Abstract
Background and Aims Most pollinators are generalists and therefore are likely to transfer heterospecific pollen among co-flowering plants. Most work on the impacts of heterospecific pollen deposition on plant fecundity has utilized hand-pollination experiments in greenhouse settings, and we continue to know very little about the reproductive effects of heterospecific pollen in field settings.
Methods We explored how patterns of naturally deposited heterospecific pollen relate to the reproductive output of Delphinium barbeyi, a common subalpine perennial herb in the Rocky Mountains (USA). We assessed a wide range of naturally occurring heterospecific pollen proportions and pollen load sizes, and linked stigmatic pollen deposition directly to seed set in individual carpels in the field.
Key Results We found that heterospecific pollen deposition in D. barbeyi is common, but typically found at low levels across stigmas collected in our sites. Neither conspecific nor heterospecific pollen deposition was related to carpel abortion. By contrast, we saw a significant positive relationship between conspecific pollen amount and viable seed production, as well as a significant negative interaction between the effects of conspecific pollen and heterospecific pollen amount, whereby the effect of conspecific pollen on viable seed production became weaker with greater heterospecific deposition on stigmas.
Conclusions To our knowledge, this is the first demonstration of a relationship between heterospecific pollen and seed production in a field setting. In addition, it is the first report of an interaction between conspecific and heterospecific pollen quantities on seed production. These findings, taken with the results from other studies, suggest that greenhouse hand-pollination studies and field studies should be more tightly integrated in future work to better understand how heterospecific pollen transfer can be detrimental for plant reproduction.
Keywords: field studies, hand-pollination studies, interspecific pollen transfer, pollen quality, seed set, stigmas
INTRODUCTION
Most pollinators are generalist foragers that can switch between plant species within a single foraging bout (Waser et al., 1996; Brosi and Briggs, 2013). This sharing of pollinators among plant species within a community can lead to the transfer of heterospecific pollen to plant stigmas. Such heterospecific pollen deposition is highly variable in nature (Ashman and Arceo-Gomez, 2013), and can represent a substantial percentage of total pollen on a stigma, often >50 % of grains (Ashman and Arceo-Gomez, 2013). Stigmatic heterospecific pollen can negatively impact plant reproductive function (reviewed in Morales and Traveset, 2008; Mitchell et al., 2009; Ashman and Arceo-Gomez, 2013), but we continue to have a limited understanding of the magnitude and mechanisms of these impacts, particularly under field conditions.
Most of what we know about the reproductive effects of heterospecific pollen comes from hand-pollination studies, which have primarily focused on mechanisms of reproductive disruption (reviewed in Morales and Traveset, 2008). Heterospecific pollen can reduce reproductive output by physically blocking conspecific pollen from adhering to the stigma (Caruso and Alfaro, 2000), by driving stigma closure (e.g. Waser and Fugate, 1986), by producing allelochemicals that limit subsequent pollen germination (Thomson et al., 1981; Kanchan and Jayachandra, 1980; Murphy and Aarssen, 1995), by interfering with pollen tube growth in the style (Arceo-Gómez and Ashman, 2011) and by usurping ovules, especially among closely related plant species (Harder and Cruzan, 1993; Burgess et al., 2008).
There is reason to suspect that these hand-pollination results, which come primarily from greenhouse or potted-plant studies, may not reflect the reality of field situations. In nature, we would expect a range of proportions of heterospecific pollen deposited on stigmas, as well as a range of pollen from different plant species, with different amounts of diversity on stigmas. Most hand-pollination experiments have applied fixed heterospecific:conspecific pollen ratios to stigmas, and the effects on plant reproduction are variable, with some finding detrimental effects of heterospecific pollen (Thomson et al., 1981; Brown et al., 2002; Larson et al., 2006) and others showing no impact (Kohn and Waser, 1985; Caruso and Alfaro, 2000). Most of these studies used only one heterospecific pollen species and in proportions not necessarily common in nature, making it difficult to apply inference from these results to plant reproduction in the field. Moreover, we know of only one study (Thomson et al., 1981) that assessed the impact of heterospecific pollen over a continuous range of experimental heterospecific pollen proportions—a situation that is likely common in nature—and that was a hand-pollination study that held conspecific pollen quantity constant, using a single heterospecific pollen donor species. In addition, the greenhouse or potted-plant context of most or all of these studies may not translate to the field in terms of possible interactions between resource limitation and heterospecific pollen deposition. For example, heterospecific pollen may have larger impacts on seed set in plants that are water- or nutrient-stressed relative to plants that are not facing serious resource limitation. Thus, while we know from field studies that heterospecific pollen deposition is common in nature, and from hand-pollination studies understand some of the mechanisms by which it can disrupt plant reproduction, the extent to which heterospecific pollen impacts plant reproduction in the field remains poorly understood.
