed excavations
Eight to twelve seed bags per genotype × maternal environment combination were excavated at regular intervals throughout the following year (Fig. 1). We timed the excavations just before (Sweden, 28 July 2011 and 20 July 2012; Italy, 4 August 2011 and 17 September 2012) and during (Sweden, 9 September 2011 and 31 August 2012; Italy, 8 November 2011 and 11 November 2012) the germination period, and before (Sweden, 3 May 2012; Italy, 30 January 2012) and during (Sweden, 20 June 2012; Italy, 29 April 2012) the seed maturation period of the local populations.
Fig. 1.

Mean weekly soil temperature (A) and soil moisture (B), and the seed maturation (dashed boxes) and germination period (filled boxes) of the natural populations (C) of Arabidopsis thaliana at the two burial sites in Italy (red) and Sweden (blue). Seeds of the burial experiment were sampled at six different time points (arrows upwards) after burial (arrows downwards).
Seed bags were brought to the lab, where we separated the seeds from soil particles and counted the number of healthy seeds (brown and filled) and dead seeds (seeds that had turned black) following Montesinos et al. (2009), and the number of seeds consumed by seed predators (empty seed coats). The change in seed colour from brown to black was taken as an indication of seed mortality. Seeds turned black mainly in Italy, and germination tests demonstrated that the brown seeds of all lines in Italy were still viable at the end of the experiment (brown seeds from the final excavation showed 82–99 % germination after 8 weeks in the growth room), while the black seeds given the same treatment as brown seeds never germinated. We cannot exclude the possibility that some non-germinating brown seeds were dead rather than dormant, although we consider this unlikely because viability tests (germination test on agar after cold stratification) have demonstrated that brown normal-looking seeds of these genotypes consistently have high viability even when the standard germination test on filter paper without stratification indicates low germinability (Postma and Ågren 2015). At the first excavation at each burial site, the number of empty seed coats was not recorded. For each seed batch, we calculated the proportions of black and predated seeds by dividing the number of black and predated seeds, respectively, by the total number of seeds (healthy, dead and predated seeds). Total seed mortality was calculated by dividing the sum of black and predated seeds by the total number of seeds.
The germinability of all seed batches was tested within 1–3 d after excavation. A separate seed batch was tested around the time of seed burial to estimate seed dormancy levels at the start of the burial experiment. For the germination test, all healthy and black seeds of a seed batch were sown on two layers of filter paper in a 9 cm Petri dish. The Petri dishes were placed in a growth room (20 °C 16 h light with photosynthetically active radiation of 150 μmol m–2 s–1 and 16 °C 8 h dark) and watered every second to third day to saturation of the filter paper (Baskin and Baskin, 2014). Emerging seedlings were recorded and removed weekly for 8 weeks, except for the seeds from the first excavation at both sites and the seeds sown at the moment of burial, for which the germination tests were conducted for only 1 week. No seeds that were classified as dead germinated. For each seed batch, the germination proportion was calculated by dividing the number of germinated seeds by the number of healthy seeds. Quantification of seed dormancy should be based on a germination test conducted for a maximum of 4 weeks, as dormancy can be broken by warm stratification during longer germination tests (Baskin and Baskin, 2014). However, in studies of A. thaliana, seed dormancy is most commonly quantified based on germination after 1 week (e.g. Alonso-Blanco et al., 2003; Clerkx et al., 2004; Bentsink et al., 2010; Huang et al., 2010). We therefore quantified the dormancy levels of the seeds of each batch as the germination proportions after 1 week and, if available, after 4 weeks in the growth room.
Data analysis
For each burial site, we examined the effect of time since seed burial (categorical variable), seed genotype, maternal environment and their two-order interactions on the germination proportion after 1 and 4 weeks in the growth room using generalized linear models (GLMs). The genotype × maternal environment interaction could not be tested for the Italian burial site, as field-produced seeds of only the Italian genotype were available at this site. For each burial site, we also examined whether the proportion of black seeds, the proportion of predated seeds and total seed mortality increased with burial time (continuous variable), and were affected by genotype, maternal environment and their two-order interactions using GLMs. For all GLMs, a binomial distribution with logit link function was used, with quasi-likelihood to account for overdispersion. The significance of each explanatory variable and interaction term was determined by comparing the change in deviance between the full model and reduced model with an F-test. All statistical analyses were performed in R version 3.2.1 (R Core Team, 2014).
