Abstract
Background
Diagnosed diabetes mellitus (DM) is a consistently documented risk factor for ischemic stroke in patients with atrial fibrillation (AF).
Objectives
To assess the association between duration of diabetes and elevated HbA1c with risk of stroke among diabetics with AF.
Methods
We assessed this association in the ATRIA California community-based cohort of AF patients (study years 1996-2003) where all events were clinician adjudicated. We used Cox proportional hazards regression to estimate the rate of ischemic stroke in diabetic patients according to time-varying measures of estimated duration of diabetes (≥3 years compared to <3 years) and HbA1c values (≥9.0% and 7.0-8.9% compared to <7.0%), focusing on periods where patients were not anticoagulated.
Results
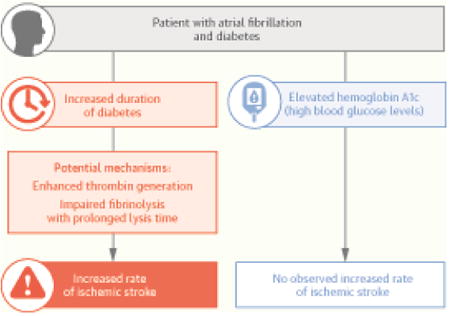
There were 2,101 diabetic patients included in the duration analysis, 40% with duration <3 years and 60% with duration ≥3 years at baseline. Among 1933 diabetic patients included in the HbA1c analysis, 46% had HbA1c <7.0%, 36% between 7.0 and 8.9%, and 19% ≥9.0% at baseline. Duration of diabetes ≥ 3 years was associated with an increased rate of ischemic stroke compared to duration <3 years (adjusted HR: 1.74, 95% CI: 1.10-2.76). The increased stroke rate was observed in older (≥75 years) and younger (<75 years) individuals. Neither poor glycemic control (HbA1c ≥9.0%, adjusted HR: 1.04, 95% CI: 0.57-1.92) or moderately increased HbA1c (7.0-8.9%, adjusted HR: 1.21, 95% CI: 0.77-1.91) were significantly associated with an increased rate of ischemic stroke compared with patients who had HbA1c <7.0%.
Conclusions
Duration of diabetes is a more important predictor of ischemic stroke than glycemic control in patients who have diabetes and AF.
Keywords: diabetes mellitus, stroke, atrial fibrillation
Graphical abstract
Introduction
Atrial fibrillation (AF) is the most common clinically significant arrhythmia and is associated with a 4- to 5-fold increase in the risk for ischemic stroke (1,2). Roughly 15% of patients with AF also carry the diagnosis of diabetes (3,4). Diagnosed diabetes mellitus (DM) is a consistently documented risk factor for ischemic stroke in patients with AF (5,6). A diagnosis of DM is included in the CHADS2 (Congestive heart failure, Hypertension, Age, Diabetes, Prior Stroke) and CHA2DS2-VASc stroke risk scoring systems for AF patients that are widely used to guide decision making for anticoagulation therapy (7,8). However, it is unclear which aspects of diabetes, including duration and glycemic control, may be associated with an increased risk of stroke in AF patients as prior studies have only assessed the diagnosis of DM as a risk factor (5,6).
The association between duration of diabetes and ischemic stroke risk has recently been studied in a general population of patients, with longer duration of diabetes associated with increased risk of stroke compared to non-diabetic patients (9,10). The association between hemoglobin A1c (HbA1c), a measure of glycemia during the prior 3 months, and ischemic stroke among DM patients has been investigated in general populations (11-13). Elevated HbA1c at baseline was an independent risk factor for stroke in DM patients with risk ratios of 1.17 and 2.33 for the highest two tertiles of HbA1c compared with the lowest tertile over 10 years of follow-up (12). Despite evidence for an association between elevated HbA1c and duration of diabetes with risk of stroke among diabetics in general, predominantly non-AF populations, it is unknown whether there is an association among diabetic patients with AF since the pathophysiology of stroke may be different in these populations.
To address these knowledge gaps, we aimed to assess the association between duration of diabetes and glycemic control with the rate of ischemic stroke in AF patients off anticoagulation therapy in clinical care within a large community-based cohort of patients with AF and comprehensive follow-up.
Methods
Study Population
Assembly of the Anticoagulation and Risk Factors in Atrial fibrillation (ATRIA) cohort has been described in detail previously (14). In brief, the cohort includes 13,559 adults aged 18 and older with diagnosed non-valvular AF who received care within Kaiser Permanente (KP) of Northern California (3). Cohort members were identified by searching electronic inpatient, outpatient, and electrocardiographic databases for physician-assigned International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9) diagnostic codes of AF (427.31, 427.32) between July 1996 and December 1997. We included all patients ≥18 years old with either ≥2 outpatient AF diagnoses or 1 outpatient AF diagnosis with ECG validation. Included patients were followed through September 2003. Since we were interested in non-transient, nonvalvular AF, we excluded AF patients with diagnosed mitral stenosis, valvular repair or replacement, transient postoperative AF, or concurrent hyperthyroidism.