Another gap in the literature on heterospecific pollen is assessment of possible interactions between heterospecific and conspecific pollen, i.e. whether the impact of a fixed amount of heterospecific pollen has varying impacts on plant reproduction depending on conspecific pollen deposition. Such interactions could arise from mechanisms driven either by the heterospecific pollen or by the plant on which the heterospecific pollen is deposited. Mechanisms mediated by heterospecific pollen include stigma clogging, stylar clogging, allelopathic inhibition and ovule usurpation (Morales and Traveset, 2008). One common feature of these mechanisms is that heterospecific pollen is likely to have stronger impacts if it is deposited before conspecific pollen, especially if it is in more contact with the receptive stigmatic surface (Waser and Fugate, 1986; Morales and Traveset, 2008). In most realistic scenarios of pollen deposition, the more heterospecific pollen that is proportionally present on a stigma, the greater the chance that it arrived early, enhancing its negative impact, whereas with more conspecific pollen the chance of an early arrival and concomitant deleterious effects is reduced. Second, there is at least one documented mechanism driven by the plant receiving the heterospecific pollen, which is stigma closure, i.e. stigmatic lobes closing in response to heterospecific pollen, effectively ruling out subsequent seed production in that flower (Waser and Fugate, 1986; Morales and Traveset, 2008), and plants could also hypothetically drive active inhibition of pollen tube growth (both conspecific and heterospecific), or ovule or carpel abortion, in response to heterospecific pollen. To effect such active mechanisms, flowers must be able to detect the presence of heterospecific pollen. If they can also detect the quantity or proportion of heterospecific pollen grains, plants could potentially use this signal when actively disrupting pollination at various points in the process (stigma closure; inhibition of pollen tube growth; ovule or carpel abortion), ultimately as a means to conserve resources by not investing in flowers that may have low-quality seed production or quality.
In this study, to begin to understand the impact of heterospecific pollen in natural systems, we used a field approach linking stigmatic pollen deposition to seed set in the same individual carpels in wild plants that had been naturally pollinated (Waser and Price, 1991a, b). In contrast to hand-pollination studies, this approach allowed us to assess stigmatic pollen loads varying greatly in conspecific pollen and heterospecific pollen quantities (but varying by definition over a range that is found in nature), while achieving a relatively large sample size and replication across space. This approach also allowed us to assess interactions between conspecific and heterospecific pollen in assessing plant reproduction.
We examined how the total amount of naturally deposited heterospecific pollen covaried with the reproductive output of Delphinium barbeyi (Ranunculaceae), a common subalpine flower species in the Rocky Mountains of Colorado, USA. D. barbeyi receives visits from several species of bumble bees (Apidae: Bombus) that are known to visit many co-flowering species within a community (Elliott and Irwin, 2009; Brosi and Briggs, 2013). We asked the following specific questions. (1) How variable is heterospecific pollen deposition in naturally occurring D. barbeyi populations? (2) Is conspecific pollen and/or heterospecific pollen deposition related to whether or not a carpel will abort? (3) How are the amounts of conspecific pollen and heterospecific pollen deposition related to seed production in (non-aborted) carpels? We hypothesized that there would be a positive relationship between heterospecific pollen deposition and carpel abortion rates and a negative relationship between heterospecific pollen deposition and seed set. In particular, we predicted that the effect of heterospecific pollen would vary depending on conspecific pollen deposition, with heterospecific pollen having a larger negative impact on stigmas with lower conspecific pollen deposition.
MATERIALS AND METHODS
Plant study system
Delphinium barbeyi is a long-lived perennial herb found in sub-alpine meadows in western North America. Its flowers are protandrous and deep purple in colour, with elongated corollas. A flower generally has three carpels. The plants are typically large, with multiple racemes per plant. The total number flowers per plant vary greatly, but the plants in our plots had an average of 12·3 flowers per raceme (median = 11, maximum = 52, minimum = 1). D. barbeyi is generally in bloom from mid-June to late July in our field sites and is a predominately outcrossing but self-compatible species (Williams et al., 2001). The primary pollinators of D. barbeyi are worker bumble bees (particularly Bombus appositus and Bombus nevadensis). D. barbeyi is known to receive heterospecific pollen, as the majority of its pollinators are generalist bumble bees, all of which also visit several other co-flowering species (Elliott and Irwin, 2009; Brosi and Briggs, 2013). While hummingbirds are common in this system and are important pollinators of the early-season congener Delphinium nuttallianum, we made only one observation of a Selasphorus rufus hummingbird visiting D. barbeyi in our sites in thousands of hours of field time (H. Briggs, pers. obs.).
Field sites
We worked in five study sites in subalpine meadows surrounding the Rocky Mountain Biological Laboratory in Gothic, Colorado (38°57·5′ N, 106°59·3′ W, 2740–3065 m above sea level), in the Gunnison National Forest, western Colorado, USA. Study sites were located at least 1 km apart. We selected sites with comparable densities of D. barbeyi (i.e. those in which D. barbeyi was the most abundant or the second most abundant plant species). The sites all occur within 10 km of each other and were similar in terms of both biotic and abiotic conditions (though it was beyond the scope of this study to quantify the abiotic conditions of each site). Each site had on average 9·2 plant species in bloom at the time of data collection (median = 10, maximum = 12, minimum = 5). Each site consisted of a single 20 × 20 m plot, which was chosen within the site to maximize D. barbeyi density.
