Abstract
Background
The advent of integrated genomics has fundamentally changed our understanding of medulloblastoma. Although survival differences exist among the 4 principal subgroups, this has yet to be elucidated in a North American cohort of irradiated patients.
Methods
Ninety-two consecutive patients between the ages of 3 and 17 treated with surgery, craniospinal irradiation, and chemotherapy were identified at the Hospital for Sick Children. Molecular subgrouping was performed using nanoString.
Results
Two treatment periods were identified: prior to 2006 as per the protocols of the Children's Oncology Group, and after 2006 per the St Jude Medulloblastoma 03 protocol. Five-year progression-free survival (PFS) over the entire cohort was 0.801 (95% CI: 0.692–0.875) with no significant difference between treatment protocols. Strikingly, we found that Group 4 patients had excellent 5-year PFS of 0.959 (95% CI: 0.744–0.994) for average risk and 0.887 (95% CI: 0.727–0.956) across all Group 4 patients. Group 3 patients had 5-year PFS of 0.733 (95% CI: 0.436–0.891). Sonic hedgehog patients did poorly across both treatment protocols, with 5-year PFS of 0.613 (95% CI: 0.333–0.804), likely owing to a high proportion of TP53 mutated patients in this age group.
Conclusions
In a cohort of irradiated patients over 3 years of age, PFS for Group 4 patients was significantly improved compared with initial reports. The impact of subgroup affiliation in these children needs to be assessed in large prospectively treated cooperative protocols to determine if more than just WNT patients can be safely selected for de-escalation of therapy.
Keywords: chemotherapy, medulloblastoma, radiation, subgroups
Medulloblastoma is the most common malignant brain tumor of childhood. Current treatment in children over age 3 years is multimodal, consisting of maximal safe resection followed by risk-adapted radiotherapy and adjuvant cisplatin-based chemotherapy.1 This has resulted in 5-year survival rates of ∼85% in children with average-risk disease and up to 70% in children with high-risk disease consisting of metastatic dissemination or residual tumor >1.5 cm2.2–5 Several chemotherapy regimens are available, whereby the majority of modern protocols are based on cisplatin and vincristine with the addition of either lomustine or cyclophosphamide. At our institution, treatment for average-risk medulloblastoma prior to 2006 was as per the Children's Oncology Group (COG) protocol for average-risk disease, consisting of 23.4 Gy craniospinal irradiation followed by 6–9 cycles of cisplatin, lomustine, and vincristine (CCG9961 Regimen A or ACNS0331).2 High-risk patients were treated as per the POG9031 and POG9631 high-risk protocols.3 After 2006 our institution started enrolling patients on the St Jude's Medulloblastoma 03 (SJMB03) protocol, consisting of risk-adapted radiotherapy followed by 4 cycles of intensified high-dose cyclophosphamide-based chemotherapy and autologous stem cell support.5
The past 10 years have led to a dramatic increase in our knowledge of medulloblastoma biology. It is now currently accepted that there are at least 4 distinct subgroups of medulloblastoma with distinct demographics, genetics, recurrence patterns, and outcomes.1,6–11 Early reports from retrospective data collection have shown distinct outcomes for the 4 subgroups, with the WNT subgroup having an excellent prognosis and Group 3 patients having a dismal prognosis across several studies.10–13 However, these estimates did not take into account treatment allocation and in particular the use of radiation and chemotherapy protocols.
A very well studied group of medulloblastoma patients in cooperative studies are children between the ages of 3 and 21, and efforts to reduce radiation doses and volume over the past 20 years have resulted in several different chemoradiotherapy regimens. Indeed, current national studies are still focused on this population, whereby therapy de-escalation for WNT patients, targeted therapy for Sonic hedgehog (SHH) patients, and treatment intensification for Groups 3 and 4 patients are currently being evaluated (NCT01878617). However, the relationship between treatment modalities and molecular subgroup is unclear. Specifically, currently reported subgroup-specific outcomes combine all age groups, and as such could be significantly biased by treatment, especially with the inclusion of infants, who are almost exclusively SHH and Group 3.1,10,11,13 A recent copy number–based risk stratification within each medulloblastoma subgroup suggested the existence of high- and low-risk groups within both Group 3 and Group 4 patients; however, this cohort did not include details on therapy.14
Ours is the major tertiary care referral center for children under the age of 18 with brain tumors in southern Ontario, representing a population of ∼8 million. As such, we sought to determine the subgroup-specific outcomes in children between the ages of 3 and 17 in a large single-center cohort.