We focused these analyses on patients with diagnosed diabetes mellitus at baseline or who were diagnosed during follow-up. The presence of diabetes mellitus was assessed from a validated longitudinal regional health plan diabetes registry (1,15) which used relevant inpatient and outpatient diagnoses (ICD-9 250.0 – 250.8), an abnormal (>6.7%) hemoglobin A1c (HbA1c) level, or a filled prescription for oral hypoglycemic agents or insulin to identify patients with diabetes. The patient's index date for these analyses was either the beginning of follow-up in the ATRIA cohort for those with diabetes at baseline, or the date of diabetes onset for those diagnosed with diabetes during follow-up in the ATRIA cohort
Measures
Exposures
Duration of diabetes was assigned both according to duration at baseline and as a time-varying exposure and categorized as <3 years and ≥3 years. An a priori three-level categorization was initially explored (<3, 3-8, >8 years), but the longer duration categories were collapsed due to similar effect sizes (Online Table 1). For analyses among diabetics, duration <3 years was considered as the reference category. We conducted additional analyses where AF patients without diabetes were considered as the reference category. Diabetes onset date for duration calculations was determined based on information either by self-report from respondents with diabetes from the diabetes registry or by using information based on meeting the first qualifying criteria from diagnoses, laboratory test results or medications. Patients without a diabetes onset date were excluded (n=106) from all analyses. Glycemic control was measured with hemoglobin HbA1c values from the regional health plan laboratory database based on HbA1c tests that were ordered as part of clinical management. HbA1c values were considered both using the baseline HbA1c value only as well as HbA1c values updated over time. Time-varying HbA1c values were considered valid for up to 1-year. At baseline, we looked back over the prior year for the most recent HbA1c value. If there was no value during this time period, the time between baseline and the first HbA1c value after baseline was excluded. If this time period between baseline and the first HbA1c value was longer than 1 year, then the patient was excluded from analysis (n=171). When considering HbA1c values as time-varying, if the time between HbA1c values during follow-up exceeded 1-year, then the time from 1-year follow-up until the next HbA1c value was excluded. HbA1c values were categorized at common clinical cut points of: <7.0%, 7.0% - 9.0%, and >9.0%. We also performed a sensitivity analysis regarding the missing time-varying HbA1c values, where HbA1c values were considered valid for only 6-months, for up to 1.5 years, and where values were assigned using linear interpolation between HbA1c tests.
Outcome
Ischemic stroke events were identified by searching hospitalization and billing claims databases for relevant ICD-9 codes for ischemic stroke found in the primary discharge diagnosis position (3). Potential events were adjudicated by a Clinical Outcomes Committee composed of physicians using medical records review, with a final decision made by a consulting neurologist if consensus was not reached by the committee. A valid ischemic stroke was defined as a documented acute neurological deficit lasting > 24 hours that was not explained by other causes (e.g., primary hemorrhage, trauma, infection, or vasculitis).
Covariables
Patient Characteristics
Data on patient age (continuous), gender, and self-reported race/ethnicity (white, black/African American, Asian/Pacific Islander, Hispanic ethnicity, other/unknown) were obtained from administrative databases. Educational attainment status and income status were assigned from geocoding based on U.S. Census block group data.
Periods off anticoagulation
To examine the role of glycemic control and duration of diabetes on risk of ischemic stroke, we focused on periods of follow-up off anticoagulation. To identify periods off warfarin therapy, we used a previously validated method to assign use of warfarin based on data from prescriptions and outpatient international normalized ratio measurements found in pharmacy and laboratory databases, respectively (3). Longitudinal warfarin exposure was based on number of days of supply per prescription and intervening international normalized ratios. For any 2 consecutive prescriptions with a gap of up to 60 days, a patient was considered to be continuously on warfarin. For gaps >60 days, we considered the patient to be continuously on warfarin if there was intervening international normalized ratio measurements at least every 42 days. Otherwise, the patient was considered off warfarin from day 31 after the end date of the first prescription until the start date of the next prescription. This grace period of 30 days at the end of each warfarin period was given because changes in warfarin dose are common.
Health-related variables
History of comorbid conditions, including prior ischemic stroke, chronic heart failure, coronary disease, and hypertension, were collected from clinical inpatient and ambulatory databases using validated algorithms and were assessed using data during the five years prior to the patient's index date and were updated during the follow-up period. Kidney dysfunction (defined as estimated glomerular filtration rate [eGFR] < 45 ml/min/1.73 m2) was calculated from laboratory values using the Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration formula (16). Patients without an eGFR value in the prior year were considered to have normal renal function and patients on dialysis were considered to have kidney dysfunction. Proteinuria was defined as a urine dipstick protein result of ≥1+ (30 mg/dL or higher) in the absence of potential urinary tract infection found in laboratory databases (17). Patients without a urine dipstick protein laboratory result in the prior year were considered to not have proteinuria. Insulin use was time-varying and assessed from prescription databases. All health-related variables were dichotomized. For all patients, the CHADS2 (7), CHA2DS2-VASc (8), and ATRIA (18) stroke risk scores were calculated. Functional disability at the time of hospital discharge was determined through review of notes in the medical record. Disability was categorized using a modified Rankin Scale into fatal inpatient event, major/severe disability (deficits that prevented independent living, corresponding to Rankin scores of 3-5), minor disability (residual deficit that did not interfere with independent living, equivalent to Rankin scores of 1-2), and no disability (Rankin score 0) (19). Assignment of modified Rankin scores in the ATRIA study was validated by post-hospital fatality rates (20).