Stigma and seed collection and quantification
We collected data from each site once during the summer of 2013. At each plot we bagged 50 D. barbeyi racemes (from different plant individuals) with incipient flower buds. Two to three days later, we opened 15–20 bags to expose female receptive but unpollinated flowers, and labelled two receptive flowers per raceme. We allowed pollination to occur uninhibited for a standardized 4-h period before reclosing the bags (Brosi and Briggs, 2013). D. barbeyi flowers are typically female-receptive for ∼2 d (N. Waser, UC Riverside, USA, pers. comm.) Some of our stigmas had extremely large pollen loads, and our expectation is that if we had assessed stigmas over the full 48-hour period of receptivity we might have seen a few additional individuals with slightly larger pollen loads. Still, we expect that we captured most of the variation in pollen deposition that is naturally present in the system with a 4-h visitation window.
We followed a protocol to allow us to link pollen deposition and seed set in the same individual carpels in the field (Waser and Price, 1991a, b). Approximately 4 d after each receptive flower was pollinated (i.e. after pollen tube growth and fertilization had occurred and flowers had wilted, but while the germinated pollen exines were still adhering to the stigma), we harvested floral stigmas, carefully leaving remaining floral structures undamaged. At this time we also marked individual developing carpels (typically three per flower) with a permanent marker. We mounted the harvested stigmas from each plot on slides with fuchsin jelly in the field (Kearns and Inouye, 1993). In the laboratory, we counted the total numbers of conspecific (D. barbeyi) and heterospecific pollen grains on each stigma. With the help of pollen reference collections housed at the Rocky Mountain Biological Laboratory, we were able to differentiate D. barbeyi pollen from the heterospecific pollen on our slides. It was beyond the scope of this study to identify the heterospecific pollen grains to species. Finally, we returned to each site 7–15 d later to collect the mature fruits. We dissected the fruits and counted the developed and undeveloped seeds per ovary, allowing us to match the total seed set per carpel with the pollen load of each previously harvested stigma.
Data analysis
We examined how well total heterospecific pollen predicted the reproductive output in D. barbeyi by running two primary analyses: first, an assessment of the proportion of carpels that had fully aborted (i.e. contained no developed seeds); and second, an assessment of seed set in carpels that had not aborted (i.e. those with one or more developed seeds). We ran these analyses separately because different factors (e.g. resource limitation) may drive a plant to fully abort a carpel versus invest in it (Burd, 1998). In addition, we assessed the collinearity between conspecific and heterospecific pollen deposition using the non-parametric Spearman’s ρ. We used a non-parametric test because the data did not meet assumptions of linearity. Because the correlation was low (ρ = 0·001) and not statistically significant (p = 0·86), we used both as covariates. We conducted all analyses using the R statistical programming language (R Development Core Team, 2013).
We used generalized linear mixed-effects models (GLMMs) because of hierarchical lack of independence in our data (Bolker et al., 2009). Plants within sites are not independent, given similar resource conditions, and are likely genetically related. Similarly, flowers within a plant clearly do not represent independent samples. Thus, we included nested random effects in the model, with flower nested within plant nested within site. We assessed carpel abortion as counts of aborted versus non-aborted carpels, and seed production as the counts of developed (fertilized) seeds versus undeveloped seeds, both of which are structurally binomial variables. Relative to a binomial distribution, however, our data for both analyses were overdispersed, which we corrected by including an individual-level (i.e. carpel-level) random effect (Elston et al., 2001). We ran GLMMs with binomial errors using the default logit link in the lme4 package for R (Bates et al., 2012).
We assessed the relative contributions of the quantities of heterospecific and conspecific pollen to reproductive output and carpel abortion using a model-comparison framework. In these models conspecific pollen and heterospecific pollen, as well as their interaction, are the fixed effects that we assessed in different combinations. We specifically compared five mixed-effects models: (1) full model including an interaction between the two fixed effects (conspecific pollen and heterospecific pollen); (2) additive model (in which the fixed effects were added but interactions were not assessed); (3) amount of conspecific pollen only; (4) amount of heterospecific pollen only; and (5) a model including no fixed effects (i.e. random effects only). For all analyses, we used Akaike’s information criterion corrected for small sample size (AICc) (Burnham et al., 2010) to determine the best model(s). We reported all models within two ΔAICc points of the best model. We used the AICcmodavg package in R (Mazerolle, 2012) for model selection.
We excluded observations in which there was seed set with either zero stigmatic pollen recorded (3 carpels out of 619) or zero proportion of conspecific pollen (12 carpels). We assume that these observations were due to loss of stigmatic pollen in the field between fertilization and stigma collection.