Methods
Patient Cohort
Between 2000 and 2012, ninety-five patients between the ages of 3 and 17 years with newly diagnosed medulloblastoma treated with craniospinal irradiation were identified at the Hospital for Sick Children. Three patients were excluded. Of these, one patient with ataxia-telangiectasia and an SHH tumor declined therapy postoperatively. The parents of a second patient declined upfront radiation in a 10 year old with a Group 4 tumor. A third patient, with neurofibromatosis type 1, declined therapy when he developed a cerebellar primitive neuroectodermal tumor 10 years after radiation for an optic pathway glioma. All remaining patients were treated in a coordinated multidisciplinary pediatric neuro-oncology program, where preoperative imaging of the entire craniospinal axis is performed in all suspected medulloblastoma, and postoperative neuroimaging is conducted within 72 h of surgery to assess for residual disease. Moreover, lumbar CSF examinations are performed in all patients 14 days postoperatively, and craniospinal irradiation is initiated as much as possible within 28 days of surgery. Prior to 2006 all patients were treated as per protocols of the COG (or its predecessors the Pediatric Oncology Group and Children's Cancer Group), specifically CCG9961 regimen A or ACNS0331 for average-risk patients and POG9631 or POG9031 for high-risk patients. One patient after 2006 was enrolled in ACNS0331 and received 18 Gy of craniospinal irradiation. After March 2006 all patients except 4 were treated on the SJMB03 study of intensified chemotherapy with autologous stem cell support. Three of the 4 patients were treated on ACNS0332, the open COG high-risk protocol, and the fourth was an average-risk Group 4 patient whose parents declined chemotherapy after completion of 23.4 Gy craniospinal irradiation. One patient with M1 disease also had concurrent Duchenne muscular dystrophy and was given an individualized therapy consisting of 23.4 Gy craniospinal irradiation followed by an abbreviated version of POG9631 consisting of 3 cycles of cisplatin/etoposide and 3 cycles of etoposide monotherapy. He was previously reported as an isolated case report and has no evidence of disease 5 years postcompletion of therapy.15 All samples and clinical annotations were obtained in accordance with the Research Ethics Board at the Hospital for Sick Children.
Molecular Diagnostics
Molecular subgroup was determined using nanoString as previously described from both formalin-fixed paraffin-embedded and frozen tissue.6,16 TP53 mutational status was determined by Sanger sequencing as previously described.17,18
Statistical Analysis
Progression-free survival (PFS) was right-censored at 5 or 10 years and analyzed by the Kaplan–Meier method; P-values were reported using the log-rank test. Survival data are presented as survival ± 95% CIs. Data were right-censored at 5 years, as patients on the SJMB03 protocol had shorter follow-up times compared with those treated prior to 2006. Associations between covariates and risk groups were tested by Fisher's exact test. All statistical analyses were performed in the R statistical environment (v3.1.2), using the R packages ‘survival’ (v2.37–7) and ‘ggplot2’ (v1.0.0).