RESULTS
Soil temperature and moisture
The mean weekly soil temperature was on average 8·9 °C warmer at the Italian field site compared with the Swedish field site, while the seasonal variation was comparable between the two sites, with a mean difference of 22·9 °C (Italy) and 19·4 °C (Sweden) between the minimum and maximum mean weekly soil temperatures during 2011 and 2012 (Fig. 1). The length of the ‘dry season’ (period with mean weekly soil water potential less than –30 kPa), which lasts from the end of spring until the end of summer, was twice as long in Italy (2011, 32 weeks; 2012, 34 weeks) compared with Sweden (2011, 16 weeks; 2012, 14 weeks; Fig. 1). The mean weekly soil water potential during the ‘dry season’ was also much lower in Italy (–277 kPa) than in Sweden (–123 kPa), while the water availability was similar during the ‘wet season’ (–18 kPa at both sites). In the natural population in Italy, seeds mature in mid-April and germinate in October–November. In the Swedish population, seeds mature in late June and germination occurs in August–September. The period from seed maturation to germination is thus more than twice as long in the natural population in Italy compared with Sweden and overlaps with the ‘dry season’ at both sites (Fig. 1).
Dormancy behaviour
Seeds produced at the field sites showed strong primary dormancy at the start of the seed burial experiment, as shown by the low germination proportions (Fig. 2A, B). In contrast, the glasshouse-produced seeds, which had after-ripened for 17 and 22 weeks before burial at the Italian and Swedish field site, respectively, had lost almost all primary dormancy (Fig. 2A, B). Only the seeds of the Italian genotype matured in the glasshouse and planted at the Italian site had some dormancy left at burial (Fig. 2A).
Fig. 2.
The proportion of Arabidopsis thaliana seeds germinating after different durations of seed burial at the Italian (A, C) and Swedish site (B, D; mean ± s.e.). Germination of seeds of the Italian and Swedish genotype produced in the glasshouse and at the site of burial was quantified after 1 week (A, B) and 4 weeks (C, D) in a growth room under standard conditions. For the seeds tested at the moment of seed burial and the seeds from the first excavation, the germination assays were conducted for only 1 week. The seed maturation and germination period of the natural populations at the two burial sites in Italy and Sweden are indicated on the x-axis together with the moments of seed burial (downward-pointing arrow) and the six sampling occasions (upward-pointing arrows).
Seeds of the Italian genotype produced in the glasshouse quickly gained dormancy after burial in the field (Fig, 2A, B). At the Swedish site, the germination proportion dropped from 100 % to 10 % within 3 weeks (Fig. 2B). In contrast, the Swedish genotype increased the level of seed dormancy substantially only during the following winter and spring (Fig. 2A, B). This indicates a genetically based difference in the induction of secondary dormancy between the two populations. The non-dormant glasshouse-produced seeds of both genotypes showed lower dormancy levels than the field-produced seeds of the same genotype during the first year of burial, but they had very similar germinability to the field-produced seeds in the second year (Fig. 2).
At both burial sites, the level of seed dormancy varied significantly with time since burial, seed genotype and maternal environment (Fig. 2; Supplementary Data Table S1), and the time since burial × genotype, time since burial × maternal environment and genotype × maternal environment interactions were highly significant (Table S1). Seeds from all genotype × maternal environment combinations showed annual variation in dormancy, i.e. dormancy cycles, with the exception of the field-produced seeds of the Italian genotype buried at the Swedish site that never germinated (Fig. 2B, D).