RESULTS
Overview
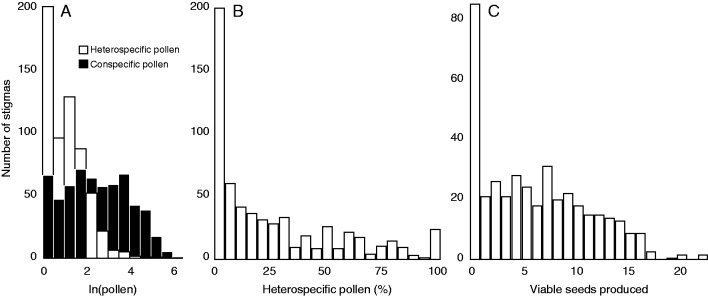
We counted >2400 seeds and linked reproductive output to pollen deposition in 604 carpels from 216 flowers on 131 plants. Each non-aborted carpel produced a mean of 6 viable seeds (median 5; range 0–22; standard deviation 5·12). Of the 604 carpels we examined, 510 (85 %) of them had at least some heterospecific pollen on the stigma (median = 3 pollen grains; mean = 4·6; range = 0–222; standard deviation = 10·85; Fig. 1) and 211 (35 %) had fully aborted. Many of the stigmas in our study received more conspecific pollen (median = 11 pollen grains; mean = 33·6; range = 0–656; standard deviation = 57·30) than is strictly necessary for fertilization based on ovule number (median ovule number per carpel = 10; mean = 8·7; range = 0–26; standard deviation = 7·26). Finally, conspecific pollen and heterospecific pollen counts were not correlated at the level of the stigma (Spearman’s ρ = 0·001; p = 0·86; Fig. 2).
Fig. 1.
Natural pollen deposition in Delphinium barbeyi. (A) Total amount of heterospecific and conspecific pollen naturally deposited on stigmas in the field. (B) Total amount of heterospecific pollen/total pollen deposited on stigmas in the field. (C) Total number of viable seed produced in non-aborted carpels.
Fig. 2.
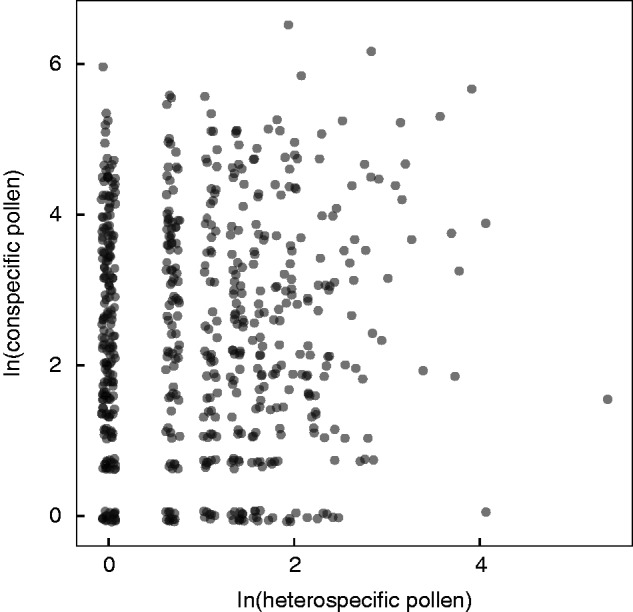
Relationship between heterospecific pollen and conspecific pollen. Counts of conspecific and heterospecific pollen are shown with natural log transformation to better visually depict the relationship, and are jittered to improve visualization of overlapping data points. The two variables are uncorrelated (raw data, non-parametric Spearman’s ρ = 0·007, P = 0·86).
Carpel abortion
We found three candidate models that were indistinguishable (i.e. within two ΔAIC points) as the best predictors of carpel abortion: (1) a random-effects only model (lowest AIC); (2) a model with conspecific pollen as the only fixed effect; and (3) a model with heterospecific pollen as the only fixed effect (see Table 1 for model comparison). For the second- and third-ranked models, neither the conspecific pollen amount (p = 0·90) nor the heterospecific pollen amount (p = 0·97; see Table 1 for model coefficients) was significantly associated with carpel abortion. Thus, neither heterospecific nor conspecific pollen deposition seems to be a primary driver of carpel abortion in D. barbeyi in our system.
Table 1.
Results of generalized linear mixed-effects models with binomial errors (viable seed production and carpel abortion results).
| Candidate models | K | AICc | ΔAIC | AICcWeight |
|---|---|---|---|---|
| 1) Proportion of Viable Seeds | ||||
| HP × CP | 8 | 1727·74 | 0 | 0·91 |
| HP + CP | 7 | 1733·86 | 6·11 | 0·04 |
| 2) Proportion of Carpels Fully Aborted | ||||
| full random effects | 5 | 243·52 | 0 | 0·5 |
| CP | 6 | 245·18 | 1·66 | 0·22 |
| HP | 6 | 245·74 | 2·23 | 0·17 |
| Best model results | Fixed effects | Coefficient | s.e. | P value |
| 1) Proportion of Viable Seeds | ||||
| HP × CP | (Intercept) | –0·207 | 0·308 | 0·501 |
| HP | 0·006 | 0·014 | 0·692 | |
| CP | 0·006 | 0·002 | 0·001*** | |
| HP:CP | –0·0004524 | 0·0001786 | 0·011* | |
| 2) Proportion of Carpels Fully Aborted | ||||
| full random effects | (Intercept) | 12·609 | 6·490 | 0·052* |
K, total number of estimable parameters; AICcWeight, indicates the level of support in favour of the model being the most parsimonious among the candidate model set; HP, heterospecific pollen; CP, conspecific pollen.