Statistical analyses
We conducted descriptive and stratified analyses to examine the distribution of potential confounders among duration and HbA1c categories. The associations between comorbid conditions and diabetes characteristics with duration and HbA1c categories were adjusted for age, gender, and race in logistic regression models (Table 1). To construct person-time data, follow-up began on the first day the patient held a diagnosis of diabetes and was not receiving warfarin therapy and ended at the first occurrence of any of the following: ischemic stroke, death, disenrollment from the health plan, or end of the follow-up period. Additionally, follow-up was stopped if the patient initiated warfarin therapy and resumed if there was another period without warfarin therapy. Cox proportional hazards regression was used to estimate the unadjusted and adjusted hazard ratio (HR) for the association between HbA1c and ischemic stroke, as well as with duration of diabetes and ischemic stroke (SAS, version 9.3 [SAS Institute, Cary, North Carolina]). We constructed models that used only baseline exposure (HbA1c or duration) values as well as models that used time-varying exposure values. To further assess and control for confounding, we added race, education, income, and time-varying CHA2DS2-VASc (which includes all the CHADS2 risk factors) and ATRIA stroke risk score factors to our multivariable models. For the HbA1c analysis, we also adjusted for time-varying use of insulin and duration of diabetes. For the duration of diabetes analyses, we did not adjust for HbA1c, use of insulin, proteinuria, or kidney dysfunction since we believed these variables to be on the causal pathway from diabetes duration to ischemic stroke. Duration analyses were stratified by age (<75 years and ≥75 years). To assess the presence of additive interaction between HbA1c and diabetes duration, we calculated the relative excess rate due to interdependence (RERI) (21).
Table 1. Characteristics of diabetic patients by estimated diabetes duration and HbA1c values at baseline.
| Diabetes Duration* | HbA1c† | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Non-Diabetic (n=8356) | 0-3 years (n=837) | ≥3 years (n=1264) | P-Value§ | HbA1c < 7.0% (n=883) | HbA1c 7.0-8.9% (n=690) | HbA1c ≥ 9.0% (n=360) | P-Value§ | |
| Age, mean (SD), years | 71.8 (12.7) | 69.0 (11.0) | 71.7 (8.9) | <0.001 | 71.5 (9.6) | 70.5 (9.7) | 67.9 (9.8) | <0.001 |
| <65 | 1903 (22.8%) | 244 (29.2%) | 251 (19.9%) | <0.001 | 184 (20.8%) | 161 (23.3%) | 118 (32.8%) | <0.001 |
| 65-74 | 2422 (29.0%) | 311 (37.2%) | 494 (39.1%) | 326 (36.9%) | 277 (40.1%) | 144 (40.0%) | ||
| ≥ 75 | 4031 (48.2%) | 282 (33.7%) | 519 (41.1%) | 374 (42.4%) | 252 (36.5%) | 98 (27.2%) | ||
| Gender, female | 3714 (44.5%) | 321 (38.4%) | 501 (39.6%) | 0.55 | 325 (36.8%) | 273 (39.6%) | 153 (42.5%) | 0.16 |
| Race | <0.001 | 0.02 | ||||||
| White | 7226 (86.5%) | 700 (83.6%) | 1071 (84.7%) | 764 (86.5%) | 591 (85.7%) | 283 (78.6%) | ||
| Black/African American | 306 (3.7%) | 28 (3.4%) | 73 (5.8%) | 31 (3.5%) | 35 (5.1%) | 28 (7.8%) | ||
| Asian/Pacific Islander | 416 (5.0%) | 75 (9.0%) | 74 (5.9%) | 55 (6.2%) | 44 (6.4%) | 33 (9.2%) | ||
| Hispanic | 193 (2.3%) | 23 (2.8%) | 43 (3.4%) | 27 (3.1%) | 15 (2.2%) | 14 (3.9%) | ||
| Other/Unknown | 215 (2.6%) | 11 (1.3%) | 3 (0.2%) | 6 (0.7%) | 4 (0.6%) | 3 (0.8%) | ||
| Education (< high school graduation) | 677 (8.1%) | 92 (10.9%) | 136 (10.7%) | 0.93 | 83 (9.4%) | 79 (11.5%) | 49 (13.6%) | 0.10 |
| Income (annual household income < $35,000) | 907 (10.9%) | 98 (11.7%) | 160 (12.6%) | 0.68 | 100 (12.0% | 87 (13.4%) | 44 (13.0%) | 0.52 |
| Comorbidities and Diabetes Characteristics | ||||||||
| Prior ischemic stroke | 632 (7.6%) | 52 (6.2%) | 173 (13.7%) | <0.001‡ | 92 (10.4%) | 70 (10.1%) | 35 (9.7%) | 0.98‡ |
| Chronic heart failure | 2141 (25.6%) | 251 (30.0%) | 583 (46.1%) | <0.001‡ | 342 (38.7%) | 264 (38.3%) | 146 (40.6%) | 0.36‡ |
| Coronary disease | 2117 (25.3%) | 261 (31.2%) | 581 (46.0%) | <0.001‡ | 351 (39.8%) | 287 (41.6%) | 134 (37.2%) | 0.56‡ |
| Peripheral arterial disease | 157 (1.9%) | 18 (2.2%) | 63 (5.0%) | 0.005‡ | 34 (3.9%) | 26 (3.8%) | 14 (3.9%) | 0.86‡ |
| Hypertension | 3852 (46.1%) | 497 (59.4%) | 901 (71.3%) | <0.001‡ | 601 (68.1%) | 475 (68.8%) | 220 (61.1%) | 0.02‡ |
| Proteinuria | 926 (11.1%) | 103 (12.3%) | 366 (29.0%) | <0.001‡ | 185 (21.0%) | 146 (21.2%) | 91 (25.3%) | 0.26‡ |