The dormancy cycles corresponded to the natural germination periods of the local populations: germination proportions were high in autumn and low during winter and spring (Fig. 2). Buried seeds of the local genotype produced at its home site showed the highest germination proportions when excavated at the time of seed germination in the natural population (Fig. 2). For the Swedish genotype this was true whether the dormancy level was estimated based on germination proportions after 1 week or 4 weeks in the growth room (Fig. 2). Seeds of the Italian genotype buried at the Italian site and excavated at the time of seed germination in the natural population had the highest germination proportion only after 4 weeks in the growth room (Fig. 2C). In general, the Italian genotype germinated more slowly than did the Swedish genotype, as indicated by a larger increase in germination between records taken after 1 and 4 weeks in the growth room (Fig. 2A, C).
At the Swedish field site, the field-produced seeds of the Swedish genotype germinated to higher proportions than did the Italian genotype during the natural germination period (Fig. 2B, D). Especially in the second year of burial, the difference between the two populations was very large. At the Italian field site, we did not have seeds from the Swedish genotype produced in the field to compare with the field-produced seeds of the Italian genotype.
We observed large differences in the dormancy levels of field-produced seeds between the first and second year of burial. In the first year, only 30·3 ± 6·1 % (mean ± s.e., Swedish site) and 9·7 ± 6·1 % (Italian site) of the field-produced seeds of the local genotype lost primary dormancy during the germination peak in autumn (Fig. 2C, D). In contrast, in the second year, the germination proportions were as high as 76·9 ± 6·9 % in Sweden and 69·3 ± 11·1 % in Italy (Fig. 2C, D). Also the speed of germination of seeds excavated during the natural germination period was much higher in the second year compared with the first year of burial for field-produced seeds of the local genotype (Supplementary Data Fig. S1). This means that a large proportion of the seeds did not lose primary dormancy during the first year but only during the second year, which could represent a bet-hedging strategy. For glasshouse-produced seeds, differences in dormancy levels between the two years were much smaller (Fig. 2). These seeds had lost primary dormancy at the moment of burial, so any dormancy was due to secondary dormancy in both years.
Seed mortality
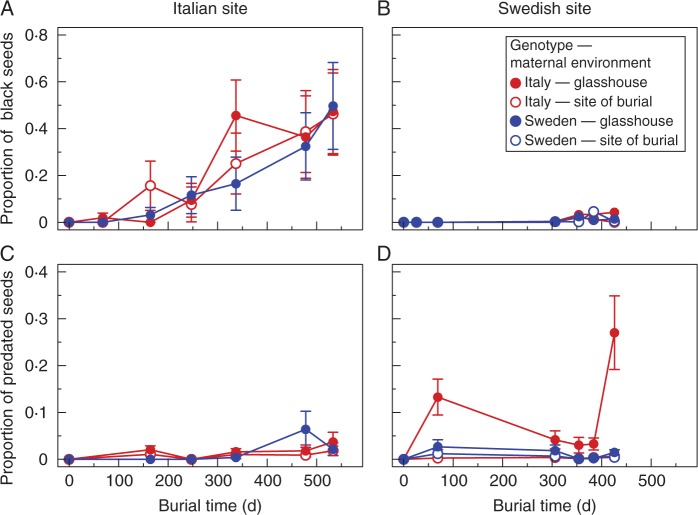
Total seed mortality (the sum of the black and predated seeds) increased with burial time at a much higher rate in Italy than in Sweden. After almost 1 year of burial, the proportion of black seeds was >10-fold higher in Italy compared with Sweden (mean ± s.e., 0·301 ± 0·078 vs. 0·028 ± 0·008; Fig. 3A, B), and varied among genotype × maternal environment combinations at the Swedish but not at the Italian burial site (Supplementary Data Table S2). The rate at which seeds turned black did not depend on seed genotype, but in Sweden the proportion of seeds that turned black was significantly higher among seeds produced in the glasshouse than among seeds produced in the field (Table S2). In Italy, the predation rate was very low for all genotype × maternal environment combinations (Fig. 3C, D; Table S2). In Sweden, the predation rate varied significantly among genotype × maternal environment combinations, with seeds of the Italian genotype produced in the glasshouse having the highest proportion of predated seeds (Fig. 3D; Table S2).
Fig. 3.
