Abstract
Background and Aims There is abundant evidence that leaf mechanical traits deter feeding by insect herbivores, but little is known about which particular traits contribute to defence across feeding guilds. We investigated the contribution of multiple mechanical traits from shear, punch and tear tests to herbivore deterrence across feeding guilds.
Methods Visible damage from miners and external chewers was measured and sucker feeding density estimated in mature leaves of 20 species of forest shrubs and small trees. Cafeteria trials were undertaken using a generalist chewer (larvae of Epiphyas postvittana, Lepidoptera). Damage was compared with leaf mechanical traits and associated nutrient and chemical defence traits.
Key Results Damage by external chewers in the field and by E. postvittana correlated negatively with mechanical traits. Hierarchical partitioning analysis indicated that the strongest independent contribution to chewing damage was by the material trait of specific work to shear, with 68 % of total variance explained by the combination of specific work to shear (alone explaining 54 %) and tannin activity in a regression model. Mining damage did not correlate with mechanical traits, probably because miners can avoid tissues that generate high strength and toughness in mature leaves. Mechanical traits correlated more strongly with chewing damage in the field than chemical defences (total phenolics and tannin activity) and nutrients (nitrogen and water), but nutrients correlated strongly with diet selection in the cafeteria trial. Surprisingly, sucker feeding density correlated positively with mechanical traits and negatively with nutrients.
Conclusions Mechanical traits of mature leaves influenced insect feeding guilds differentially, reflecting differences in life history and feeding modes. For external chewers, energy (work) to fracture in shearing tests, at both structural and material levels, was strongly predictive of damage. Knowing which leaf mechanical traits influence insect feeding, and in which guilds, is important to our wider understanding of plant–herbivore interactions.
Keywords: Feeding guilds, insect herbivory, leaf strength, leaf toughness, plant–insect interactions
INTRODUCTION
Not all green plant tissues are palatable (Sinclair, 1975; Abe and Higashi, 1991), due partly to the wide array of structural defences (Hanley et al., 2007). All folivores experience leaf mechanical traits since they must penetrate the leaf in some way to obtain nutrients (Abe and Higashi, 1991; Sanson, 2006), and may not encounter some chemical defences until mechanical barriers have been partially overcome. Insects feeding on ‘tough’ leaves may experience mandibular wear (Raupp, 1985) and reduced feeding rates (Tanton, 1962; Raupp, 1985; Clissold et al., 2009), which leads to poor growth (Rausher, 1981; Clissold et al., 2009; Dimarco et al., 2012), probably reduced fecundity (Raupp, 1985), and high mortality (Tanton, 1962; Larsson and Ohmart, 1988). Leaf mechanical traits contribute significantly to defence from insect herbivores (Hanley et al., 2007), but little is known about which mechanical traits contribute most to defence for different feeding guilds.
Insect herbivores often prefer young leaves, which are typically softer, often with higher nutrient concentrations, but sometimes higher levels of chemical defence (Coley, 1983; Lowman and Box, 1983; Meldau et al., 2012). Mature leaves are typically stronger and tougher (Choong, 1996; Brunt et al., 2006; Peeters et al., 2007): traits such as fracture strength (the maximum force to fracture the leaf) and fracture toughness (the work or energy to fracture the leaf) potentially protect mature leaves from herbivores with inadequate adaptations to overcome the physical barriers, or because the energy investment in acquiring and processing the leaf tissue is too high. Strong, tough leaves may also impose indirect costs, such as the consequences of more time spent on leaf processing instead of activities related to reproduction and predator avoidance (e.g. Mueller and Dearing, 1994).
Different animals fracture plants in differing ways and at differing scales, depending on their nutritional physiology, mouthparts and size (Lucas et al., 2000; Sanson, 2006; Peeters et al., 2007). Even small insects can vary substantially in their mode of accessing nutrients (Peeters et al., 2001; Peeters, 2002b). Since little is known about the modes of action used by insect mouthparts to fracture leaf material during feeding (Clissold, 2007) and how plant tissues fracture under loads exerted by insects, it is not simple to predict which foliar mechanical traits are most limiting. Previous studies of defensive roles of mechanical traits have often measured the force to fracture leaves using a simple penetrometer (Coley, 1983; Lowman and Box, 1983; Hagen and Chabot, 1986) or a tensile test (Pérez-Harguindeguy et al., 2003); these traits relate to strength but sometimes are reported as ‘toughness’ (Choong et al., 1992; Wright and Vincent, 1996; Sanson et al., 2001). More rarely, fracture toughness has been measured (Choong, 1996; Clissold et al., 2009; Ibanez et al., 2013). Few studies have investigated both strength and toughness (Malishev and Sanson, 2015) or used multiple test types, e.g. tensile versus shear traits.
Most studies use some form of cutting test (shearing or punching) to explore associations between leaf mechanical traits and insect herbivory (Choong, 1996; Clissold et al., 2009; Ibanez et al. 2013). These tests are probably multimodal in terms of mode of fracture (Vincent, 1990), but reflect the effective leaf strength and toughness. It is uncertain whether ‘structural’ traits (normalized for width but not thickness) are more relevant than ‘material’ traits, normalized to leaf thickness. Both are potentially relevant, depending on the size and mode of action of the mouthparts and the style of feeding. Some insects feed by penetrating the full thickness of the leaf, in which case structural traits may be more important than material traits alone. While it is known that insects can be influenced by physical leaf traits at a much finer scale, i.e. at the tissue level (Hagen and Chabot, 1986; Casher, 1996; Wright and Vincent, 1996), we seek to provide analytical tools that may be useful at a community-wide level in ecological studies where such fine-scale studies may not be feasible.
Insights into effects of leaf structure on abundance of insect herbivore guilds have been provided by a detailed field study of co-occurring plant species (Peeters et al., 2001, 2007). That study found, for example, that the density of total chewing insects on mature leaves was correlated with a range of mechanical traits (including strength and toughness), but that density of total suckers was correlated with toughness but not strength. However, leaf damage was not measured, so here we extend that study by investigating (1) the contribution of mechanical traits to defence across insect feeding guilds, and more specifically, (2) the relative importance of strength versus toughness and structural (whole leaf) versus material (per unit leaf thickness) traits to defence, and (3) which test types (punch, shear or tear) correlate most strongly with leaf damage. To our knowledge, this is the first systematic investigation of the association of different leaf mechanical traits with herbivore damage by different insect guilds.
These questions were addressed by measuring herbivore damage on leaves of forest understorey species at the study sites of Peeters et al. (2001, 2007) and Peeters (2002a), and correlating it with mechanical traits derived from several forms of fracture tests (punch, shear and tear). Mechanical traits relate to a variety of functions, e.g. support and vascular transport as well as protection from abiotic and biotic damage. Here we investigate only fracture traits, since herbivory most often involves fracturing tissue for acquisition and processing. In addition, cafeteria trials were undertaken using a generalist chewer (e.g. Schädler et al., 2003) and damage was compared with leaf mechanical traits. Mechanical traits can be correlated with cell wall concentration across species (Choong et al., 1992; Read and Sanson, 2003), typically indigestible by folivorous insects (Abe and Higashi, 1991), and with nutrients such as nitrogen (Choong et al., 1992; Read and Sanson, 2003) and water, and with chemical defences (Read et al., 2009). Hence some of these associated leaf traits were also measured to aid interpretation of the independent role of mechanical traits.
MATERIALS AND METHODS
The field study was undertaken in eucalypt forest in Bunyip State Park (37°59ʹ S, 145°48ʹ E, 170–220 m asl), ∼80 km southeast of Melbourne (Victoria, Australia). Twenty species of evergreen understorey shrubs and small trees from ten families were studied, including 17 of the 18 species studied previously (Table 1). Plants were included from two sites to include a range of leaf forms: a damp south-facing gully and a drier north-facing site (within 2 km), both on sandy clay loam over Devonian granite. The climate is temperate, with a mean maximum temperature in summer months of 23–26 °C, mean minimum in winter months of 4–5 °C (Warragul, 27 km southeast, 143 m asl) and annual rainfall of 952 mm (Labertouche, 12 km south, 77 m asl) (Bureau of Meteorology, Australia).
Table 1.