| Significant kidney dysfunction | 1166 (14.0%) | 94 (11.2%) | 280 (22.2%) | <0.001‡ | 150 (17.0%) | 119 (17.2%) | 58 (16.1%) | 0.92‡ |
| Insulin use | - | 38 (4.5%) | 490 (38.8%) | <0.001‡ | 142 (16.1%) | 226 (32.8%) | 149 (41.4%) | <0.001‡ |
| HbA1c Value | <0.001‡ | |||||||
| < 7.0% | - | 417 (49.8%) | 461 (36.5%) | - | - | - | ||
| 7.0-8.9% | - | 285 (34.1%) | 508 (40.2%) | - | - | - | ||
| ≥9.0% | - | 135 (16.1%) | 295 (23.3%) | - | - | - | ||
| Duration of diabetes | <0.001‡ | |||||||
| 0-3 years | - | - | - | 458 (51.9%) | 226 (32.8%) | 89 (24.7%) | ||
| ≥3 years | - | - | - | 425 (48.1%) | 464 (67.2%) | 271 (75.3%) | ||
| Predicted Stroke Risk | ||||||||
| CHADS2, mean (SD) | 1.4 (1.1) | 2.4 (1.1) | 2.9 (1.2) | <0.001 | 2.7 (1.2) | 2.6 (1.1) | 2.5 (1.0) | 0.01 |
| CHA2DS2-VASc, mean (SD) | 2.8 (1.7) | 3.8 (1.6) | 4.5 (1.6) | <0.001 | 4.3 (1.6) | 4.2 (1.6) | 4.0 (1.6) | 0.01 |
| ATRIA stroke risk score, mean (SD) | 4.9 (2.8) | 5.5 (2.7) | 6.8 (2.6) | <0.001 | 6.4 (2.7) | 6.2 (2.6) | 5.8 (2.7) | 0.008 |
Population excludes n=106 without a diabetes onset date
Population excludes n=171 with initial gap > 1 year and n=106 without a diabetes onset date (n=274 total)
Adjusted for age, gender, race
P-Values represent comparisons among diabetic patients (Reference categories are duration ≤ 3 years and HbA1c < 7%). Compared to non-diabetic patients, diabetics with duration ≤ 3 years were significantly different (p<0.05) in all categories except for income status, peripheral arterial disease, prior ischemic stroke, and significant kidney dysfunction. Diabetics with duration > 3 years were significantly different in all categories except mean age. SD: standard deviation
This research was approved by the Institutional Review Boards at Massachusetts General Hospital and at Kaiser Foundation Research Institute. Waiver of informed consent was obtained because of the nature of the study.
Results
Among 2101 diabetic patients included in the diabetes duration analysis, 837 (39.8%) had estimated diabetes duration for ≤ 3 years at baseline, while 1264 (60.2%) had estimated diabetes duration for > 3 years at baseline (Table 1). The mean duration of diabetes at baseline was 7.5 years (SD: 9.6, Median: 4.7). Patients who had diabetes for > 3 years were older than those with diabetes for 0-3 years. In addition, they were more likely to have had a prior ischemic stroke, to have diagnosed chronic heart failure, coronary disease, peripheral arterial disease, hypertension, proteinuria, significant kidney dysfunction, to be using insulin, and to have HbA1c ≥ 9.0% compared to patients with duration ≤ 3 years at baseline after adjusting for age, gender, and race. Patients with longer duration of diabetes also had increased risk of stroke according to CHADS2, CHA2DS2-Vasc, and ATRIA stroke risk scores.
Among 1,933 diabetic patients included in the HbA1c analysis, 883 (45.7%) had a HbA1c value <7.0% at baseline, while 690 (35.7%) had a HbA1c value between 7.0 and 8.9%, and 360 (18.6%) had a HbA1c value ≥ 9.0% (Table 1). Patients with HbA1c ≥ 9.0% were younger and less likely to be white. Patients with HbA1c ≥ 9.0% were also less likely to have diagnosed hypertension, were more likely to be using insulin, and had diabetes for a longer period of time compared to patients with lower HbA1c values at baseline after adjusting for age, gender, and race. There were small, but statistically significant, differences in CHADS2, CHA2DS2-Vasc, and ATRIA stroke risk scores among HbA1c categories, with HbA1c ≥ 9.0% having the lowest stroke risk scores.
Follow-up
In the duration of diabetes analysis, there was 5219.7 person-years off of warfarin therapy included (mean [SD]: 2.48 [2.23] years per patient) among diabetics. Non-diabetics contributed 25,806.7 person-years off warfarin therapy (mean [SD]: 3.09 [2.48] years per patient). For both analyses, diabetic patients without a known diabetes onset date were excluded, but they only represented 102.2 person-years.
There were a total of 7217 HbA1c measurements during follow-up. The median number of days between HbA1c measurements was 143 (interquartile range 89-240) days, with median 154 days among HbA1c < 7.0% group; median 135 days among HbA1c 7.0%-8.9%; and median 135 days among HbA1c ≥ 9.0%). The HbA1c analysis included 3818.0 person-years off of warfarin therapy (mean [SD]: 1.98 [1.98] years per patient). Due to gaps between HbA1c measures that exceeded 1-year, 1236.8 person-years off-warfarin were excluded from analyses with time-varying HbA1c values.