*P < 0·05; ***P < 0·001.
Seed production
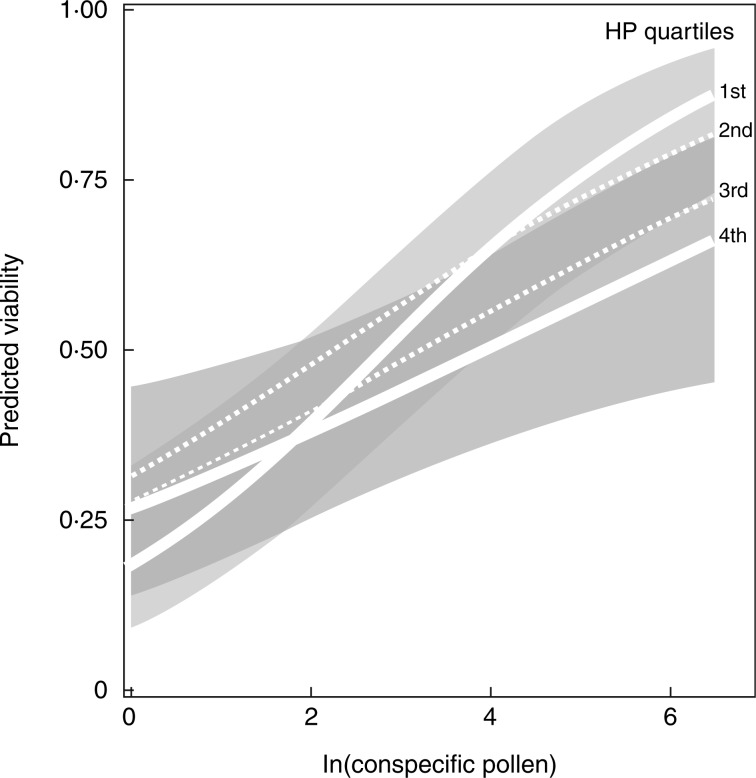
The model that best explained the probability of viable seed production in non-aborted carpels was the full model that included an interaction term between heterospecific and conspecific pollen quantities (Fig. 3, Table 1). This model included a significant positive relationship between conspecific pollen amount and viable seed production; no significant effect of heterospecific pollen on its own; and a significant negative interaction between conspecific pollen and heterospecific pollen amount (Fig. 3, also see Table 1 for model coefficients) such that the effect of conspecific pollen deposition on viable seed production gets weaker (i.e. shallower slope) with greater heterospecific pollen deposition. For example, according to the model predictions, if a stigma had 54 grains of conspecific pollen and no heterospecific pollen, 60 % of the seeds would be viable. By contrast, for a stigma with the same 54 grains of conspecific pollen but also 50 grains of heterospecific pollen, the model predicts that only 45 % of the seeds would be viable.
Fig. 3.
Effect of conspecific and heterospecific pollen deposition on probability of viable seed production. Lines depict the best-fit estimate from the GLMM that describes the interaction between conspecific and heterospecific pollen deposition and viable seed production. First and 4th quartiles are solid white lines with 95 % confidence intervals, while 2nd and 3rd quartiles are depicted as thin dashed lines without confidence intervals for clarity. Note the difference between the fits for the 1st and 4th quartiles at the higher values of the x-axis. For the purpose of visualization we used ln(conspecific pollen) in this model to depict a more even spread across the conspecific pollen axis. See Table 1 for estimates based on continuous variation in heterospecific pollen. Heterospecific pollen quartiles: 1st, 0·0–1·0; 2nd, 1·0–2·5; 3rd, 2·5–5·0; 4th, 5·0–222·0.
DISCUSSION
To understand the association between naturally deposited heterospecific pollen and seed production, we used a field approach linking stigmatic pollen deposition to seed set in the same individual carpels in wild plants. Heterospecific pollen deposition was highly variable on D. barbeyi stigmas in the field, and while some degree of heterospecific pollen was found on most sampled flowers it was typically present in low amounts (Fig. 1). Neither heterospecific pollen nor conspecific pollen was a good predictor of carpel abortion in our sites. Conspecific pollen deposition was positively related to viable seed production, but we also found a significant negative interaction between heterospecific pollen and conspecific pollen. That is, with increasing heterospecific pollen the positive relationship between conspecific pollen deposition and viable seed production in D. barbeyi became weaker (Fig. 3).