Plant species used in this study, their abbreviations (codes used in figure legends) and location within the study area (D, dry; W, wet). Species nomenclature is taken from the Australian Plant Census (http://www.chah.gov.au/apc/index.html, accessed on 29 September 2015)
| Family, species | Site | Code |
|---|---|---|
| Asteraceae | ||
| Olearia lirata | W | ol |
| Fabaceae | ||
| Pultenaea muelleriP | W | pm |
| Goodeniaceae | ||
| Goodenia ovata | W | go |
| Lamiaceae | ||
| Prostanthera lasianthosP | W | pl |
| Mimosaceae | ||
| Acacia genistifoliaP | D | ag |
| Acacia myrtifoliaP | D | am |
| Monimiaceae | ||
| Hedycarya angustifoliaP | W | ha |
| Myrtaceae | ||
| Leptospermum continentale | D | lc |
| Proteaceae | ||
| Banksia marginataP | D | bm |
| Banksia spinulosa var. cunninghamiiP | D | bs |
| Grevillea barklyanaP | W | gb |
| Hakea decurrens subsp. physocarpaP,1 | D | hd |
| Hakea ulicinaP | D | hu |
| Lomatia fraseriP | W | lf |
| Rhamnaceae | ||
| Pomaderris asperaP | W | pa |
| Spyridium parvifoliumP | D | sp |
| Rutaceae | ||
| Boronia muelleriP | W | bo |
| Correa reflexa var. reflexaP | W | cr |
| Leionema bilobumP,2 | W | lb |
| Zieria arborescens subsp. arborescensP | W | za |
1Nomenclature change from H. sericea
2Nomenclature change from Phebalium bilobum
PIncluded in the study by Peeters et al. (2001, 2007), with two nomenclature changes indicated
Assessing herbivore damage
Five plants per species were selected at the study sites in February (austral summer). On each plant, three leaves or phyllodes that expanded in the current growing season were tagged. Since we wished to measure damage to mature leaves, damage was first assessed on recently matured leaves, then re-assessed after 12 months. The percentage leaf area that was visibly damaged was estimated by eye when ≤1 %, or by image analysis of digital photographs (Mix image analysis software, R. Stolk and G. Sanson, Monash University). Damage was assessed separately for external chewing and mining. Sucker feeding density was assessed by counting scales and scars from stylet penetration, examining both leaf surfaces, and expressed per single-sided leaf area. However, this estimate was only undertaken at the final assessment when leaves were removed from the plants, so it is uncertain at what stage of leaf development the feeding occurred. Where branchlets had died or disappeared by the second assessment, a leaf of the same cohort was chosen randomly. Damage may have been underestimated if whole leaves were removed by insect herbivores prior to branchlet death.
Acceptability to larvae of the generalist chewer, the light brown apple moth (LBAM) (Epiphyas postvittana, Lepidoptera: Tortricidae) was assessed in a cafeteria trial in May–June. The larvae were grown in containers with a medium of yeast and vitamin supplements, supplied by the Department of Primary Industries, Victoria (Knoxfield). Half of the larvae were reared in a controlled environment cabinet at 15 °C until reaching ∼1 cm in length (‘small larvae’), and the others were reared at 21 °C to increase their growth rate until they reached a length of ∼3 cm (‘large larvae’).
Leaves that developed in the previous growing season were collected from sunlit branches of tagged plants, ∼0·5–2 m above the ground, over a 2-week period in May–June. They were wrapped in moist paper and sealed in plastic bags. In the laboratory, samples of 1 cm2 were cut from the leaves, or multiple cut leaves were used in small-leaved species. Leaf area was measured by image analysis before and after each trial to determine the area eaten. Feeding chambers were created by inverting plastic containers onto a piece of plain unbleached cotton cloth marked with a grid of 20 locations for leaf samples and intersection points for LBAM larvae. Moist paper was inserted to increase humidity. Leaf samples of each species were randomly positioned (using random numbers), and ten larvae were randomly positioned on the intersection points of the grid. Five replicate chambers were set up separately for small and large larvae, with each plant species used in each feeding trial. Feeding chambers were placed in a controlled environment cabinet at 21 °C and larvae were allowed to feed for 24 h.
Measurement of leaf traits
The same sampling protocol was used over 5 weeks in May–June to measure leaf traits, with a replicate of each species included in each of five collection batches. Leaves were stored overnight at 2 °C and allowed to reach room temperature prior to measuring mechanical traits, water concentration and leaf area, within 24 h of collection. Leaves or leaflets were randomly selected for each test. Samples for chemical analyses were freeze-dried and ground to a powder.
Mechanical tests
Punch, shear and tear tests were undertaken using a modified universal testing machine (Chatillon Universal Tension and Compression Tester, model UTSE-2) following Read and Sanson (2003), with traits derived using LeafME software (M. Logan, Monash University). Strength (the force to fracture divided by the area of leaf over which the force was applied) was measured in punch and tear tests. Toughness (work to fracture, derived from the area under the force–displacement curve) was determined in punch, shear and tear tests. Traits were also expressed per unit leaf thickness to derive material traits (i.e. specific strength, specific work/toughness).
For shear tests, guillotine blades, with a horizontal cutting edge on the lower blade and with the upper blade closing at a 20° angle, were used to shear a leaf strip transversely. A longitudinal test strip, up to 5 mm wide, was cut from one side of the leaf to avoid the midrib and leaf margins, but for five species the leaves were too narrow and the whole leaf was used (Acacia genistifolia, Hakea decurrens, Hakea ulicina, Leptospermum continentale and Pultenaea muelleri). The cut was made at a random distance along the length of the strip and the thickness at the cut surface was measured with a micrometer. Work to shear was calculated per unit width of the test strip, and specific work to shear as work to shear divided by strip thickness. For the spine-like leaves of H. decurrens, specific work to shear was calculated using the diameter of the leaf to estimate the cross-sectional area.
In punch tests, leaves were punched at five random positions with a flat-sided punch of 0·5 mm diameter and a clearance of 0·05 mm with the die. The midrib (except in H. decurrens), margin and the apical and basal 10 percentiles were avoided, and punches were made at least 5 mm apart. Leaves were punched from the lower to upper surface so that revolute margins could be avoided, and the tissue type (secondary veins or inter-vein lamina) was noted. Leaf thickness at the punch position was measured with a micrometer. Traits were calculated from the average of the five punches, and also from the inter-vein lamina alone (the weakest part of the leaf). Secondary veins were closely spaced and could not be avoided in H. decurrens and P. muelleri.
For tear tests, a longitudinal strip up to 5 mm wide was cut from one side of a leaf to avoid the midrib and margins, with a length of 10 times the strip width to avoid effects of necking (Vincent, 1990). Strips were notched on one side (∼1/10th of strip width) to direct the position of fracture and each end was glued into the slot of a cheesehead screw with cyanoacrylate glue. The screws holding the test strip were mounted onto the force-tester and tension was applied. The fracture length was measured with digital callipers, and thickness at the torn edge was measured with a micrometer. Testing could not be undertaken in the narrow-leaved species A. genistifolia, H. decurrens and L. continentale, and in H. ulicina and P. muelleri the leaves could only be torn across the entire leaf, including the midrib.
Specific leaf area and chemical analyses
The leaf area of one leaf per replicate plant was measured by image analysis, after weighing the fully hydrated leaf. The leaf was then dried at 105 °C and reweighed. Specific leaf area (SLA, leaf area per unit dry mass) and water concentration per unit dry mass were then calculated. Cell wall concentration was measured as neutral detergent fibre (NDF) following Van Soest et al. (1991) after sonicating in acetone and centrifuging to remove tannins. Total phenolics were estimated by the Prussian blue method (Price and Butler, 1977) as modified by Graham (1992), after extraction in 50 % acetone (Cork and Krockenberger, 1991), and expressed as gallic acid equivalents (GAE) per leaf dry mass. Tannin activity was estimated from precipitation of protein (bovine γ-globulin) by the phenolic extract using the blue BSA (bovine serum albumin) method (Asquith and Butler, 1985) and reported per unit leaf dry mass. Nitrogen concentration was measured by a Leco CHN-200 Auto-Analyser (Leco Corp., St Joseph, MI, USA).
Statistical analyses
Differences in leaf traits and herbivore damage among species were tested with ANOVA. Pearson correlation was used to determine associations between leaf traits and field-based damage, with log-transformation used when necessary. Spearman correlation was used to estimate associations in the cafeteria trial, but the frequency of zero values limited further analysis. Principal components analysis (PCA) was used to reduce the set of leaf traits (excluding tear tests, which were not available for three species, and average punch data) to determine whether field leaf damage correlated better with the main components than with individual variables. Since many leaf traits correlated with external chewing damage but were often strongly intercorrelated, hierarchical partitioning was used to identify variables with a strong independent effect to include in regression models. This function calculates goodness-of-fit measures across all variable combinations in a hierarchy and then uses the algorithm of Chevan and Sutherland (1991) to calculate the independent contribution (I) of each independent variable, with statistical significance derived by a randomization procedure (Mac Nally and Horrocks, 2002; Walsh and Mac Nally, 2013). Nine leaf traits (since the use of more than nine traits can lead to rounding errors) were selected that correlated with chewing damage, initially including only variables for which data were recorded for all species, and analysed using hier.part (Walsh and Mac Nally, 2013) in R v. 2.14.2 (R Development Core Team, 2011). A critical value of α = 0·05 was used for hypothesis testing, and SYSTAT™ v. 12 was used for all analyses except hierarchical partitioning.