The proportion of black and predated Arabidopsis thaliana seeds of the Italian and Swedish genotype produced in the glasshouse and at the site of burial after different durations of burial in Italy (A, C) and Sweden (B, D; mean ± s.e.). Data on the proportion of predated seeds from the first excavation at both the Italian and Swedish site are missing.
DISCUSSION
Many plant species have seeds that can stay viable in the soil for several years, but little is known about the relative importance of genetically and environmentally induced variation in seed bank dynamics. We investigated the extent to which genetic and environmental factors affect dormancy cycling and seed mortality by conducting a reciprocal seed burial experiment between two natural populations of Arabidopsis thaliana from Italy and Sweden. Our results demonstrate genetic differences in the dormancy cycle between the two study populations in terms of both secondary dormancy induction and rate of dormancy release. At both sites, field-produced seeds of the local genotype had low germinability in the first year, suggesting that a considerable fraction of the seeds enter the seed bank, which can be interpreted as a bet-hedging strategy in an environment where conditions for seedling establishment vary among years. However, the reproductive value of seeds in the seed bank may differ between the two sites. In Sweden, the mortality rate of buried seeds was very low throughout the experiment, whereas in Italy seed mortality increased rapidly during the second year.
Interspecific variation in the dormancy cycling behaviour of buried seeds has been well described (Baskin and Baskin, 2014), but, to the best of our knowledge, this is the first demonstration of intraspecific genetic differentiation in annual seed dormancy cycles. A seed burial experiment in Spain revealed variation in the germination behaviour between montane and coastal A. thaliana populations, but it was not possible to distinguish between genetic and environmental effects underlying the observed differences (Montesinos et al., 2009).
The correspondence between the dormancy cycle of the local population and seasonal changes in conditions for seedling establishment suggest that the differences are adaptive. In Italy, the high dormancy level of the local genotype is likely to be favoured by selection because of the long and dry summers with periods of drought occurring until October (Fig. 1). In Sweden, on the other hand, buried seeds of the Italian genotype did not germinate at any time of the year. The Swedish summer may be too short to relieve the strong dormancy of the Italian genotype. However, we may have overestimated dormancy levels to some extent, because germination assays were performed under standard germination conditions (mean daily temperature of 18·7 °C) that are, although recommended for seed dormancy studies (Bentsink and Koornneef, 2011; Footitt and Finch-Savage, 2011), very warm compared with the mean soil temperatures during the natural germination period in the field sites (Italy, 12·6 °C; Sweden, 11·3 °C). Previous studies have shown that A. thaliana seeds first gain the ability to germinate at low temperatures when dormancy is gradually relieved (Baskin and Baskin, 2014).
There were also marked differences in the dormancy cycle between the two burial sites. Seasonal variation in soil temperature is considered to be the main factor regulating annual dormancy cycles, but soil moisture conditions may also play a role (Benech-Arnold et al., 2000; Batlla and Luis Benech-Arnold, 2007; Footitt et al., 2011, 2013). High temperatures usually lead to a faster loss of dormancy during summer in winter annuals such as A. thaliana (Footitt et al., 2011; Baskin and Baskin, 2014). The soil temperature was higher in Italy than in Sweden year-round (Fig. 1), and dormancy levels were indeed generally lower at the Italian site than in Sweden.
The temporal changes in the germinability of the non-dormant seeds that were buried indicated both genetic and environmental effects on secondary dormancy induction. At both sites, secondary dormancy was induced when seeds were exposed to the soil environment, but the Italian genotype responded faster than did the Swedish genotype (Fig. 2). Cold stratification is considered to be the most important mechanism for secondary dormancy induction in winter annuals (Bewley et al., 2013; Baskin and Baskin, 2014), and has been demonstrated for A. thaliana (Penfield and Springthorpe, 2012; Debieu et al., 2013). However, warm stratification can also induce secondary dormancy in A. thaliana (Huang et al., 2010; Montesinos-Navarro et al., 2012), and may explain much of the increased dormancy of the non-dormant Italian seeds that were dispersed in spring and quickly became dormant after exposure to warm summer temperatures in the soil.