Results
Cohort Characteristics
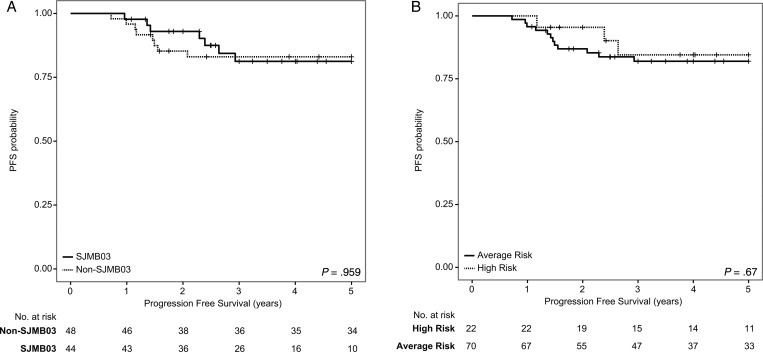
Demographic and clinical details of the cohort are presented in Table 1. Five-year PFS for the entire cohort was 0.801 (95% CI: 0.692–0.875) and 5-year overall survival (OS) was 0.851 (95% CI: 0.748–0.915). As treatment paradigms changed significantly at our center in 2006, PFS was determined as per SJMB and COG protocols without any significant differences, with SJMB having 5-year PFS of 0.771 (95% CI: 0.578–0.883) and COG having 5-year PFS of 0.822 (95% CI: 0.676–0.907) (P = .959; Fig. 1A). When restricted to Group 4, which comprised almost half of all patients, we observed no difference in survival between the 2 regimens (P = .131; Supplementary Fig. S1). There were 70 patients with average-risk disease and 22 patients with high-risk disease, with 5-year PFS of 0.791 (95% CI: 0.662–0.876) and 0.833 (95% CI: 0.568–0.943), respectively (Fig. 1B). We observed no significant difference in PFS between these 2 groups (P = .67). In the COG cohort, 8 average-risk patients were treated with 36 Gy of craniospinal irradiation on the POG9631 protocol due to the presence of diffuse anaplasia. Four of these 8 patients relapsed, 3 of whom had SHH tumors with TP53 mutations and 1 a Group 3 tumor. As such, we conclude that in a single-center cohort of consecutive patients, there is no significant difference in treatment between intensified and non-intensified chemotherapy.
Table 1.
Demographics and clinicopathological variables of the 2 treatment cohorts
| Non-SJMB03 (n = 48) | SJMB03 (n = 44) | P | |
|---|---|---|---|
| Male | 31 (64.6%) | 28 (63.6%) | 1 |
| Age at diagnosis, y, median (range) | 7.3 (3–15.3) | 8.3 (3.6–17.2) | .26 |
| High-risk disease, n | 15 (31.3%) | 7 (15.9%) | .094 |
| Metastatic dissemination, n | 11 (22.9%) | 7 (15.9%) | .45 |
| Incomplete resection >1.5 cm2, n | 5 (10.4%) | 0 | .058 |
| CSI dose, Gy, na | .044 | ||
| 18 | 1 (2.1%) | 0 | |
| 23.4 | 27 (57.4%) | 35 (79.5%) | |
| 31 | 0 | 1 (2.3%) | |
| 36–39 | 19 (40.4%) | 8 (18.2%) | |
| Treatment failures, n | 12 (25%) | 7 (15.9%) | .31 |
| LCA morphology, n | 8 (16.7%) | 14 (31.8%) | .14 |
| MYC amplificationb, n | 0 | 0 | |
| MYCN amplificationb, n | 1 (3.8%) | 2 (5.2%) | 1 |
| Subgroup, n | .073 | ||
| WNT | 3 (6.3%) | 10 (22.7%) | |
| SHH | 10 (20.8%) | 7 (15.9%) | |
| Group 3 | 9 (18.8%) | 10 (22.7%) | |
| Group 4 | 26 (54.2%) | 17 (38.6%) |
Abbreviations: CSI, craniospinal irradiation; LCA, large-cell anaplastic.
aOne patient treated as per SJMB03 with a suspicious suprasellar metastasis treated with 31 Gy CSI.
bNMYC and CMYC amplification status available on 38 patients treated with SJMB03 and 26 patients treated on non-SJMB03.
Fig. 1.
Kaplan–Meier survival analysis comparing PFS across treatment protocols and risk levels across 92 medulloblastomas. (A) Comparison of PFS in patients treated with intensified chemotherapy (“SJMB03”) vs protocols of the COG (“Non-SJMB03”). (B) Comparison of PFS in patients with average-risk disease vs those with high-risk disease. P-values determined using the log-rank method.