RESULTS
Leaf traits
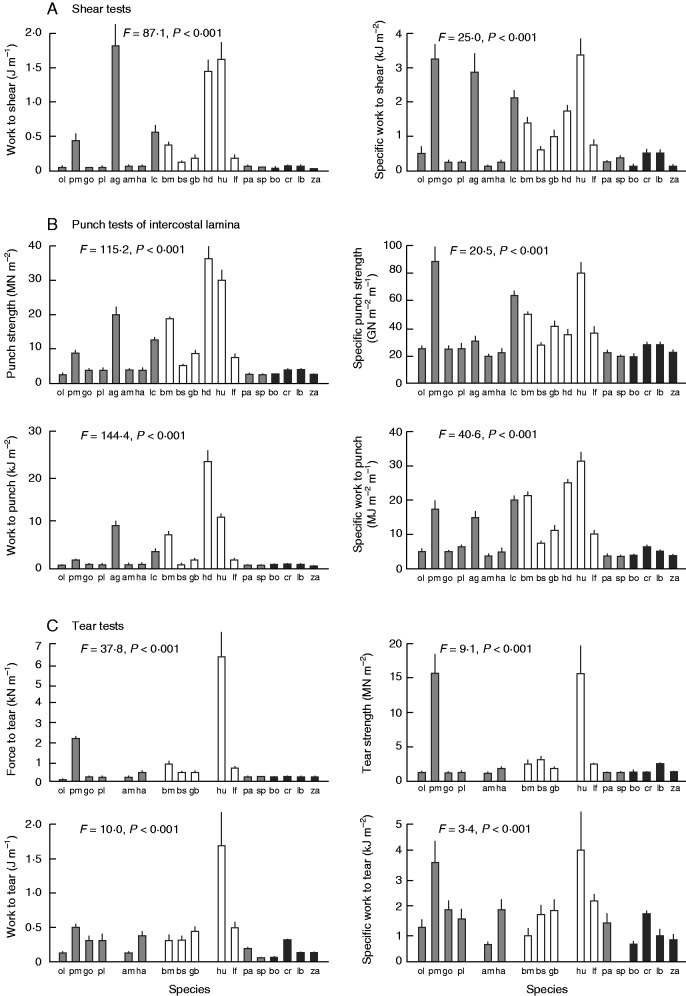
Species varied significantly in all mechanical traits (Fig. 1). There was more variability in structural traits such as punch strength and work to fracture (punch, tear or shear tests) than in material traits (those expressed per unit leaf thickness). Work to shear varied ∼90-fold among species being highest in A. genistifolia and Hakea spp. and lowest in Zieria arborescens and specific work to shear varied ∼20-fold (Fig. 1A). Punch tests of inter-vein lamina and averaged values across the leaf (including secondary veins) were strongly correlated (Pearson correlation coefficient RP = 0·99; P < 0·001), so only the former are shown (Fig. 1B). Punch strength (lamina-only and averaged values) varied 15- to 17-fold (highest in Hakea spp.) but specific punch strength varied only 4-fold among species. Work to punch varied ∼50- to 60-fold, being highest in H. decurrens (midrib could not be avoided), and specific work to punch was highest in H. ulicina, with 8-fold variation among species (Fig. 1B). High values of tearing traits were recorded in H. ulicina and P. muelleri, both of which have parallel secondary veins, with 9- to 55-fold variation across species in tearing traits (Fig. 1C).
Fig. 1.
Variation in leaf mechanical traits among plant species. (A) Shear tests, (B) punch tests and (C) tear tests. Specific strength and specific work are material traits; all others are structural. The values are means ± s.e., n = 5. All species were tested, except in the tearing tests (Acacia genistifolia, Leptospermum continentale and Hakea decurrens). Values for some species were so low as to be indistinguishable on these axes. The results of ANOVA are given (all variables were log-transformed). Bars for two common families are shaded differently: Proteaceae, open bars; Rutaceae, black bars. Species codes are given in Table 1.
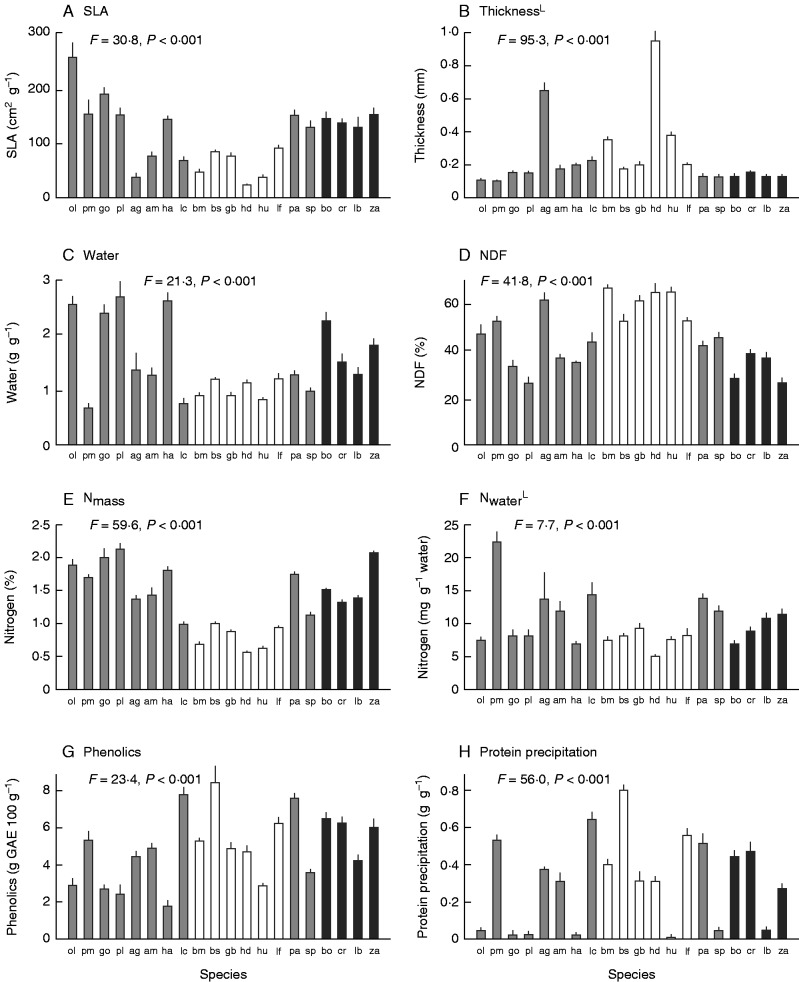
There was also considerable variation among species in leaf morphology and chemistry (Fig. 2). SLA and leaf thickness varied 8- to 9-fold among species (Fig. 2A, B), and water concentration varied ∼4-fold (Fig. 2C). NDF varied ∼3-fold, with relatively high values in Proteaceae species (Fig. 2D). Both N per dry mass (Nmass) and N per unit water (Nwater) varied 4-fold among species (Fig. 2E) but the latter was generally more similar across species and families. Total phenolics varied 5-fold (Fig. 2G), but tannin activity (protein precipitation) varied 90-fold among species (Fig. 2H).
Fig. 2.
Variation in leaf morphology and chemistry among plant species. (A) SLA, (B) thickness, (C) water, (D) NDF, (E) Nmass, (F) Nwater, (G) total phenolics and (H) protein precipitation (tannin activity). The values are means ± s.e., n = 5. The results of ANOVA are given. L, data were log-transformed for analysis. Bars for two common families are shaded differently: Proteaceae, open bars; Rutaceae, black bars. Species codes are given in Table 1.
Patterns among traits
Most mechanical traits were strongly intercorrelated (Table 2). In particular, leaves that were strong also tended to be tough, e.g. punch strength was highly correlated with work to punch and work to shear (toughness) (RP = 0·96–0·99). This trend also applied to material traits, with specific punch strength strongly correlated with specific work to punch and specific work to shear (RP = 0·88). Second, structural traits were significantly correlated with material traits. For example, leaves that were strong were on average strong per unit leaf thickness (RP = 0·74, punch test), and tough leaves were tough per unit leaf thickness (RP = 0·93–0·94, punch and shear tests). These correlations were strongest in punch and shear tests. Third, strength and toughness correlated significantly between different test types, particularly for punch and shear tests (Table 2). Traits measured in tear tests correlated slightly less strongly with those from punch and shear tests, and specific work to tear tended to be more weakly correlated with most mechanical traits (Table 2).
Table 2.