Duration of Diabetes and Ischemic Stroke
During follow-up, there were 137 validated ischemic stroke events among diabetics included in the duration of diabetes analysis. The rate of ischemic stroke during follow-up was higher among patients with estimated duration of diabetes ≥3 years (2.9/100 person-years) compared with patients who had an estimated duration of diabetes less than 3 years (1.7/100 person-years) (Table 2). In unadjusted analyses, diabetes duration ≥3 years was associated with an increased rate of ischemic stroke compared with duration <3 years (unadjusted hazard ratio (HR): 2.04, 95% CI: 1.27-3.26). After adjustment for stroke risk factors in multivariable analyses, the increased rate of ischemic stroke associated with diabetes duration ≥ 3 years remained elevated (adjusted HR: 1.74, 95% CI: 1.10-2.76). In analyses stratified by age (<75 and ≥75 years), the increased rate of ischemic stroke in patients with duration ≥ 3 years was present in both age groups, but was larger in the older age group (<75 years, Baseline Duration – adjusted HR: 1.52, 95% CI: 0.92-2.52, Time-Varying Duration - adjusted HR: 1.75, 95% CI: 0.81-3.80; ≥75 years, Baseline Duration – adjusted HR: 1.55, 95% CI: 0.92-2.62, Time-Varying Duration - adjusted HR: 1.71, 95% CI: 0.96-3.06). Additive interaction between duration and age was small and not statistically significant (RERI: 0.34, 95% CI: -0.57-1.24). Since subjects with a prior stroke may be at increased risk of incident ischemic stroke, we performed analyses (both unstratified and stratified by age) which excluded those with prior ischemic stroke and results were similar (data not shown). Compared with non-diabetics, diabetics with an estimated duration >3 years had an increased rate of ischemic stroke (adjusted HR: 1.62, 95% CI: 1.31-2.00), while diabetics with a duration < 3 years did not (adjusted HR: 0.90, 95% CI: 0.58-1.40) (Table 2). Results were similar when using only baseline duration values. Analyses with a three-level categorization of estimated diabetes duration (<3, 3-8, >8 years) showed a similarly increased rate of ischemic stroke in both the 3-8 and >8 years categories (Online Table 1). At the time of discharge, 52.4% of patients with estimated diabetes duration <3 years had major deficits or worse compared to 64.7% of patients with estimated diabetes duration of three of more years, though this difference was not statistically significant.
Table 2. Association between estimated duration of diabetes and incidence of ischemic stroke.
| Non-Diabetic | Estimated Diabetes Duration < 3 years | Estimated Diabetes Duration ≥3 years | |
|---|---|---|---|
| Baseline Duration Values | |||
|
| |||
| Ischemic stroke events | 463 | 44 | 93 |
|
| |||
| Person-years (py) | 25,801.8 | 2299.9 | 2919.8 |
|
| |||
| Rate | 1.8/100 py | 1.9/100py | 3.2/100py |
|
| |||
| Among Diabetics Only | |||
|
| |||
| Hazard Ratio (unadjusted) | n/a | Reference | 1.75 (1.22-2.50) |
|
| |||
| Hazard Ratio (adjusted*) | n/a | Reference | 1.51 (1.05-2.17) |
|
| |||
| Among All Patients | |||
|
| |||
| Hazard Ratio (unadjusted) | Reference | 1.10 (0.81-1.50) | 1.80 (1.44-2.25) |
|
| |||
| Hazard Ratio (adjusted*) | Reference | 1.15 (0.84-1.58) | 1.63 (1.29-2.05) |
|
| |||
| Time Varying Duration Values | |||
| Ischemic stroke events | 463 | 21 | 116 |
| Person-years (py) | 25,801.8 | 1260.6 | 3959.1 |
| Rate | 1.8/100 py | 1.7/100 py | 2.9/100 py |
| Among Diabetics Only | |||
| Hazard Ratio (unadjusted) | n/a | Reference | 2.04 (1.27-3.26) |
| Hazard Ratio (adjusted*) | n/a | Reference | 1.74 (1.10-2.76) |
| Among All Patients | |||
| Hazard Ratio (unadjusted) | Reference | 0.84 (0.55-1.30) | 1.75 (1.43-2.15) |
| Hazard Ratio (adjusted*) | Reference | 0.90 (0.58-1.40) | 1.62 (1.31-2.00) |
Adjusted for age, gender, race, education, income, congestive heart failure, hypertension, prior ischemic stroke, coronary artery disease, and peripheral arterial disease
Py = person-years
HbA1c and Ischemic Stroke
Using time-varying HbA1c, there were 104 validated ischemic stroke events during follow-up included in the HbA1c analysis. The rate of ischemic stroke during follow-up was 2.7/100 person-years (unadjusted HR: 1.11, 95% CI: 0.63-1.98) in patients with HbA1c ≥9.0%, 3.0/100 person-years (unadjusted HR: 1.22, 95% CI: 0.80-1.87) in patients with HbA1c between 7.0% and 8.9%, compared to a rate of 2.5/100 person-years in patients with HbA1c <7.0% (Table 3A). In multivariable models adjusting for stroke risk factors, insulin use, and diabetes duration, neither poor glycemic control (HbA1c ≥ 9.0%, adjusted HR: 1.04, 95% CI: 0.57-1.92) or moderately increased HbA1c (7.0%-8.9%, adjusted HR: 1.21, 95% CI: 0.77-1.91) were significantly associated with an increased rate of ischemic stroke compared with patients who had HbA1c < 7.0%. There was similarly no significant increase in rate of ischemic stroke when only using baseline HbA1c values. Results were similar when using different rules for classifying time-varying HbA1c values (i.e. valid for 6 months, valid for 1.5 years, and assessed using linear interpolation) (Table 3B). Additionally, the results were similar in multivariable models that did not adjust for insulin use (data not shown). Since the rate of ischemic stroke did not vary significantly by HbA1c level among diabetics, we do not present analyses with non-diabetics as the reference group. There was no clear pattern in the proportion of patients discharged with major deficits or worse by HbA1c category (<7.0%: 64.6%, 7.0%-8.9%: 52.9%, ≥9.0%: 68.7) and the observed differences were not significantly different.