We found that heterospecific pollen on D. barbeyi stigmas is widespread and highly variable, and typically occurs at relatively low levels. In terms of variability, heterospecific pollen represented anywhere from 0 to 97 % of the grains present in a pollen load. The widespread nature of heterospecific pollen deposition is reflected by the fact that 85 % of stigmas had some heterospecific pollen present. Still, most stigmas received only low levels of heterospecific pollen deposition (median of only 3 grains per stigma, relative to 11 grains for conspecific pollen). These results are broadly consistent with patterns reported in a comprehensive review of studies assessing the impact of heterospecific pollen transfer, including 77 species from 17 different sources (Ashman and Arceo-Gomez, 2013). This review found that, although receipt of heterospecific pollen was variable, all species received at least some heterospecific pollen on their stigmas. Similarly, in a community-wide analysis of pollen transfer, Fang and Huang (2013) found that among the 57 plant species they surveyed heterospecific pollen deposition was common but highly variable, representing 0–66 % of the total pollen on stigmas. The co-flowering plant community and pollinator behaviour are just two of the factors that might influence variation in heterospecific pollen receipt between plants of the same species within and between sites. Variation in heterospecific pollen receipt within species or even within a population obviously has important implications for the evolution of coping mechanisms and remains an area ripe for more research (Ashman and Arceo-Gomez 2013).
A large proportion of carpels aborted in our study (nearly 35 %), yet contrary to our expectations we found that neither heterospecific pollen nor conspecific pollen was a good predictor of carpel abortion in D. barbeyi. Thus, it seems likely that resource limitation, rather than pollen limitation, drove carpel abortion in our system. Extrinsic factors such as weather conditions, flower phenology, herbivory, competition (both intra- and inter-specific) and disease can lead to within plant-variation in carpel abortion (Burd, 1998; Niesenbaum, 1999; Marshall et al., 2010). Furthermore, intrinsic factors, including architecture (i.e. location of flower on the raceme), plant size, developmental constraints or allocation strategies, are also known to affect patterns of carpel abortion (Delph, 1986, 1993; Guitian et al., 1996; Burd, 1998).
In contrast to carpel abortion, both conspecific pollen and heterospecific pollen played a role in seed production in D. barbeyi. As expected, conspecific pollen deposition on its own was positively related to viable seed production. Heterospecific pollen on its own did not affect seed set, but there was a significant negative heterospecific pollen × conspecific pollen interaction. In other words, with greater heterospecific pollen deposition a fixed amount of conspecific pollen would result in lower seed set. Because this was a correlational study, we were not able to tease apart other factors that may have led to a decrease in seed production, such as diversity of heterospecific pollen. To our knowledge, this is the first demonstration of an interaction between heterospecific pollen and conspecific pollen in their effects on plant reproduction. We hypothesized that we would find such an interaction as we expected that the negative effects of heterospecific pollen might occur only in the context of conspecific pollen. In the case of comparing deposition of a small versus a large heterospecific pollen load in stigmas with no conspecific pollen, we would expect the same result: zero seeds produced. Following the same logic, we would expect the impact of depositing a medium-sized load of heterospecific pollen on a stigma would differ in stigmas with just a few conspecific pollen grains versus a large conspecific pollen load (relative to ovule number). We are aware of only one previous assessment of the statistical interaction between stigmatic pollen load quantity and the proportion of heterospecific pollen grains (Arceo-Gómez and Ashman, 2011). This study found no evidence for a pollen quantity × proportion of heterospecific pollen interaction, but the study design was oriented towards a different objective (assessing diversity of heterospecific pollen donors) and thus included just two heterospecific pollen load sizes, which corresponded to 28 and 16 % of total pollen load proportions. Finally, we were not able to distinguish between self and outcrossed conspecific pollen in this study. Self pollen has been shown to intensify the negative impact of heterospecific pollen receipt in Mimulus guttatus and self pollen has been shown to produce fewer seeds in D. barbeyi (Williams et al., 2001, Arceo-Gómez and Ashman 2014). More studies examining how mixed-mating plant species cope with heterospecific pollen receipt would be valuable, particularly in a field setting where plants may receive heterospecific pollen from a variety of plant species.
Putting this result in the context of previous findings highlights that there has been a gulf between field studies and hand-pollination studies in greenhouse settings, a gulf that, if bridged, would improve our understanding of the effects of heterospecific pollen transfer in nature. Future hand-pollination studies should contribute to bridging the gap by integrating what we have learned from field studies so far. First, hand-pollination studies should be designed using parameters explicitly drawn from field studies, in terms of heterospecific and conspecific pollen amounts, and heterospecific pollen diversity, considering not only means but also variability. To date, most hand-pollination studies have used only one proportion of heterospecific pollen (typically 50 %), and we are aware of only one hand-pollination study (Thomson et al., 1981) that specifically applied a full range of heterospecific pollen deposition. Second, the results from our field study, particularly our finding of a heterospecific pollen × conspecific pollen interaction in seed production, highlights the advantage of understanding this result mechanistically, via hand-pollination studies that vary the quantity of both conspecific pollen and heterospecific pollen factorially. Third, our finding that neither conspecific pollen nor heterospecific pollen was strongly linked to carpel abortion rates underscores that hand-pollination studies that control both pollen and resource limitation (e.g. with limited watering and/or limited soil nutrients) in a greenhouse setting could greatly improve our understanding of how these factors interact.