Correlations of mechanical traits from the punch, shear and tear tests. The data are Pearson correlation coefficients (RP), with asterisks indicating the significance level (*P < 0·05; **P < 0·01; ***P < 0·001) (n = 20 for all traits except those from tearing tests, for which n = 17). All data were log-transformed for statistical analyses. Specific strength and specific work are material traits; all others are structural traits
| Punch tests: lamina |
Shear tests |
Tear tests |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Strength | Specific strength | Work | Specific work | Work | Specific work | Force per width | Strength | Work | |
| Punch tests (lamina) | |||||||||
| Strength | |||||||||
| Specific strength | 0·74*** | ||||||||
| Work | 0·99*** | 0·66** | |||||||
| Specific work | 0·96*** | 0·88*** | 0·93*** | ||||||
| Shear tests | |||||||||
| Work | 0·96*** | 0·78*** | 0·95*** | 0·96*** | |||||
| Specific work | 0·83*** | 0·88*** | 0·79*** | 0·91*** | 0·94*** | ||||
| Tear tests | |||||||||
| Force per width | 0·91*** | 0·90*** | 0·88*** | 0·93*** | 0·92*** | 0·83*** | |||
| Strength | 0·77*** | 0·91*** | 0·72** | 0·85*** | 0·87*** | 0·87*** | 0·94*** | ||
| Work | 0·82*** | 0·77*** | 0·81*** | 0·82*** | 0·80*** | 0·68** | 0·83*** | 0·71** | |
| Specific work | 0·59* | 0·72** | 0·54* | 0·66** | 0·67** | 0·68** | 0·68** | 0·70** | 0·89*** |
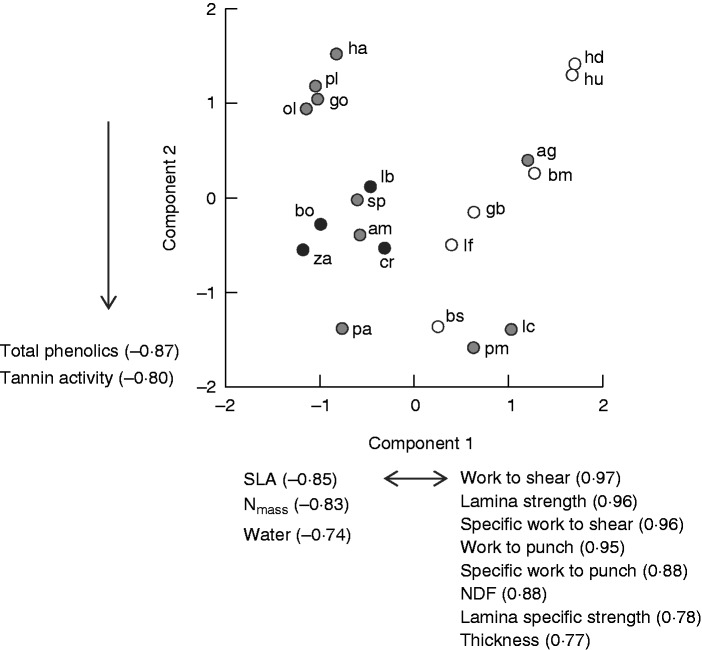
All mechanical traits from punch and shear tests, and tearing force per width, correlated negatively with both SLA and Nmass (Table 3). In particular, SLA was strongly correlated with punch strength and work (Table 3). All mechanical traits except work to tear and specific work to tear correlated negatively with water concentration and all except specific work to tear correlated positively with NDF (Table 3). No mechanical traits correlated with total phenolics or tannin activity (Table 3). The first two components of the PCA explained 79 % of the total variance. The first axis was strongly correlated with mechanical traits, NDF, Nmass, SLA, thickness and water concentration, explaining 61 % of the total variance, and the second axis, explaining only 17 % of the total variance, was correlated with total phenolics and tannin activity (Fig. 3).
Table 3.
Correlations of mechanical traits with other leaf traits. The data are Pearson correlation coefficients (RP), with asterisks indicating the significance level (*P < 0·05; **P < 0·01; ***P < 0·001; n = 20 for all traits except those from tearing tests, for which n = 17). All mechanical traits were log-transformed for statistical analysis
| Mechanical traits | SLA | Water | NDF | Nmass | Total phenolics | Tannin activity |
|---|---|---|---|---|---|---|
| Shear tests | ||||||
| Work | –0·76*** | –0·62** | 0·84*** | –0·70** | 0·04 | 0·24 |
| Specific work | –0·60** | –0·66** | 0·85*** | –0·64** | 0·01 | 0·27 |
| Punch tests: lamina | ||||||
| Strength | –0·83*** | –0·57** | 0·80*** | –0·73*** | –0·10 | 0·02 |
| Specific strength | –0·47* | –0·63** | 0·65** | –0·52* | 0·09 | 0·25 |
| Work | –0·84*** | –0·53* | 0·79*** | –0·74*** | 0·02 | 0·18 |
| Specific work | –0·73*** | –0·61** | 0·80*** | –0·70** | 0·07 | 0·26 |
| Tear tests | ||||||
| Force per width | –0·63** | –0·50* | 0·65** | –0·55* | –0·10 | 0·10 |
| Strength | –0·44 | –0·52* | 0·61* | –0·42 | –0·08 | 0·10 |
| Work | –0·47 | –0·22 | 0·54* | –0·38 | –0·14 | 0·08 |
| Specific work | –0·13 | –0·15 | 0·43 | –0·13 | –0·12 | 0·08 |
Fig. 3.
PCA plot of leaf traits showing the traits correlating strongly with the first two components. Component loadings are given in brackets. Data points for two common families are shaded differently: Proteaceae, open points; Rutaceae, black points. Species codes are given in Table 1.
Herbivory of field-grown leaves and correlations with leaf traits
Total estimated leaf damage due to external chewers and miners varied from <1 to 15 %, being highest in the soft-leaved Pomaderris aspera and in the moderately tough-leaved Lomatia fraseri. Least total damage was recorded in some of the species with high values of mechanical traits: H. decurrens, A. genistifolia, L. continentale and P. muelleri (Fig. 4A, B). Total damage correlated negatively with most punch and shear traits, but not with any tear traits (Table 4). However, stronger patterns emerged when total damage was separated into damage due to external chewing and mining. Damage due to mining varied from ≤1 % in 13 species to 13 % in L. fraseri (Fig. 4A), and did not correlate with any measured leaf traits (Table 4). However, damage by external chewers, ranging from ≤1 % in 8 species to 13 % (Fig. 4B), correlated strongly (negatively) with many mechanical traits, particularly with shear traits (work to shear and specific work to shear) (Table 4). Damage was not significantly correlated with work to tear or specific work to tear, and was only weakly correlated with force to tear (Table 4). Damage by external chewers also correlated weakly and negatively with NDF, leaf thickness and tannin activity, and positively with SLA, water and Nmass (Table 4). Sucker feeding density varied from ≤1 cm–2 to ≥10 cm–2 and, in contrast to chewing damage, correlated positively with most mechanical traits and NDF and negatively with SLA, water and Nmass (Table 4).
Fig. 4.
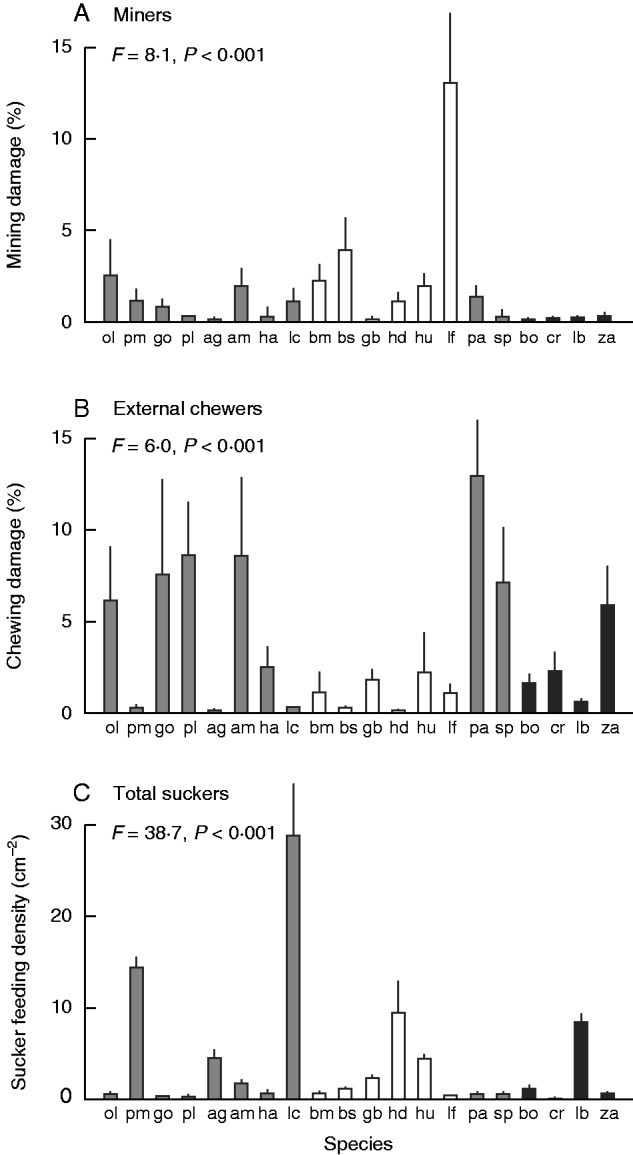
Variation in damage among plant species by feeding guilds. (A) Miners, (B) external chewers and (C) total sucker feeding density. The values are means ± s.e., n = 5 plants. ANOVA results are given (all variables were log-transformed). Bars for two common families are shaded differently: Proteaceae, open bars; Rutaceae, black bars. Species codes are given in Table 1.