Molecular Subgroup Is Highly Prognostic
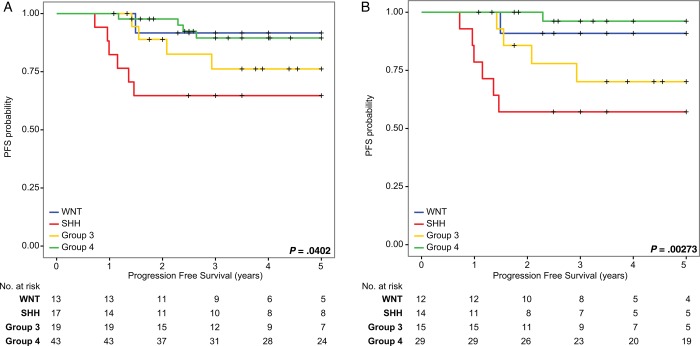
In order to determine the effect of molecular subgroup on outcome in a consecutive cohort, cases were subgrouped using nanoString limited gene expression profiling. When 5-year PFS was determined across the entire cohort of 92, there was a significant difference across the 4 subgroups in a pooled analysis. Specifically, 5-year PFS for the WNT subgroup was 0.895 (95% CI: 0.454–0.985), for the SHH subgroup 0.613 (95% CI: 0.333–0.804), for Group Three 0.733 (95% CI: 0.436–0.891), and for Group Four 0.887 (95% CI: 0.727–0.956) (P = .0402 across all 4 subgroups; Fig. 2A). In a pairwise comparison of Group 4 versus Group 3 and SHH, Group 4 did significantly worse compared with SHH (P = .009). When restricting the analysis to only average-risk patients, 5-year PFS for WNT was 0.8824 (95% CI: 0.411–0.983), for SHH 0.52 (95% CI: 0.228–0.749), for Group Three 0.60 (95% CI: 0.290–0.810), and for Group Four 0.959 (95% CI: 0.744–0.994) (P = .0027 across all 4 subgroups; Fig. 2B). In a pairwise comparison, Group 4 did considerably worse than SHH (P = .001) and Group 3 (P = .015); however, after correcting for multiple testing, only Group 4 versus SHH remained significantly different. Previously we showed that Group 4 patients recur later.6 In light of this finding, we looked at 10-year PFS for Group 4 and found that although there were 2 additional failures, survival was 0.837 (95% CI: 0.639–0.932) (Supplementary Fig. S2A). There was one late Group 4 failure consisting of one average-risk patient randomized to 18 Gy of craniospinal irradiation as per ACNS0331 who relapsed with disseminated leptomeningeal disease 6.7 years postdiagnosis. An additional failure, of an SHH patient, was noted at 10.5 years postdiagnosis. Ten-year OS for the WNT subgroup was 1, for the SHH subgroup 0.613 (95% CI: 0.333–0.804), for Group Three 0.733 (95% CI: 0.436–0.891), and for Group Four 0.837 (95% CI: 0.639–0.932) (P = .00665 across all 4 subgroups; Supplementary Fig. S2B). One Group 3 patient died of lymphoma 3.8 years postdiagnosis for average-risk medulloblastoma, and one Group 4 patient died of a biopsy-proven secondary glioblastoma in the previously irradiated posterior fossa 9.5 years after diagnosis. Neither of these patients with secondary malignancies had germline testing for a secondary malignancy. As such we conclude that in our single-center cohort, Group 4 patients had considerably better outcomes than previously reported, particularly those with average-risk disease.
Fig. 2.
Kaplan–Meier survival analysis comparing PFS in a subgroup-specific manner across (A) the entire cohort of 92 medulloblastomas and (B) average-risk medulloblastoma only. P-values determined using the log-rank method.