Table 3a. Association between HbA1c values and incidence of ischemic stroke.
| HbA1c < 7.0% | HbA1c 7.0-8.9% | HbA1c ≥ 9.0% | |
|---|---|---|---|
| Baseline HbA1c Values | |||
|
| |||
| Ischemic stroke events | 58 | 50 | 29 |
|
| |||
| Person-years (py) | 2198.6 | 1754.2 | 1009.8 |
|
| |||
| Rate | 2.6/100py | 2.9/100py | 2.9/100py |
|
| |||
| Hazard Ratio (unadjusted) | Reference | 1.09 (0.75-1.60) | 1.10 (0.70-1.72) |
|
| |||
| Hazard Ratio (adjusted*) | Reference | 1.03 (0.69-1.54) | 1.05 (0.66-1.66) |
|
| |||
| Time-Varying HbA1c Values | |||
| Ischemic stroke events | 42 | 46 | 16 |
| Person-years (py) | 1690.8 | 1545.3 | 581.9 |
| Rate | 2.5/100 py | 3.0/100 py | 2.7/100 py |
| Hazard Ratio (unadjusted) | Reference | 1.22 (0.80-1.87) | 1.11 (0.63-1.98) |
| Hazard Ratio (adjusted*) | Reference | 1.21 (0.77-1.91) | 1.04 (0.57-1.92) |
Adjusted for age, gender, race, education, income, congestive heart failure, hypertension, prior ischemic stroke, coronary artery disease, peripheral arterial disease, proteinuria, significant kidney dysfunction, insulin use, and diabetes duration
Py = person-years
Table 3b. Association between time-varying HbA1c values and incidence of ischemic stroke using different rules for classifying time-varying HbA1c values (i.e. valid for 6 months, valid for 1.5 years, and assessed using linear interpolation).
| HbA1c < 7.0% | HbA1c 7.0-8.9% | HbA1c > 9.0% | |
|---|---|---|---|
| 6-Months | |||
| Ischemic stroke events | 32 | 33 | 7 |
| Person-years (py) | 1229.7 | 1173.5 | 431.8 |
| Rate | 2.6/100 py | 2.8/100 py | 1.6/100 py |
| Hazard Ratio (unadjusted) | - | 1.15 (0.70-1.88) | 0.62 (0.27-1.41) |
| Hazard Ratio (adjusted*) | - | 1.13 (0.67-1.92) | 0.68 (0.29-1.62) |
| 1.5 Years | |||
| Ischemic stroke events | 48 | 51 | 16 |
| Person-years (py) | 1905.7 | 1684.9 | 660.0 |
| Rate | 2.5/100 py | 3.0/100 py | 2.4/100 py |
| Hazard Ratio (unadjusted) | - | 1.24 (0.83-1.85) | 0.96 (0.55-1.69) |
| Hazard Ratio (adjusted*) | - | 1.24 (0.81-1.88) | 0.95 (0.53-1.72) |
| Linear Interpolation | |||
| Ischemic stroke events | 55 | 59 | 20 |
| Person-years (py) | 1953.3 | 1953.1 | 712.8 |
| Rate | 2.8/100 py | 3.0/100 py | 2.8/100 py |
| Hazard Ratio (unadjusted) | - | 1.10 (0.77-1.59) | 0.94 (0.57-1.57) |
| Hazard Ratio (adjusted*) | - | 1.12 (0.76-1.63) | 0.93 (0.55-1.58) |
Adjusted for age, gender, race, education, income, congestive heart failure, hypertension, prior thromboembolism, coronary artery disease, peripheral arterial disease, proteinuria, significant kidney dysfunction, insulin use, and diabetes duration. Py = person-years
Interaction between HbA1c and Duration of Diabetes
Among diabetics, additive interaction between HbA1c and duration of diabetes was assessed with HbA1c dichotomized at both 7.0% (RERI: 0.16, 95% CI: -0.80 – 1.12) and 9.0% (RERI: 0.001, 95% CI: -1.32 – 1.33) and in both cases the magnitude of interaction was small.
Discussion
Within a large, ambulatory cohort of adults with atrial fibrillation and diabetes, we found that longer estimated duration of diabetes was strongly associated with an increase in adjusted rate of ischemic stroke, while elevated hemoglobin A1c values were not significantly associated with an increase in ischemic stroke among diabetics. The increased rate of ischemic stroke in those who have had diabetes for an estimated 3 or more years appeared to be independent of age as the association was present in both older (≥75 years) and younger (<75 years) subjects.
Our results for diabetes duration are consistent with prior research conducted within a general population of patients which found an increased rate of ischemic stroke as duration increased compared to non-diabetic patients (9,10). However, our results for HbA1c in diabetics with AF are not consistent with prior research conducted in diabetic patients in general. In our study, increased HbA1c did not have a substantial impact on the rate of ischemic stroke, while elevated HbA1c was significantly associated with ischemic stroke in predominantly non-AF populations (11-13). A possible reason for HbA1c having no association with ischemic stroke in diabetic patients with AF is the difference in the primary mechanism for stroke in diabetic patients with and without AF. Among patients with diabetes without AF, stroke is often due to underlying atherosclerosis (22,23). This mechanism may not be as important among diabetic patients with AF since the primary mechanism for ischemic stroke is atrio-embolic (24). Duration of diabetes may be most important among AF populations due to enhanced thrombin generation, impaired fibrinolysis with prolonged lysis time, and unfavorably altered plasma fibrin clot structure which may increase the risk of thrombotic events (25,26).
Current stroke risk scoring systems for AF patients include a diagnosis of diabetes mellitus as a stroke risk factor (7,8,18). While the diagnosis of diabetes has been shown to be a risk factor for stroke, the magnitude of this association has varied among different studies and has often been quite small. For example, the association between diabetes and stroke was small in the Swedish Atrial Fibrillation cohort study (adjusted HR: 1.19) and the UK General Practice Research Database (adjusted RR: 1.33), while the association was larger in the ATRIA cohort (HR: 1.57) and the AF Investigators pooled analyses of warfarin trials (RR = 1.7) (4,18,27,28). Our study suggests that stroke risk schemes for AF patients could potentially be improved by accounting for how long a patient has had diabetes.