Table 4.
Correlations of all leaf traits with herbivore damage in the field. The data given are Pearson correlation coefficients (RP), with asterisks indicating the significance level (*P < 0·05; **P < 0·01; ***P < 0·001; n = 20 for all traits except those from tearing tests, for which n = 17). ‘L’ indicates data were log-transformed for analysis
| Leaf traits | Mining damageL | Chewing damageL | Total damageL | Sucker feeding densityL |
|---|---|---|---|---|
| Mechanical/physical traits | ||||
| Work to shearL | 0·15 | –0·73*** | –0·57** | 0·64** |
| Specific work to shearL | 0·18 | –0·74*** | –0·56* | 0·68** |
| Average punch strengthL | 0·20 | –0·62** | –0·47* | 0·55* |
| Average punch specific strengthL | 0·29 | –0·57** | –0·34 | 0·58** |
| Average work to punchL | 0·19 | –0·61** | –0·47* | 0·52* |
| Average specific work to punchL | 0·27 | –0·66** | –0·45* | 0·61** |
| Lamina punch strengthL | 0·17 | –0·65** | –0·50* | 0·56* |
| Lamina punch specific strengthL | 0·27 | –0·57** | –0·34 | 0·59** |
| Lamina work to punchL | 0·16 | –0·65** | –0·50* | 0·54* |
| Lamina specific work to punchL | 0·25 | –0·68** | –0·47* | 0·62** |
| Force to tearL | 0·31 | –0·54* | –0·29 | 0·55* |
| Tear strengthL | 0·28 | –0·62** | –0·37 | 0·67** |
| Work to tearL | 0·33 | –0·34 | –0·10 | 0·16 |
| Specific work to tearL | 0·29 | –0·37 | –0·12 | 0·18 |
| SLA | –0·09 | 0·55* | 0·41 | –0·52* |
| Leaf thicknessL | 0·06 | –0·47* | –0·43* | 0·36 |
| Chemical traits | ||||
| Water | –0·19 | 0·51* | 0·29 | –0·61** |
| NDF | 0·31 | –0·51* | –0·30 | 0·45* |
| Nmass | –0·21 | 0·50* | 0·28 | –0·46* |
| NwaterL | –0·10 | –0·16 | –0·19 | 0·32 |
| Total phenolics | 0·21 | –0·37 | –0·05 | 0·19 |
| Tannin activity | 0·30 | –0·54* | –0·14 | 0·24 |
| PCA1 | 0·23 | –0·70** | –0·49* | 0·63** |
| PCA2 | –0·14 | 0·25 | 0·00 | –0·20 |
Component 1 of the PCA correlated negatively with chewing damage and positively with sucker feeding density, but not more strongly than with some individual leaf traits (Table 4). The second component did not correlate with estimates of damage or sucker feeding density. Of the variables included in the hierarchical partitioning analysis, only specific work to shear provided a significant independent contribution (I = 19–20 %) to the explained variance in chewing damage (Table 5), although work to shear and tannin activity also had high but non-significant I values (Table 5). When these traits were included in a regression model, 54 % of the total variance in chewing damage was explained by specific work to shear (with only 1 % additional variation explained by work to shear), and 68 % was explained when tannin activity was added.
Table 5.
Hierarchical partitioning analysis of leaf trait effects on chewing damage in the field trial (n = 20). The data presented are I, the independent contribution of leaf traits to variance in chewing damage, expressed as a percentage of the explained variance, with statistical significance (*P < 0·05) derived by randomization procedures (Walsh and Mac Nally, 2013). ‘L’ indicates data were log-transformed for analysis. Chewing damage was also log-transformed. When mechanical traits were analysed alone to include tear tests (n = 17), again, only specific work to shear made a significant independent contribution (I = 19 %; P < 0·05) to chewing damage
| Leaf trait | I (%) |
|---|---|
| Work to shear L | 14·5 |
| Specific work to shear L | 19·9* |
| Lamina punch strength L | 8·4 |
| Lamina punch specific strength L | 6·9 |
| Lamina work to punch L | 8·5 |
| Lamina specific work to punch L | 9·3 |
| NDF | 10·7 |
| Nmass | 4·8 |
| Tannin activity | 17·0 |
Cafeteria trials with LBAM larvae and correlations with leaf traits
The leaf area consumed by LBAM larvae in the cafeteria trials varied widely among species (Fig. 5). Consumption by small and large LBAM larvae varied from <1 % of leaf area in 11 species to 35 %. The area eaten was strongly negatively correlated with some mechanical traits, particularly shear traits, and with NDF (Table 6). Correlations were strongest in large larvae, and no significant correlations were recorded with work to tear and specific work to tear, or with total phenolics or tannin activity. Area eaten was strongly positively correlated with SLA, water and Nmass, but not Nwater (Table 6). Again, correlations were stronger in large LBAM larvae than in small larvae. Strong negative correlations were recorded with component 1 of the PCA, but correlations were only slightly stronger than with some individual leaf traits (Table 6).
Fig. 5.
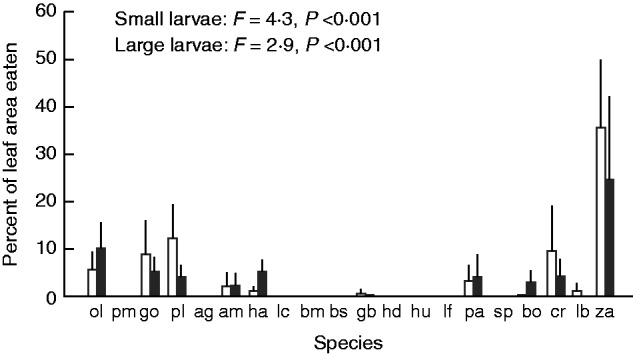
Variation in the percentage of leaf material eaten across plant species by small (open bars) and large (black bars) LBAM larvae. The values are means ± s.e., n = 5. The results of ANOVA are given (variables were log-transformed). Species codes are given in Table 1.
Table 6.
Correlations of leaf traits with herbivore damage in the cafeteria trials using LBAM larvae. The data given are Spearman correlation coefficients (RS), with asterisks indicating the significance level (*P < 0·05; **P < 0·01; ***P < 0·001) (n = 20 for all traits except those from tearing tests, for which n = 17)
| Small larvae | Large larvae | |
|---|---|---|
| Mechanical/physical traits | ||
| Lamina punch strength | –0·58** | –0·71*** |
| Lamina punch specific strength | –0·48* | –0·65** |
| Lamina work to punch | –0·63** | –0·72*** |
| Lamina specific work to punch | –0·57** | –0·66** |
| Work to shear | –0·66** | –0·77*** |
| Specific work to shear | –0·67** | –0·77*** |
| Tear strength | –0·68** | –0·72** |
| Force to tear | –0·61** | –0·68** |
| Work to tear | –0·33 | –0·43 |
| Specific work to tear | –0·14 | –0·19 |
| SLA | 0·69** | 0·80*** |
| Leaf thickness | –0·47* | –0·53* |
| Chemical traits | ||
| Water | 0·69** | 0·82*** |
| NDF | –0·68** | –0·72*** |
| Nmass | 0·71** | 0·82*** |
| Nwater | 0·01 | –0·22 |
| Total phenolics | –0·27 | –0·23 |
| Tannin activity | –0·43 | –0·39 |
| PCA1 | –0·73*** | –0·85*** |
| PCA2 | 0·06 | 0·13 |
DISCUSSION
How do mechanical traits contribute to defence across insect feeding guilds?
This study suggests a strong relationship between leaf mechanical traits and herbivory, both in the field and in the cafeteria trial. In particular, it suggests a strong negative link between external chewing damage and both strength and toughness, consistent with earlier studies (Coley, 1983; Lowman and Box, 1983). These traits would reduce diet quality of the leaves, as they potentially pose immediate mechanical problems for manipulation and penetration, and possibly digestion (Mueller and Dearing, 1994; Clissold et al., 2006). The results are also consistent with negative correlations between the density of chewers and a range of foliar mechanical traits across plant species at the same study site (Peeters et al., 2007).