Association With Presumed High-Risk Markers
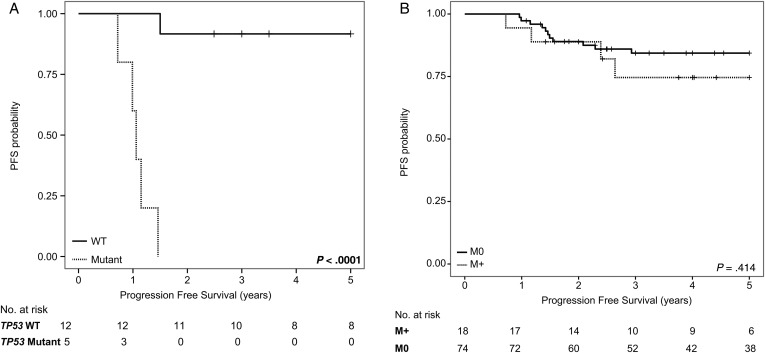
In order to determine whether previously described markers of poor prognosis had an effect on survival in our cohort, TP53 mutational status and large-cell and/or anaplastic morphology were determined in SHH, Group 3, and Group 4, respectively. Five of the 7 failures in the average-risk SHH subgroup were TP53 mutated, consistent with previous reports. When 5-year PFS for SHH was recalculated for TP53 wild type only, survival was significantly higher at 0.88 (95% CI: 0.766–0.930; P < .001) (Fig. 3, Supplementary Fig. S3). Germline TP53 status is unknown in these cases. In 22 patients with anaplastic and/or large-cell pathology, there were 5 failures, of which 3 were SHH patients harboring TP53 mutations. MYC and MYCN amplification status was known in 64 of 92 patients as determined by fluorescence in situ hybridization or Affymetrix SNP6.0 arrays. Three cases harbored MYCN amplification, one Group 4 patient had relapse-free follow-up 3.5 years postdiagnosis, one SHH patient had a TP53 mutated tumor that progressed in the tumor bed 1.5 years postdiagnosis, and a second SHH patient was relapse free 3 years postdiagnosis. No patients in our cohort had MYC amplifications. Eighteen patients had metastatic dissemination, and 5-year PFS was not significantly different, with M0 patients having 5-year PFS of 0.821 (95% CI: 0.700–0.897) and M+ having 5-year PFS of 0.71 (95% CI: 0.406–0.882; P = .41) (Fig. 3). Five patients had incomplete resections with residual disease of >1.5 cm2, and none progressed. All 5 of these incomplete resections were nonmetastatic, and 3 patients with incomplete resections were Group 4, SHH, and WNT, respectively. Median time to start of radiation from the initial surgery was 28 days (range, 14–48; interquartile range, 26.2–31). The interval between surgery and initiation of radiation was not significantly different across the 4 subgroups but was significantly shorter in the group treated as per SJMB03 (P = .15) (Supplementary Fig. S4).
Fig. 3.
Kaplan–Meier survival analysis comparing PFS for (A) TP53 wild-type (WT) vs TP53 mutant SHH medulloblastomas and (B) M0 vs M+ at diagnosis. P-values determined using the log-rank method.
Discussion
Several recent studies have shown significant prognostic implications of the 4 core medulloblastoma subgroups. Many of these studies were retrospective, and of the 2 prospective studies reported, data were incomplete, as only patients for whom sufficient material was available were included.19,20 Moreover, one of the 2 prospective studies combined infants treated with chemotherapy-only approaches with children and adults treated with craniospinal irradiation, thus resulting in significant treatment heterogeneity, particularly for patients with SHH and Group 3 tumors. The present study is the first report of subgroup-specific outcomes in a homogeneously treated cohort of 92 consecutive children who received craniospinal irradiation between the ages of 3 and 17. We find that similar to previous studies, WNT patients have an excellent prognosis; however, we find some differences where SHH patients have a very poor outcome compared with Group 3 and Group 4 patients, who have an excellent outcome, particularly those with average-risk disease. Moreover, we show that subgroup-specific outcomes between intensified chemotherapy with high-dose cyclophosphamide with autologous stem cell support as prescribed in the SJMB03 protocol and standard chemotherapy as per the protocols of the COG are not different and that in a consecutive cohort treated with risk-adapted therapy, high- and average-risk medulloblastoma have similar PFS. When the 2 chemotherapy approaches are compared in a subgroup-specific manner, our results suggest that no particular subgroup benefits from the high-dose cyclophosphamide with autologous stem cell support as prescribed in the SJMB03 protocol. Overall our outcomes are similar to multicenter cooperative studies, which have shown that average-risk medulloblastoma has 5-year PFS of 80% and high-risk medulloblastoma has 5-year PFS of 70%.