Similarly, field studies on the effects of heterospecific pollen can look to hand-pollination experiments—with their typically more mechanistic focus—for inspiration. A critical first step is to increase the number of field studies that directly link pollen deposition and seed production within the same carpel (Waser and Price, 1991a, b). To our knowledge the work we present here is the first in which this approach has been applied to understanding the effects of heterospecific pollen, which should be repeated in a wide range of plant species with different mating systems (Ashman and Arceo-Gomez, 2013). Another particular need is for field studies that assess multiple sites. We continue to have a poor understanding of how plant genotype and environmental factors interact to shape the effects of heterospecific pollen, and work along environmental gradients could be informative, especially in disentangling the relative effects of pollen versus resource limitation in shaping seed set. Similarly, experimental approaches to assessing the relative effects of resource versus pollen limitation in the context of heterospecific pollen deposition in field settings (e.g. with watering and/or fertilizer treatments) would also be a valuable research direction. Finally, hand-pollination and field approaches should be explicitly integrated by conducting more hand-pollination experiments in field settings.
Our understanding of the impact of heterospecific pollen deposition is growing, but there is still much to learn about the way that co-flowering plants interact through pollinator sharing and how heterospecific pollen deposition impacts plant reproductive fitness. In a changing world where we can expect to see both increasing disruptions in pollination (Biesmeijer et al., 2006; Potts et al., 2010; Brosi and Briggs, 2013) and the emergence of new interactions via introduced species and climate change, studies that unify both field studies and controlled hand-pollination studies will allow us to better understand the implications of heterospecific pollen deposition for reproductive output in natural plant communities.
ACKNOWLEDGEMENTS
A. Cooke and K. Niezgoda provided field assistance, and C. Chu, A. Graham, E. Lake, B. Lawley and A. Miljanic assisted with pollen and seed counts. P. Humphrey, G. Gilbert, I. Parker and two anonymous reviewers provided constructive comments on the manuscript. This work was supported by the US National Science Foundation (DEB-1120572 to B.J.B. and DBI-0753774, DBI-1262713, DBI-1034780, DBI-0432544, DBI-0420910, OIA-0963529, DBI-1219635 to the Rocky Mountain Biological Lab and I. Billick), the Rocky Mountain Biological Laboratory (to B.J.B. and H.M.B.), Emory University (to B.J.B. and L.M.A.), the University of California, Santa Cruz, and the Jean H. Langenheim Fellowship in Plant Ecology and Evolution (to H.M.B.).
LITERATURE CITED
- Arceo-Gómez G, Ashman T-L. 2011. Heterospecific pollen deposition: does diversity alter the consequences? New Phytologist 192: 738–746. [DOI] [PubMed] [Google Scholar]
- Arceo-Gómez G, Ashman T-L. 2014. Patterns of pollen quantity and quality limitation of pre-zygotic reproduction in Mimulus guttatus vary with co-flowering context. Oikos 123: 1261–1269. [Google Scholar]
- Ashman TL, Arceo-Gomez G. 2013. Toward a predictive understanding of the fitness costs of heterospecific pollen receipt and its importance in co-flowering communities. American Journal of Botany 100: 1061–1070. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B. 2012. lme4: linear mixed-effects models using S4 classes. R Package Version 0.999375-39. http://CRAN.Rproject.org/package=lme4. [Google Scholar]
- Biesmeijer JC, Roberts SPM, Reemer M, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313: 351–354. [DOI] [PubMed] [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution 24: 127–135. [DOI] [PubMed] [Google Scholar]
- Brosi BJ, Briggs HM. 2013. Single pollinator species losses reduce floral fidelity and plant reproductive function. Proceedings of the National Academy of Sciences of the USA 110: 13044–13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown BJ, Mitchell RJ, Graham SA. 2002. Competition for pollination between an invasive species (purple loosestrife) and a native congener. Ecology 83: 2328–2336. [Google Scholar]
- Burd M. 1998. “Excess” flower production and selective fruit abortion: a model of potential benefits. Ecology 79: 2123–2132. [Google Scholar]
- Burgess KS, Morgan M, Husband BC. 2008. Interspecific seed discounting and the fertility cost of hybridization in an endangered species. New Phytologist 177: 276–283. [DOI] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, Huyvaert KP. 2010. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behavioral Ecology and Sociobiology 65: 23–35. [Google Scholar]