However, correlations of leaf mechanical traits with herbivory and with densities of insect feeding guilds may not reflect a simple causal relationship (Peeters et al., 2007). For example, most mechanical traits showing strong negative correlations with chewing damage were negatively correlated with Nmass, and performance of insect herbivores and herbivore damage is likely to increase on protein-rich tissues (Mattson, 1980; Loranger et al., 2012; Barbehenn et al., 2013). Chewing damage in the field correlated more strongly with some mechanical traits than with nitrogen in this study, and densities of chewing insects at the same study site were not correlated with nitrogen concentrations (Peeters, 2002a; Peeters et al., 2007), suggesting a causal role of mechanical traits in defence. However, some caution is necessary as nitrogen concentration does not necessarily reflect availability and quality for these herbivores, since it potentially includes nitrogen in insoluble forms as well as in defences such as alkaloids (McNeill and Southwood, 1978; Mattson, 1980). Furthermore, in the cafeteria experiment, correlations of damage were similarly strong with Nmass, NDF, water and some mechanical traits.
For chewing insects, mechanical traits may act independently or in concert with other traits, including nutrients, to influence diet choice (Hanley et al., 2007; Carmona et al., 2011; Ibanez et al., 2013). Furthermore, for insects that are unable to digest much cell wall, the latter may act as a diluent (Timmins et al., 1988; Clissold et al., 2004; Lee et al., 2004) as well as contributing to mechanical traits, and some components may reduce digestibility (Swain, 1979). Hence, a strong and tough leaf may be difficult to bite and energetically expensive to process (Schofield et al., 2011), and more of it may be needed to obtain dietary requirements, including through effects on ratios of assimilated nutrients (Clissold et al., 2006). Some of these strong, tough leaves are also thick, and some insect herbivores may be limited by gape size (Nahrung et al., 2001). Herbivore damage did not correlate with total phenolics across plant species, irrespective of feeding guild, and only chewing damage correlated (weakly) with tannin activity (see also Carmona et al., 2011). However, some of these plants have other forms of chemical defences, including alkaloids and terpenes, which may show different trends, individually or summatively. We found no clear evidence of syndromes of co-adapted traits (Agrawal and Fishbein, 2006), but a larger suite of morphological and chemical traits is probably needed to gain insights into this issue. In this study, mechanical traits were not correlated with total phenolics or tannin activity (see also Read et al., 2009; Moles et al., 2013). Some other traits did co-vary, but although Nmass correlated negatively with mechanical traits, this was probably a consequence of dilution by cell wall (mechanical traits were not correlated with Nwater). In addition, NDF (and SLA) is related to mechanical traits via cell wall mass (Choong, 1996). That is, investment in structure enhances mechanical traits and inevitably reduces levels of nutrients, water and chemical defences on a dry mass basis, for insects that do not digest cell wall.
In contrast to external chewing, leaf mining was not negatively correlated with mechanical traits of mature leaves. Adult females of some insects may probe and puncture the leaf surface, often feeding prior to ovipositing (Parrella, 1987), and if the leaf is unsuitable (e.g. due a thick epidermal wall) the female may move to another leaf (Wei et al., 2000). This might result in females more often choosing young leaves on which to oviposit. If deposited as eggs into the mesophyll of young leaves that become tough at maturity, miners may never have to penetrate the ‘tougher’ outer layers of mature leaves to feed, except to exit the leaf as an adult. Eggs of surface-laying insects are commonly deposited on the lower leaf surface (Reavey and Gaston, 1991), which often has a thinner cuticle and lower epidermis, and is less often associated with potential barriers such as lignified epidermis, hypodermis or sclerenchyma. However, some plant species with strong and tough mature leaves may have dense mesophyll, which may constrain feeding (Wei et al., 2000), or lignified bundle sheath extensions that act as barriers to internal movement and feeding (Hagen and Chabot, 1986; Casher, 1996). Very thin leaves may also restrict the choice of tissue feeding location except for the youngest larvae (Reavey and Gaston, 1991).
There were no negative correlations between sucker feeding density and mechanical traits (see also Fiene et al., 2013). Notably, feeding density gives only a coarse index of damage, with no indication of impact on the plant. Mechanical traits may be less important to sessile phloem feeders that use salivary enzymes to enhance foliar penetration by their narrow stylets (Peeters et al., 2007). Furthermore, sessile suckers tend to use their stylet tracks repeatedly, so energetic costs of stylet penetration may be less limiting (Peeters et al., 2007). Nevertheless, total suckers and phloem suckers were less common on tough leaves in the field survey (Peeters et al., 2007), in contrast to the higher total sucker feeding density recorded on stronger and tougher leaves in this study. These observations may not be inconsistent if much of the feeding by suckers in our study had occurred on young expanding leaves (Walker, 1985). For example, higher densities of mobile phloem feeders were more often recorded by Peeters et al. (2001) in samples containing new leaves than in those with only mature leaves.
The positive correlations of sucker feeding density with leaf mechanical traits and negative correlations with nitrogen and water concentrations may reflect the growth environment of the plants. Species with tougher leaves were common on the drier site with a more open canopy, and so exposed to higher levels of light than soft-leaved species, which generally occurred on the moister site. This may lead to higher concentrations of non-structural carbohydrates in leaves (including immature leaves) of tougher species. Furthermore, suckers are less likely to be influenced by concentration of nutrients per bulk leaf mass than chewers. Consistent with this suggestion, there was a (non-significant) positive association between sucker feeding density and Nwater. We did not distinguish stylet scars associated with vascular tissue from those associated with mesophyll. Peeters et al. (2001) found a high density of sessile phloem feeders on P. muelleri compared with co-occurring plant species, but not high densities of sessile mesophyll feeders, mobile phloem feeders or mobile mesophyll feeders. Hence a complex pattern of feeding preference may occur among types of suckers.
The LBAM trials indicated negative correlations between the amount consumed and leaf strength and toughness (structural and material traits) across species, consistent with field-based data from this and other studies of chewing damage across plant species (Coley, 1983). Small larvae may be less able to manipulate and feed on strong and tough leaves or parts (Ohmart et al., 1987; Mueller and Dearing, 1994; Malishev and Sanson, 2015), so stronger trends were expected in small larvae. However, no overall difference in species choice was detected between small and large larvae, and stronger correlations with mechanical and nutrient traits were recorded in larger than smaller larvae, but the two size-groups may be eating different types of tissues, e.g. smaller larvae selecting softer and more nutritious tissue at a finer scale (Shade and Wilson, 1967).
Which mechanical traits correlate best with herbivore damage?
Since studies commonly measure a single mechanical trait, most often force to fracture (Coley, 1983; Raupp, 1985; Braby, 1994), it is important to understand any differences in effects of different mechanical traits on herbivores. However, the strong correlations among many of the mechanical traits limited our capacity to discriminate individual effects. In particular, strength, despite being the maximum force to fracture, was highly correlated with toughness, a summative measure of the tissues cut (see also Read and Sanson, 2003; Read et al., 2005; Malishev and Sanson, 2015). In addition, relatively little difference was recorded between structural and material traits in their correlations with chewing damage, either in the field survey or the cafeteria trial. Leaf thickness was negatively correlated with chewing damage, suggesting a slightly stronger influence of structural traits, but may be just a reflection of positive correlations between structural and material traits in this study. Hierarchical partitioning analysis suggested that the strongest independent contribution was by the material trait of specific work to shear, although work to shear also correlated highly with chewing damage. Hence, energy appears to be more limiting to these chewers than the force required to fracture leaves in this study (see also Malishev and Sanson, 2015), but this is not always the case (Wright and Vincent, 1996). Nevertheless, even if energy limitation rather than force limitation is widespread among chewing insects, given the strong correlations of specific work to fracture with some other mechanical traits, ecologists may choose to use alternative correlated traits for convenience. We note, however, that discriminatory power may be lost and, most importantly, the correlations recorded in this and other studies may not be universal. Notably, tear tests measure the resistance of the strongest elements, i.e. the veins, and because of the energy stored in the veins will overestimate the strength to a varying degree (Wright, 1992), depending on the leaf venation pattern. Hence tear tests are less likely to reflect herbivore susceptibility of the whole leaf, especially as tearing is unlikely to be a significant mode of fracture employed by insects when consuming plant tissue.
There was marked variation in responses of contrasting feeding guilds, with miners and suckers showing no evidence of negative effects of any mechanical traits of mature leaves on feeding. In this regard, it would be valuable to know what leaf stage was selected for oviposition by adult miners, and for feeding by suckers. In addition, the estimate of sucker feeding density used may be too coarse as a measure of leaf acceptability since it did not distinguish among types of suckers, their mode and location of feeding, and duration of feeding. It is also possible that some scars judged as caused by sucker feeding were instead oviposition scars.