This study was strengthened by including a large number of AF patients with diabetes from a community-based cohort with comprehensive follow-up. Furthermore, our large cohort is distinctive in having information on HbA1c values and duration of diabetes. The registry we utilized to identify patients with diabetes has been demonstrated to be highly sensitive (96% with self-reported diabetes from survey as gold standard) and specific (2% false positives) (15). All events were adjudicated by physicians or nurses using a standardized medical record review protocol which minimizes errors in case ascertainment. For both HbA1c and diabetes duration, we also were able to consider these variables as time-varying to minimize misclassification when assessing their association with ischemic stroke.
This study has several potential limitations. We are unable to separate type 1 and type 2 diabetes. However, given the age of onset in this population and the prevalence of type 2 diabetes, we expect the vast majority are type 2 diabetics. As this was a clinical practice-derived cohort, there was no structured protocol to screen for diabetes, so there may be some misclassification about the actual duration of diabetes, especially given that there is often a lag between diabetes onset and diagnosis (29). Although this study improves upon prior studies by analyzing repeated measures of HbA1c, it is possible that misclassification and/or selection bias may have occurred. Since not all patients received regularly-spaced HbA1c lab monitoring, our primary analyses allowed an HbA1c value to be valid for up to 1 year. This approach was meant to minimize misclassification by using a single HbA1c value for an overly long period of time. We examined the potential for selection bias by comparing patients who did vs. did not go more than 1-year between HbA1c measurements and found having a gap between a patient's HbA1c measures of ≥1 year was not associated with HbA1c value, a finding which alleviates concerns about selection bias. Additionally, we performed several sensitivity analyses using different approaches for handling time-varying HbA1c values. Considering an HbA1c value valid for 6 months, 18 months, and using a linear interpolation approach (which did not exclude person-time based on gaps in measurements) all had similar results to our primary approach. Further, we also present analyses using only baseline HbA1c values and our results were confirmed. It remains possible that our follow-up period of up to 7 years may not have been long enough to observe an association between HbA1c and thromboembolism. A final concern is that there may be residual confounding in these analyses. Confounding by age was of particular concern since increased duration is correlated with increased age. In addition to controlling for age in our multivariable models, we also stratified our duration analyses by age and found that duration of diabetes was associated with ischemic stroke in both younger and older patients, although was only statistically significant in older patients due to wide confidence intervals.
Conclusions
In conclusion, our study demonstrates that duration of diabetes is a more important predictor of ischemic stroke than glycemic control in patients who have both diabetes and atrial fibrillation. Given the relatively small association between the diagnosis of diabetes and ischemic stroke in several published studies, accounting for duration of diabetes may improve stroke risk models for patients with atrial fibrillation.
Supplementary Material
Perspectives.
Competency in Medical Knowledge
Among patients with diabetes and atrial fibrillation, the duration of diabetes is a more important predictor of ischemic stroke than glycemic control.
Translational Outlook
Future studies should address whether incorporating the duration of diabetes in atrial fibrillation stroke risk assessment tools improves prediction compared to the current models that incorporate only the diagnosis of diabetes.
Acknowledgments
Funding Sources: This study was supported by the National Institute on Aging (R01 AG15478 and K23 AG028978), the National Heart, Lung and Blood Institute (U19 HL91179 and RC2HL101589), and the Eliot B. and Edith C. Shoolman fund of the Massachusetts General Hospital (Boston, MA). The funding sources had no role in study design, data collection, data analysis, data interpretation, or preparation of this manuscript. Jeffrey Ashburner, PhD, MPH had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- AF
Atrial fibrillation
- CI
Confidence interval
- DM
Diabetes mellitus
- ECG
Electrocardiogram
- eGFR
Estimated glomerular filtration rate
- HbA1c
Hemoglobin A1c
- HR
Hazard ratio
- RERI
Relative excess rate due to interdependence
Appendix 1
Association between duration of diabetes and incidence of ischemic stroke with 3 duration categories.
| Non-Diabetic | Duration < 3 years | Duration 3-8 years | Duration > 8 years | |
|---|---|---|---|---|
| Baseline Duration Values | ||||
| Ischemic stroke events | 463 | 44 | 42 | 51 |
| Person-years (py) | 25,801.8 | 2299.9 | 1496.3 | 1423.5 |
| Rate | 1.8/100 py | 1.9/100py | 2.8/100py | 3.6/100py |
| Among Diabetics Only | ||||
| Hazard Ratio (unadjusted) | n/a | Reference | 1.55 (1.02-2.35) | 1.96 (1.31-2.94) |
| Hazard Ratio (adjusted*) | n/a | Reference | 1.36 (0.89-2.08) | 1.66 (1.10-2.51) |
| Among All Patients | ||||
| Hazard Ratio (unadjusted) | Reference | 1.10 (0.81-1.50) | 1.59 (1.16-2.18) | 2.02 (1.51-2.70) |
| Hazard Ratio (adjusted*) | Reference | 1.16 (0.85-1.59) | 1.46 (1.05-2.03) | 1.80 (1.33-2.42) |
| Time Varying Duration Values | ||||
| Ischemic stroke events | 463 | 21 | 53 | 63 |
| Person-years (py) | 25,801.8 | 1260.6 | 1830.4 | 2128.7 |
| Rate | 1.8/100 py | 1.7/100 py | 2.9/100 py | 3.0/100 py |