The conditions during seed maturation on the mother plant are known to affect the primary dormancy level of seeds (Donohue, 2009; Postma and Ågren, 2015). We have in a previous study demonstrated that these maternal effects result in higher dormancy levels of seeds produced at the Italian and Swedish field site compared with seeds produced under standard glasshouse conditions (Postma and Ågren, 2015). Within 1 year after burial, the after-ripened non-dormant glasshouse seeds had the same dormancy behaviour as the seeds dispersed with natural levels of primary dormancy, although they continued to have somewhat higher germination proportions than the field-matured seeds (Fig. 2). Although we cannot separate the effects of the after-ripening history of the seeds from the maternal environmental conditions, the results demonstrate that A. thaliana seeds can adjust their dormancy levels to current environmental conditions, and suggest that any maternal effects on dormancy cycles are small.
The germination proportions of field-matured seeds of the local genotypes were markedly lower during the first germination season compared with the second season at both sites, indicating that many seeds would have ended up in the seed bank. This is in line with observations indicating that almost a third of the established plants in Norwegian A. thaliana populations resulted from seeds in the persistent seed bank (Falahati-Anbaran et al., 2014). Our findings suggest that primary dormancy should contribute to the formation of a persistent seed bank by preventing a considerable portion of the seeds from germinating in the first season.
The soil environment was much more hostile to buried seeds in Italy compared with Sweden. Seed mortality after 1 year of burial was 10-fold higher in Italy compared with Sweden, even though the predation rate was higher in Sweden. Seed aging is the time-dependent deterioration of seeds and eventually leads to the loss of viability (Walters, 1998). The rate of seed ageing increases with temperature, while both high and very low moisture content can be associated with a high rate of ageing (Walters, 1998; Walters et al., 2005; Bewley et al., 2013). The high soil temperature in combination with a long dry period may have contributed to the high proportion of black seeds in Italy (Fig. 1), although black seeds may also have died for reasons other than ageing, including microbial decay or fungal infections. Montesinos et al. (2009) found that 25 % of the A. thaliana seeds buried in Spanish populations had turned black after 1·5 years, which is comparable with our findings in Italy.
Predation of A. thaliana seeds below the soil surface has not previously been quantified, and was much higher in Sweden compared with Italy. Montesinos et al. (2009) did not mention any seed predation in their seed burial experiment with nylon mesh bags. Baskin and Baskin (1983) buried nylon bags containing seeds in plastic containers, and Footitt et al. (2011, 2013, 2014) actively decreased the mortality risk by treating the seeds with fungicide and placing the seeds in soda lime Ballotini balls before burial. In contrast, our seeds experienced local water conditions, and were in contact with soil particles and soil biota, although only seed predators small enough to get through the mesh size of our nylon bags could access the seeds. Nematodes were the only potential seed predators observed inside seed bags at excavation. Glasshouse-produced seeds of the Italian genotype were significantly more predated than other genotype × maternal environment combinations, which might be related to the thickness of the seed coat (Gardarin et al., 2010) or variation in secondary metabolites (Donkin et al., 1995).
Our findings have practical implications for weed control in agricultural systems. Most weed species of arable lands have physiological dormancy, and their dormancy cycles play an important role in the periodicity of weed emergence (Benech-Arnold et al., 2000; Batlla and Luis Benech-Arnold, 2007; Baskin and Baskin, 2014). Much effort has been put into predicting changes in dormancy levels of weeds because strategic timing of agronomic practices can highly improve weed control (Dyer, 1995; Colbach et al., 2005; Batlla and Luis Benech-Arnold, 2007). Our results demonstrate that genetic variation in dormancy cycles between populations from different geographic regions should be taken into account in weed emergence models. Moreover, variation in seed mortality among habitats should affect weed eradication strategies. More knowledge about the genetic variability of dormancy and seed persistence in the soil is still needed, and poses a future challenge for weed modelling (Grundy, 2003; Neve et al., 2009).