Comparisons of field-based herbivory and laboratory trials
A wide diversity of insect herbivores occurs on these plants, including a range of fine-scale feeding guilds, although density varies considerably among morphospecies (Peeters et al., 2001), but it is uncertain how the different species are contributing to the damage recorded. We found that correlations of field-based herbivore chewing damage with NDF and SLA were weak compared with correlations with mechanical traits, which is relevant given their use as surrogates for defensive mechanical traits (Choong et al., 1992; Hanley et al., 2007). The former is surprising since NDF can contribute to defence by its influence on mechanical traits (Choong, 1996) and possibly by dilution (Timmins et al., 1988) and reduction of digestibility (Swain, 1979; Rausher, 1981; Clissold et al., 2006). While SLA and NDF correlated with mechanical traits, other factors influence mechanical traits, including the way cells are arranged in leaf tissues (Read et al., 2000). The stronger influence of SLA, NDF and nitrogen in the cafeteria trial is an interesting contrast with the field-based survey of leaf damage. The field-based chewing damage is likely to partly reflect oviposition choice by females (Prudic et al., 2005), whereas the cafeteria trial reflects only choices by feeders. However, while a laboratory-based trial might provide a better indication of the actual feeding choices by removing effects of oviposition choices, it also removes aspects of the whole-plant morphology that may play a role in herbivore deterrence. For example, factors influencing oviposition choices are part of the suite of plant defence traits. In addition, in the field, leaf strength and toughness may be correlated with traits (for both oviposition and feeding choices) such as leaf size or arrangement on the plant, and when effects of such traits are removed in laboratory-based trials nutrient concentrations may appear more important than they are under field conditions, at least in this type of vegetation. It should also be noted that the cafeteria trial used a single species of generalist herbivore, whereas the field survey by its nature included multiple species adapted to feeding on these species. Hence it should not be surprising that chewing damage in cafeteria trials, using a generalist herbivore, is less tightly associated with mechanical traits than among adapted insect species in the more complex field environment. Notably, leaves produced in different seasons can differ in nutrients and defence (Matsuki et al., 2004), further complicating comparison of a cafeteria trial with herbivory data from a full growth season.
Conclusions
Three major conclusions were reached in this study. First, a strong defensive role of leaf mechanical traits was identified, with work (energy) to fracture (structural plus material) accounting for 55 % of the total variance in damage by external chewers in the field, and correlating strongly with leaf consumption by a generalist herbivore. In particular, specific work to shear was strongly and independently associated with reduced damage in mature leaves and may prove to be the best predictive measure of mechanical defence for this feeding guild. Second, trait associations of mature leaves with insect damage differed substantially among feeding guilds, probably largely due to differences in feeding mode and life history. No evidence of a defensive role of mechanical traits of mature leaves was found in sapsuckers or leaf miners. For sapsuckers, any defensive role of mechanical traits may have been obscured due to uncertainty of the timing of feeding with respect to leaf age. For miners, tissues that generate high strength and toughness in mature leaves can sometimes be avoided. Third, consequences of leaf structure engender trait syndromes, whereby investment in structure has positive effects on mechanical traits and likely negative effects on levels of nutrients, water and chemical defences on a dry mass basis. This is in addition to any co-adapted traits of defences and nutrition. Disentangling this complexity of both food and forager is important to our wider understanding of plant–herbivore interactions.
ACKNOWLEDGEMENTS
We thank the Department of Sustainability and Environment (Victoria) for permission to conduct our study in Bunyip State Park, F. Richardson of the Department of Primary Industries (Victoria) for supplying LBAM larvae, F. Clissold and S. Jenkins for technical advice, and S. Jenkins and S. Kerr for quantifying phenolics. We also thank P. Peeters and F. Clissold for helpful comments on a draft of this manuscript. This research was funded by the School of Biological Sciences, Monash University.
LITERATURE CITED
- Abe T, Higashi M. 1991. Cellulose centered perspective on terrestrial community structure. Oikos 60: 127–133. [Google Scholar]
- Agrawal AA, Fishbein M. 2006. Plant defense syndromes. Ecology 87: S132–S149. [DOI] [PubMed] [Google Scholar]
- Asquith TN, Butler LG. 1985. Use of dye-labeled protein as spectrophotometric assay for protein precipitants such as tannin. Journal of Chemical Ecology 11: 1535–1544. [DOI] [PubMed] [Google Scholar]
- Barbehenn RV, Niewiadomski J, Kochmanski J. 2013. Importance of protein quality versus quantity in alternative host plants for a leaf-feeding insect. Oecologia 173: 1–12. [DOI] [PubMed] [Google Scholar]
- Braby MF. 1994. The significance of egg size variation in butterflies in relation to hostplant quality. Oikos 71: 119–129. [Google Scholar]
- Brunt C, Read J, Sanson GD. 2006. Changes in resource concentration and defence during leaf development in a tough-leaved (Nothofagus moorei) and soft-leaved (Toona ciliata) species. Oecologia 148: 583–592. [DOI] [PubMed] [Google Scholar]
- Carmona D, Lajeunesse MJ, Johnson MTJ. 2011. Plant traits that predict resistance to herbivores. Functional Ecology 25: 358–367. [Google Scholar]
- Casher LE. 1996. Leaf toughness in Quercus agrifolia and its effects on tissue selection by first instars of Phryganidia californica (Lepidoptera: Dioptidae) and Bucculatrix albertiella (Lepidoptera: Lyonetiidae). Annals of the Entomological Society of America 89: 109–121. [Google Scholar]
- Chevan A, Sutherland M. 1991. Hierarchical partitioning. American Statistician 45: 90–96. [Google Scholar]
- Choong MF. 1996. What makes a leaf tough and how this affects the pattern of Castanopsis fissa leaf consumption by caterpillars. Functional Ecology 10: 668–674. [Google Scholar]
- Choong MF, Lucas PW, Ong JSY, Pereira B, Tan HTW, Turner IM. 1992. Leaf fracture toughness and sclerophylly: their correlations and ecological implications. New Phytologist 121: 597–610. [Google Scholar]
- Clissold FJ. 2007. The biomechanics of chewing and plant fracture: mechanisms and implications. Advances in Insect Physiology 34: 317–372. [Google Scholar]
- Clissold FJ, Sanson GD, Read J. 2004. Indigestibility of plant cell wall by the Australian plague locust, Chortoicetes terminifera. Entomologia Experimentalis et Applicata 112: 159–168. [Google Scholar]
- Clissold FJ, Sanson GD, Read J. 2006. The paradoxical effects of nutrient ratios and supply rates on an outbreaking insect herbivore, the Australian plague locust. Journal of Animal Ecology 75: 1000–1013. [DOI] [PubMed] [Google Scholar]
- Clissold FJ, Sanson GD, Read J, Simpson SJ. 2009. Gross vs. net income: how plant toughness affects performance of an insect herbivore. Ecology 90: 3393–3405. [DOI] [PubMed] [Google Scholar]
- Coley PD. 1983. Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecological Monographs 53: 209–233. [Google Scholar]
- Cork SJ, Krockenberger AK. 1991. Methods and pitfalls of extracting condensed tannins and other phenolics from plants: insights from investigations on Eucalyptus leaves. Journal of Chemical Ecology 17: 123–134. [DOI] [PubMed] [Google Scholar]
- Dimarco RD, Nice CC, Fordyce JA. 2012. Family matters: effect of host plant variation in chemical and mechanical defences on a sequestering specialist herbivore. Oecologia 170: 687–693. [DOI] [PubMed] [Google Scholar]
- Fiene J, Kalns L, Nansen C, Bernal J, Harris M, Sword GA. 2013. Foraging on individual leaves by an intracellular feeding insect is not associated with leaf biomechanical properties or leaf orientation. PLoS One 8: e80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham HD. 1992. Stabilization of the Prussian blue color in the determination of polyphenols. Journal of Agricultural and Food Chemistry 40: 801–805. [Google Scholar]
- Hagen RH, Chabot JF. 1986. Leaf anatomy of maples (Acer) and host use by Lepidoptera larvae. Oikos 47: 335–345 [Google Scholar]
- Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM. 2007. Plant structural traits and their role in anti-herbivore defence. Perspectives in Plant Ecology, Evolution and Systematics 8: 157–178. [Google Scholar]