| Hazard Ratio (unadjusted) | n/a | Reference | 2.01 (1.21-3.34) | 2.06 (1.25-3.40) |
| Hazard Ratio (adjusted*) | n/a | Reference | 1.87 (1.14-3.07) | 1.68 (1.03-2.73) |
| Hazard Ratio (unadjusted) | Reference | 0.84 (0.55-1.30) | 1.72 (1.29-2.28) | 1.78 (1.37-2.32) |
| Hazard Ratio (adjusted*) | Reference | 0.88 (0.57-1.39) | 1.67 (1.26-2.24) | 1.56 (1.19-2.04) |
Adjusted for age, gender, race, education, income, congestive heart failure, hypertension, prior ischemic stroke, coronary artery disease, and peripheral arterial disease
py=person years
Footnotes
Disclosures: Dr. Singer serves as a consultant/advisory board member for Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, CVS Heath, Johnson and Johnson, Medtronic, Pfizer, and St. Jude Medical on matters related to preventing stroke in atrial fibrillation. Additionally, Dr. Singer has research contracts with Medtronic, Inc related to atrial fibrillation and risk of stroke, with Johnson and Johnson related to stroke prevention in atrial fibrillation, and with Bristol-Myers Squibb related to atrial fibrillation and risk of stroke. Dr. Go has received a research grant from CSL Behring. No other authors have relationships with industry.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Chang Y, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 4.Van Staa TP, Setakis E, Di Tanna GL, et al. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9:39–48. doi: 10.1111/j.1538-7836.2010.04085.x. [DOI] [PubMed] [Google Scholar]
- 5.The Atrial Fibrillation Investigators. Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154:1449–1457. [PubMed] [Google Scholar]
- 6.The Atrial Fibrillation Investigators. The efficacy of aspirin in patients with atrial fibrillation. Analysis of pooled data from 3 randomized trials. Arch Intern Med. 1997;157:1237–1240. [PubMed] [Google Scholar]
- 7.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285:2864–2870. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 8.Lip GY, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee C, Moon YP, Paik MC, et al. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2012;43:1212–1217. doi: 10.1161/STROKEAHA.111.641381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janghorbani M, Hu FB, Willett WC, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses' Health Study. Diabetes Care. 2007;30:1730–1735. doi: 10.2337/dc06-2363. [DOI] [PubMed] [Google Scholar]
- 11.Moss SE, Klein R, Klein BE, Meuer SM. The association of glycemia and cause-specific mortality in a diabetic population. Arch Intern Med. 1994;154:2473–2479. [PubMed] [Google Scholar]
- 12.Selvin E, Coresh J, Shahar E, et al. Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol. 2005;4:821–826. doi: 10.1016/S1474-4422(05)70227-1. [DOI] [PubMed] [Google Scholar]
- 13.Stratton IM, Adler AI, Neil HA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Go AS, Hylek EM, Borowsky LH, et al. Warfarin use among ambulatory patients with nonvalvular atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Ann Intern Med. 1999;131:927–934. doi: 10.7326/0003-4819-131-12-199912210-00004. [DOI] [PubMed] [Google Scholar]
- 15.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–1402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Singer DE, Chang Y, Borowsky LH, et al. A new risk scheme to predict ischemic stroke and other thromboembolism in atrial fibrillation: the ATRIA study stroke risk score. J Am Heart Assoc. 2013;2:e000250. doi: 10.1161/JAHA.113.000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan PW, Jorgensen HS, Wade DT. Outcome measures in acute stroke trials: a systematic review and some recommendations to improve practice. Stroke. 2000;31:1429–1438. doi: 10.1161/01.str.31.6.1429. [DOI] [PubMed] [Google Scholar]
- 20.Fang MC, Go AS, Chang Y, et al. Long-term survival after ischemic stroke in patients with atrial fibrillation. Neurology. 2014;82:1033–1037. doi: 10.1212/WNL.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd. Philadelphia, PA: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 22.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 23.Selvin E, Coresh J, Golden SH, et al. Atherosclerosis risk in communities s. Glycemic control, atherosclerosis, and risk factors for cardiovascular disease in individuals with diabetes: the atherosclerosis risk in communities study. Diabetes Care. 2005;28:1965–1973. doi: 10.2337/diacare.28.8.1965. [DOI] [PubMed] [Google Scholar]
- 24.Hart RG, Pearce LA, Miller VT, et al. Cardioembolic vs. noncardioembolic strokes in atrial fibrillation: frequency and effect of antithrombotic agents in the stroke prevention in atrial fibrillation studies. Cerebrovasc Dis. 2000;10:39–43. doi: 10.1159/000016023. [DOI] [PubMed] [Google Scholar]
- 25.Konieczynska M, Fil K, Bazanek M, Undas A. Prolonged duration of type 2 diabetes is associated with increased thrombin generation, prothrombotic fibrin clot phenotype and impaired fibrinolysis. Thromb Haemost. 2014;111:685–693. doi: 10.1160/TH13-07-0566. [DOI] [PubMed] [Google Scholar]
- 26.Neergaard-Petersen S, Hvas AM, et al. The influence of type 2 diabetes on fibrin clot properties in patients with CAD. Thromb Haemost. 2014;112 doi: 10.1160/TH14-05-0468. [DOI] [PubMed] [Google Scholar]
- 27.Butler AC, Tait RC. Restarting anticoagulation in prosthetic heart valve patients after intracranial haemorrhage: a 2-year follow-up. Br J Haematol. 1998;103:1064–1066. doi: 10.1046/j.1365-2141.1998.01078.x. [DOI] [PubMed] [Google Scholar]
- 28.Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. doi: 10.1093/eurheartj/ehr488. [DOI] [PubMed] [Google Scholar]
- 29.Harris MI, Klein R, Welborn TA, Knuiman MW. Onset of NIDDM occurs at least 4-7 yr before clinical diagnosis. Diabetes Care. 1992;15:815–819. doi: 10.2337/diacare.15.7.815. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