- Caruso CM, Alfaro M. 2000. Interspecific pollen transfer as a mechanism of competition: effect of Castilleja linariaefolia pollen on seed set of Ipomopsis aggregata. Canadian Journal of Botany 78: 600–606. [Google Scholar]
- Delph LF. 1986. Factors regulating fruit and seed production in the desert annual Lesquerella gordonii. Oecologia 69: 471–476. [DOI] [PubMed] [Google Scholar]
- Delph LF. 1993. Factors affecting intraplant variation in flowering and fruiting in the gynodioecious species Hebe subalpina. Journal of Ecology 81: 287–296. [Google Scholar]
- Elliott SE, Irwin RE. 2009. Effects of flowering plant density on pollinator visitation, pollen receipt, and seed production in Delphinium barbeyi (Ranunculaceae). American Journal of Botany 96: 912–919. [DOI] [PubMed] [Google Scholar]
- Elston DA, Moss R, Boulinier T, Arrowsmith C, Lambin X. 2001. Analysis of aggregation, a worked example: numbers of ticks on red grouse chicks. Parasitology 122: 563–569. [DOI] [PubMed] [Google Scholar]
- Fang Q, Huang S-Q. 2013. A directed network analysis of heterospecific pollen transfer in a biodiverse community. Ecology 94: 1176–1185. [DOI] [PubMed] [Google Scholar]
- Guitian J, Guitian P, Navarro L. 1996. Fruit set, fruit reduction, and fruiting strategy in Cornus sanguinea (Cornaceae). American Journal of Botany 83: 744–748. [Google Scholar]
- Harder LD, Cruzan MB. 1993. Unilateral incompatibility and the effects of interspecific pollination for Erythronium americanum and Erythronium albidum (Liliaceae). Canadian Journal of Botany 71: 353–358. [Google Scholar]
- Kanchan SD, Jayachandra 1980. Allelopathic effects of Parthenium hysterophorus L. Plant and Soil 55: 67–75. [Google Scholar]
- Kearns CA, Inouye DW. 1993. Techniques for pollination biologists. Boulder, CO: University Press of Colorado. [Google Scholar]
- Kohn JR, Waser NM. 1985. The effect of Delphinium nelsonii pollen on seed set in Ipomopsis aggregata, a competitor for hummingbird pollination. American Journal of Botany 72: 1144–1148. [Google Scholar]
- Larson DL, Royer RA, Royer MR. 2006. Insect visitation and pollen deposition in an invaded prairie plant community. Biological Conservation 130: 148–159. [Google Scholar]
- Marshall DL, Avritt JJ, Maliakal-Witt S, Medeiros JS, Shaner MGM. 2010. The impact of plant and flower age on mating patterns. Annals of Botany 105: 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazerolle MJ. 2012. AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R package version 1.24. http://cran.rproject.org/web/packages/AICcmodavg/index.html. [Google Scholar]
- Mitchell RJ, Irwin RE, Flanagan RJ, Karron JD. 2009. Ecology and evolution of plant-pollinator interactions. Annals of Botany 103: 1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales C, Traveset A. 2008. Interspecific pollen transfer: magnitude, prevalence and consequences for plant fitness. Critical Reviews in Plant Sciences 27: 221–238. [Google Scholar]
- Murphy SD, Aarssen LW. 1995. Reduced seed set in Elytrigia repens caused by allelopathic pollen from Phleum pratense. Canadian Journal of Botany 73: 1417–1422. [Google Scholar]
- Niesenbaum RA. 1999. The effects of pollen load size and donor diversity on pollen performance, selective abortion, and progeny vigor in Mirabilis jalapa (Nyctaginaceae). American Journal of Botany 86: 261–268. [PubMed] [Google Scholar]
- Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends in Ecology and Evolution 25: 345–353. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2013) R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Thomson JD, Andrews BJ, Plowright RC. 1981. The effect of foreign pollen on ovule development in Diervilla lonicera (Caprifoliaceae). New Phytologist 90: 777–783. [Google Scholar]
- Waser NM, Fugate ML. 1986. Pollen precedence and stigma closure: a mechanism of competition for pollination between Delphinium nelsonii and Ipomopsis aggregata. Oecologia 70: 573–577. [DOI] [PubMed] [Google Scholar]
- Waser NM, Price MV. 1991a. Outcrossing distance effects in Delphinium nelsonii: pollen loads, pollen tubes, and seed set. Ecology 72: 171–179. [Google Scholar]
- Waser NM, Price MV. 1991b. Reproductive costs of self-pollination in Ipomopsis aggregata (Polemoniaeae): are ovules usurped? American Journal of Botany 78: 1036–1043. [Google Scholar]
- Waser NM, Chittka L, Price M, Williams NM, Ollerton J. 1996. Generalization in pollination systems, and why it matters. Ecology 77: 1043–1060. [Google Scholar]
- Williams CF, Ruvinsky J, Scott PE, Hews DK. 2001. Pollination, breeding system, and genetic structure in two sympatric Delphinium (Ranunculaceae) species. American Journal of Botany 88: 1623–1633. [PubMed] [Google Scholar]