In summary, while primary seed dormancy has received much attention, we are just starting to learn about the role of secondary dormancy in the germination timing of plant populations. We have demonstrated genetic differences in dormancy cycling behaviour between two populations of A. thaliana, which are likely to contribute to local adaptation. Our results suggest that primary dormancy allows a proportion of the dispersed seeds to enter the seed bank by preventing germination in the first germination season following dispersal, and also that the value of a seed bank in A. thaliana should vary among environments because of differences in the rate of seed mortality. Future work should quantify the adaptive significance of genetic differentiation in dormancy cycling, and determine the causes of seed mortality. This knowledge will not only increase our understanding of the importance of seed bank traits for local adaptation and population dynamics of natural plant populations, but can also be used to enhance weed control in agriculture.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxford-journals.org and consist of the following. Table S1: the effect of time since seed burial, genotype and maternal environment on the germination proportions after 1 and 4 weeks in the growth room. Table S2: the effect of time since burial, genotype and maternal environment on the proportion of black seeds, the proportion of predated seeds and total seed mortality. Fig. S1: the germination proportion of seeds of the local genotype produced at the site of burial (Italy and Sweden) as a function of the number of days in the growth room.
ACKNOWLEDGEMENTS
We thank Jenny Glans, Elham Sadeghayobi, Mattias Vass and Carolina Wimmergren for help with field and glasshouse work, and two anonymous reviewers for their helpful comments on the manuscript. This work was supported by grants from the Swedish Research Council to J.Å.
LITERATURE CITED
- Ågren J, Schemske DW. 2012. Reciprocal transplants demonstrate strong adaptive differentiation of the model organism Arabidopsis thaliana in its native range. New Phytologist 194: 1112–1122. [DOI] [PubMed] [Google Scholar]
- Ågren J, Oakley CG, McKay JK, Lovell JT, Schemske DW. 2013. Genetic mapping of adaptation reveals fitness tradeoffs in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 110: 21077–21082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Blanco C, Bentsink L, Hanhart CJ, Blankestijn-de Vries H, Koornneef M. 2003. Analysis of natural allelic variation at seed dormancy loci of Arabidopsis thaliana. Genetics 164: 711–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin CC, Baskin JM. 2014. Seeds: ecology, biogeography, and evolution of dormancy and germination, 2nd edn San Diego, CA: Academic Press. [Google Scholar]
- Baskin JM, Baskin CC. 1983. Seasonal changes in the germination responses of buried seeds of Arabidopsis thaliana and ecological interpretation. Botanical Gazette 144: 540–543. [Google Scholar]
- Baskin JM, Baskin CC. 1985. The annual dormancy cycle in buried weed seeds: a continuum. BioScience 35: 492–498. [Google Scholar]
- Batlla D, Benech-Arnold RL. 2007. Predicting changes in dormancy level in weed seed soil banks: implications for weed management. Crop Protection 26: 189–197. [Google Scholar]
- Benech-Arnold RL, Sánchez RA, Forcella F, Kruk BC, Ghersa CM. 2000. Environmental control of dormancy in weed seed banks in soil. Field Crops Research 67: 105–122. [Google Scholar]
- Bentsink L, Koornneef M. 2011. Identification and characterization of quantitative trait loci that control seed dormancy in Arabidopsis. In: Kermode AR, ed. Seed dormancy: methods and protocols. Totowa, NJ: Humana Press, 165–184. [DOI] [PubMed] [Google Scholar]
- Bentsink L, Hanson J, Hanhart CJ, et al. 2010. Natural variation for seed dormancy in Arabidopsis is regulated by additive genetic and molecular pathways. Proceedings of the National Academy of Sciences, USA 107: 4264–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki H. 2013. Seeds: physiology of development, germination and dormancy. New York: Springer. [Google Scholar]