- Ibanez S, Lavorel S, Puijalon S, Moretti M. 2013. Herbivory mediated by coupling between biomechanical traits of plants and grasshoppers. Functional Ecology 27: 479–489. [Google Scholar]
- Larsson S, Ohmart CP. 1988. Leaf age and larval performance of the leaf beetle Paropsis atomaria. Ecological Entomology 13: 19–24. [Google Scholar]
- Lee KP, Raubenheimer D, Simpson SJ. 2004. The effects of nutritional imbalance on compensatory feeding for cellulose-mediated dietary dilution in a generalist caterpillar. Physiological Entomology 29: 108–117. [Google Scholar]
- Loranger J, Meyer ST, Shipley B, et al. 2012. Predicting invertebrate herbivory from plant traits: evidence from 51 grassland species in experimental monocultures. Ecology 93: 2674–2682. [DOI] [PubMed] [Google Scholar]
- Lowman MD, Box JD. 1983. Variation in leaf toughness and phenolic content among five species of Australian rain forest trees. Australian Journal of Ecology 8: 17–25 [Google Scholar]
- Lucas PW, Turner IM, Dominy NJ, Yamashita N. 2000. Mechanical defences to herbivory. Annals of Botany 86: 913–920. [Google Scholar]
- Mac Nally R, Horrocks G. 2002. Relative influences of patch, landscape and historical factors on birds in an Australian fragmented landscape. Journal of Biogeography 29: 395–410. [Google Scholar]
- Malishev M, Sanson GD. 2015. Leaf mechanics and herbivory defence: how tough tissue along the leaf body deters growing insect herbivores. Austral Ecology 40: 300–308. [Google Scholar]
- Matsuki S, Sano Y, Koike T. 2004. Chemical and physical defence in early and late leaves in three heterophyllous birch species native to northern Japan. Annals of Botany 93: 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson WJ., Jr 1980. Herbivory in relation to plant nitrogen content. Annual Review of Ecology and Systematics 11: 119–161. [Google Scholar]
- McNeil S, Southwood TRE. 1978. The role of nitrogen in the development of insect/plant relationships. In: Harbourne J. ed. Biochemical aspects of plant and animal co-evolution. London: Academic Press, 77–98. [Google Scholar]
- Meldau S, Erb M, Baldwin IT. 2012. Defence on demand: mechanisms behind optimal defence patterns. Annals of Botany 110: 1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles AT, Peco B, Wallis IR, et al. 2013. Correlations between physical and chemical defences in plants: tradeoffs, syndromes, or just many different ways to skin a herbivorous cat? New Phytologist 198: 252–263. [DOI] [PubMed] [Google Scholar]
- Mueller UG, Dearing MD. 1994. Predation and avoidance of tough leaves by aquatic larvae of the moth Parapoynx rugosalis (Lepidoptera: Pyralidae). Ecological Entomology 19: 155–158. [Google Scholar]
- Nahrung HF, Dunstan PK, Allen GR. 2001. Larval gregariousness and neonate establishment of the eucalypt-feeding beetle Chrysophtharta agricola (Coleoptera: Chrysomelidae: Paropsini). Oikos 94: 358–364. [Google Scholar]
- Ohmart CP, Thomas JR, Stewart LG. 1987. Nitrogen, leaf toughness and the population dynamics of Paropsis atomaria Olivier (Coleoptera: Chrysomelidae) – a hypothesis. Journal of the Australian Entomological Society 26: 203–207. [Google Scholar]
- Parrella MP. 1987. Biology of Liriomyza. Annual Review of Entomology 32: 201–224. [Google Scholar]
- Peeters PJ. 2002a. Correlations between leaf structural traits and the densities of herbivorous insect guilds. Biological Journal of the Linnean Society 77: 43–65. [Google Scholar]
- Peeters PJ. 2002b. Correlations between leaf constituent levels and the densities of herbivorous insect guilds in an Australian forest. Austral Ecology 27: 658–671. [Google Scholar]
- Peeters PJ, Read J, Sanson GD. 2001. Variation in the guild composition of herbivorous insect assemblages among co-occurring plant species. Austral Ecology 26: 385–399. [Google Scholar]
- Peeters PJ, Sanson G, Read J. 2007. Leaf biomechanical properties and the densities of herbivorous insect guilds. Functional Ecology 21: 246–255. [Google Scholar]
- Pérez-Harguindeguy N, Díaz S, Vendramini F, Cornelissen JHC, Gurvich DE, Cabido M. 2003. Leaf traits and herbivore selection in the field and in cafeteria experiments. Austral Ecology 28: 642–650. [Google Scholar]
- Price ML, Butler LG. 1977. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. Journal of Agricultural and Food Chemistry 25: 1268–1273. [Google Scholar]
- Prudic KL, Oliver JC, Bowers MD. 2005. Soil nutrient effects on oviposition preference, larval performance, and chemical defense of a specialist insect herbivore. Oecologia 143: 578–587. [DOI] [PubMed] [Google Scholar]
- R Development Core Team . 2011. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Raupp MJ. 1985. Effects of leaf toughness on mandibular wear of the leaf beetle, Plagiodera versicolora. Ecological Entomology 10: 73–79. [Google Scholar]
- Rausher MD. 1981. Host plant selection by Battus philenor butterflies: the roles of predation, nutrition, and plant chemistry. Ecological Monographs 51: 1–20. [Google Scholar]
- Read J, Sanson GD. 2003. Characterizing sclerophylly: the mechanical properties of a diverse range of leaf types. New Phytologist 160: 81–99. [DOI] [PubMed] [Google Scholar]
- Read J, Edwards C, Sanson GD, Aranwela N. 2000. Relationships between sclerophylly, leaf biomechanical properties and leaf anatomy in some Australian heath and forest species. Plant Biosystems 134: 261–277. [Google Scholar]
- Read J, Sanson GD, Lamont BB. 2005. Leaf mechanical properties in sclerophyll woodland and shrubland on contrasting soils. Plant and Soil 276: 95–113. [Google Scholar]
- Read J, Sanson GD, Caldwell E, et al. 2009. Correlations between leaf toughness and phenolics among species in contrasting environments of Australia and New Caledonia. Annals of Botany 103: 757–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavey D, Gaston KJ. 1991. The importance of leaf structure in oviposition by leaf-mining microlepidoptera. Oikos 61: 19–28. [Google Scholar]
- Sanson G. 2006. The biomechanics of browsing and grazing. American Journal of Botany 93: 1531–1545. [DOI] [PubMed] [Google Scholar]
- Sanson G, Read J, Aranwela N, Clissold F, Peeters P. 2001. Measurement of leaf biomechanical properties in studies of herbivory: opportunities, problems and procedures. Austral Ecology 26: 535–546. [Google Scholar]
- Schädler M, Jung G, Auge H, Brandl R. 2003. Palatability, decomposition and insect herbivory: patterns in a successional old-field plant community. Oikos 103: 121–132. [Google Scholar]
- Schofield RMS, Emmett KD, Niedbala JC, Nesson MH. 2011. Leaf-cutter ants with worn mandibles cut half as fast, spend twice the energy, and tend to carry instead of cut. Behavioral Ecology and Sociobiology 65: 969–982. [Google Scholar]
- Shade RE, Wilson MC. 1967. Leaf-vein spacing as a factor affecting larval feeding behavior of the cereal leaf beetle, Oulema melanopus (Coleoptera: Chrysomelidae). Annals of the Entomological Society of America 60: 493–496. [Google Scholar]
- Sinclair ARE. 1975. The resource limitation of trophic levels in tropical grassland ecosystems. Journal of Animal Ecology 44: 497–520. [Google Scholar]
- Swain T. 1979. Tannins and lignins. In: Rosenthal GA, Berenbaum MR. eds. Herbivores: their interactions with secondary plant metabolites. New York: Academic Press, 657–718. [Google Scholar]
- Tanton MT. 1962. The effect of leaf ‘toughness’ on the feeding of larvae of the mustard beetle Phaedon cochleariae Fab. Entomologia Experimentalis et Applicata 5: 74–78. [Google Scholar]
- Timmins WA, Bellward K, Stamp AJ, Reynolds SE. 1988. Food intake, conversion efficiency, and feeding behaviour of tobacco hornworm caterpillars given artificial diet of varying nutrient and water content. Physiological Entomology 13: 303–314. [Google Scholar]
- Van Soest PJ, Robertson JB, Lewis BA. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. Journal of Dairy Science 74: 3583–3597. [DOI] [PubMed] [Google Scholar]
- Vincent JFV. 1990. Fracture properties of plants. Advances in Botanical Research 17: 235–287. [Google Scholar]
- Walker GP. 1985. Stylet penetration by the bayberry whitefly, as affected by leaf age in lemon, Citrus limon. Entomologia Experimentalis et Applicata 39: 115–122. [Google Scholar]
- Walsh C, Mac Nally R. 2013. hier.part: Hierarchical partitioning. R package version 1·0-4. https://cran.r-project.org/web/packages/hier.part/index.html (accessed 22 September 2015). [Google Scholar]
- Wei J, Zou L, Kuang R, He L. 2000. Influence of leaf tissue structure on host feeding selection by pea leafminer Liriomyza huidobrensis (Diptera: Agromyzidae). Zoological Studies 39: 295–300. [Google Scholar]
- Wright W. 1992. The fracture properties of grasses and their relevance to feeding in herbivores. PhD dissertation, University of Reading, UK. [Google Scholar]
- Wright W, Vincent J FV. 1996. Herbivory and the mechanics of fracture in plants. Biological Reviews 71: 401–413. [Google Scholar]