- Chiang GCK, Barua D, Dittmar E, Kramer EM, de Casas RR, Donohue K. 2012. Pleiotropy in the wild: the dormancy gene DOG1 exerts cascading control on life cycles. Evolution 67: 883–893. [DOI] [PubMed] [Google Scholar]
- Clerkx EJM, El-Lithy ME, Vierling E, et al. 2004. Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiology 135: 432–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbach N, Dürr C, Roger-Estrade J, Caneill J. 2005. How to model the effects of farming practices on weed emergence. Weed Research 45: 2–17. [Google Scholar]
- Debieu M, Tang C, Stich B, et al. 2013. Co-variation between seed dormancy, growth rate and flowering time changes with latitude in Arabidopsis thaliana. PLoS One 8: e61075 doi: 10.1371/journal.pone.0061075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkx MPM, Karssen CM. 1994. Are seasonal dormancy patterns in Arabidopsis thaliana regulated by changes in seed sensitivity to light, nitrate and gibberellin? Annals of Botany 73: 129–136. [Google Scholar]
- Dittmar EL, Oakley CG, Ågren J, Schemske DW. 2014. Flowering time QTL in natural populations of Arabidopsis thaliana and implications for their adaptive value. Molecular Ecology 23: 4291–4303. [DOI] [PubMed] [Google Scholar]
- Donkin SG, Eiteman MA, Williams PL. 1995. Toxicity of glucosinolates and their enzymatic decomposition products to Caenorhabditis elegans. Journal of Nematology 27: 258. [PMC free article] [PubMed] [Google Scholar]
- Donohue K. 2005. Seeds and seasons: interpreting germination timing in the field. Seed Science Research 15: 175–187. [Google Scholar]
- Donohue K. 2009. Completing the cycle: maternal effects as the missing link in plant life histories. Philosophical Transactions of the Royal Society B: Biological Sciences 364: 1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohue K, Rubio de Casas R, Burghardt L, Kovach K, Willis CG. 2010. Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics 41: 293–319. [Google Scholar]
- Dyer WE. 1995. Exploiting weed seed dormancy and germination requirements through agronomic practices. Weed Science 43: 498–503. [Google Scholar]
- Evans MEK, Dennehy JJ. 2005. Germ banking: bet-hedging and variable release from egg and seed dormancy. Quarterly Review of Biology 80: 431–451. [DOI] [PubMed] [Google Scholar]
- Falahati-Anbaran M, Lundemo S, Stenøien HK. 2014. Seed dispersal in time can counteract the effect of gene flow between natural populations of Arabidopsis thaliana. New Phytologist 202: 1043–1054. [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. 2006. Seed dormancy and the control of germination. New Phytologist 171: 501–523. [DOI] [PubMed] [Google Scholar]
- Footitt S, Finch-Savage WE. 2011. Production of seed samples for the effective molecular analysis of dormancy cycling in Arabidopsis. In: Kermode AR, ed. Seed dormancy: methods and protocols. Totowa, NJ: Humana Press, 65–79. [DOI] [PubMed] [Google Scholar]
- Footitt S, Douterelo-Soler I, Clay H, Finch-Savage WE. 2011. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proceedings of the National Academy of Sciences, USA 108: 20236–20241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Huang Z, Clay HA, Mead A, Finch-Savage WE. 2013. Temperature, light and nitrate sensing coordinate Arabidopsis seed dormancy cycling, resulting in winter and summer annual phenotypes. The Plant Journal 74: 1003–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Footitt S, Clay HA, Dent K, Finch-Savage WE. 2014. Environment sensing in spring-dispersed seeds of a winter annual Arabidopsis influences the regulation of dormancy to align germination potential with seasonal changes. New Phytologist 202: 929–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardarin A, Dürr C, Mannino MR, Busset H, Colbach N. 2010. Seed mortality in the soil is related to seed coat thickness. Seed Science Research 20: 243–256. [Google Scholar]
- Grime JP. 2000. The contribution of seedling regeneration to the structure and dynamics of plant communities, ecosystems and larger units of the landscape. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities, 2nd edn Wallingford, UK: CAB International, 361–374. [Google Scholar]
- Grundy AC. 2003. Predicting weed emergence: a review of approaches and future challenges. Weed Research 43: 1–11. [Google Scholar]
- Hilhorst HW. 1998. The regulation of secondary dormancy. The membrane hypothesis revisited. Seed Science Research 8: 77–90. [Google Scholar]
- Huang X, Schmitt J, Dorn L, et al. 2010. The earliest stages of adaptation in an experimental plant population: strong selection on QTLS for seed dormancy. Molecular Ecology 19: 1335–1351. [DOI] [PubMed] [Google Scholar]
- Kalamees R, Zobel M. 2002. The role of the seed bank in gap regeneration in a calcareous grassland community. Ecology 83: 1017–1025. [Google Scholar]
- Lundemo S, Falahati-Anbaran M, Stenøien HK. 2009. Seed banks cause elevated generation times and effective population sizes of Arabidopsis thaliana in northern Europe. Molecular Ecology 18: 2798–2811. [DOI] [PubMed] [Google Scholar]
- Luzuriaga AL, Escudero A, Olano JM, Loidi J. 2005. Regenerative role of seed banks following an intense soil disturbance. Acta Oecologica 27: 57–66. [Google Scholar]
- Montesinos A, Tonsor SJ, Alonso-Blanco C, Picó FX. 2009. Demographic and genetic patterns of variation among populations of Arabidopsis thaliana from contrasting native environments. PLoS One 4: e7213 doi: 10.1371/journal.pone.0007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesinos-Navarro A, Picó FX, Tonsor SJ. 2012. Clinal variation in seed traits influencing life cycle timing in Arabidopsis thaliana. Evolution 66: 3417–3431. [DOI] [PubMed] [Google Scholar]
- Neve P, Vila-Aiub M, Roux F. 2009. Evolutionary-thinking in agricultural weed management. New Phytologist 184: 783–793. [DOI] [PubMed] [Google Scholar]
- Oakley CG, Ågren J, Atchison RA, Schemske DW. 2014. QTL mapping of freezing tolerance: links to fitness and adaptive trade-offs. Molecular Ecology 23: 4304–4315. [DOI] [PubMed] [Google Scholar]
- Pakeman RJ, Small JL. 2005. The role of the seed bank, seed rain and the timing of disturbance in gap regeneration. Journal of Vegetation Science 16: 121–130. [Google Scholar]
- Penfield S, Springthorpe V. 2012. Understanding chilling responses in Arabidopsis seeds and their contribution to life history. Philosophical Transactions of the Royal Society B: Biological Sciences 367: 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma FM, Ågren J. 2015. Maternal environment affects the genetic basis of seed dormancy in Arabidopsis thaliana. Molecular Ecology 24: 785–797. [DOI] [PubMed] [Google Scholar]
- Core Team R. 2014. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Saatkamp A, Poschlod P, Venable DL. 2014. The functional role of soil seed banks in natural communities. In: Hillier SH, ed. Seeds: the ecology of regeneration in plant communities, 3rd edn Wallingford, UK: CAB International, 263–295. [Google Scholar]
- Seger J, Brockmann HJ. 1987. What is bet hedging? In: Harvey PH, Partridge L, eds. Oxford Surveys in Evolutionary Biology, Vol. 4 Oxford: Oxford University Press, 182–211. [Google Scholar]
- Thompson K. 2000. The functional ecology of soil seed banks. In: Fenner M, ed. Seeds: the ecology of regeneration in plant communities, 2nd edn Wallingford, UK: CAB International, 215–235. [Google Scholar]
- Thompson K, Bakker JP, Bekker RM, Hodgson JG. 1998. Ecological correlates of seed persistence in soil in the north-west European flora. Journal of Ecology 86: 163–169. [Google Scholar]
- Tielbörger K, Petruů M, Lampei C. 2012. Bet-hedging germination in annual plants: a sound empirical test of the theoretical foundations. Oikos 121: 1860–1868. [Google Scholar]
- Venable DL, Brown JS. 1988. The selective interactions of dispersal, dormancy, and seed size as adaptations for reducing risk in variable environments. American Naturalist 131: 360–384. [Google Scholar]
- Vleeshouwers LM, Bouwmeester HJ, Karssen CM. 1995. Redefining seed dormancy: an attempt to integrate physiology and ecology. Journal of Ecology 83: 1031. [Google Scholar]
- Walters C. 1998. Understanding the mechanisms and kinetics of seed aging. Seed Science Research 8: 223–244. [Google Scholar]
- Walters C, Hill LM, Wheeler LJ. 2005. Dying while dry: kinetics and mechanisms of deterioration in desiccated organisms. Integrative and Comparative Biology 45: 751–758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.