Our subgroup-specific PFS rates are significantly different from those previously reported in the literature. Specifically, SHH patients do poorly in our cohort relative to the other subgroups.19,20 The most likely reason for this discrepancy is that our cohort is limited to children aged 3–17, whereas previous reports have included infants and adults. Infant desmoplastic tumors are well known to have an excellent outcome, and these tumors are almost all exclusively SHH, which could explain why in previous cohorts SHH had an intermediate outcome. It was previously shown that TP53 mutations are known to be a marker of dismal prognosis in SHH patients, are significantly enriched in patients aged 3–17, and are rare in infants with SHH tumors, who have an excellent prognosis.17,21 In our cohort the majority of SHH failures were TP53 mutated, which explains both the very short time to progression and the high rate of failures. As such, upfront novel experimental approaches should be considered in future trial designs for this very high risk group of patients.
Group 4 patients in our cohort had outcomes significantly better than previously reported in either retrospective studies or the prospective HIT2000 and SIOP-PNET3 cohorts.10,11,14,19,20 These previous studies have placed Group 4 patients in an intermediate prognostic category. There are several possible explanations for the differences in survival noted among the various global cohorts. Previously we showed that infants with Group 4 tumors do poorly compared with older children and that Group 4 tumors require external beam irradiation to the tumor bed for local control.6,14 As such, it is possible that Group 4 patients benefit the most from timely initiation of radiation, which may not be the case in the HIT2000 or SIOP-PNET3 cohorts. Indeed, ours is a tertiary care center with a very large comprehensive pediatric neuro-oncology service, and patients are treated with strict adherence to protocol even when not enrolled. Another possibility is the inclusion of adult patients, whereby it has previously been suggested that Group 4 adult patients have a poor outcome.13 One intriguing possibility is treatment-related differences. In the SIOP-PNET3 cohort, patients were randomized to either craniospinal irradiation alone or pre-irradiation chemotherapy consisting of carboplatin, etoposide, cyclophosphamide, and vincristine. As such, it is difficult to compare SIOP-PNET3 outcomes to ours, as all our protocols are cisplatin based, and overall outcomes using current COG, International Society of Paediatric Oncology, or St Jude's protocols are significantly better than those reported in PNET3.2–5 The HIT2000 cohort was treated with risk-adapted craniospinal irradiation followed by chemotherapy consisting of cisplatin, lomustine, and vincristine. In our cohort, prior to 2006, average-risk patients were treated with Packer regimen A consisting of cisplatin, lomustine, and vincristine; however, high-risk patients were treated with cyclophosphamide-based protocols. This may explain the discrepancies in outcome, as high-risk Group 4 patients may benefit from cyclophosphamide therapy; however, we observed excellent survivals for average-risk Group 4 patients treated prior to 2006 on Packer regimen A containing lomustine. Indeed, this may also explain the relatively improved survival of Group 3 patients in our cohort. The possibility of treatment-related differences in outcome in Group 4 patients warrants additional investigation in ongoing prospective trials. As previously reported by our group, after 2006 radiation boosts to the posterior fossa were replaced with conformal radiation boosts to the tumor bed only, without any effect on survival and with a significant improvement in neurocognitive outcomes.22 Future reduction in therapies could be carefully considered for average-risk Group 4 patients, specifically a reduction in chemotherapy and/or craniospinal irradiation.
This study is limited by small numbers and a single treating institution and as such does require prospective validation in current and future multicenter prospective clinical trials. However, our study highlights the importance of banking tissue (ideally frozen) in all patients enrolled in prospective trials in order to properly conduct molecular analysis and correlate this with treatment. Indeed, in the HIT2000 cohort, only one-fifth of all patients had sufficient tissue available for molecular analysis, and within the COG studies, no prospective medulloblastoma study to date has integrated molecular biology. This further strengthens the importance of integrating biology with treatment when defining new and novel biomarkers such as molecular subgroup to risk stratification of medulloblastoma.
This study suggests that outcomes using modern treatment protocols for irradiated Group 4 patients may not be as poor as previously suggested. Indeed, our results suggest that average-risk Group 4 patients may constitute a low-risk group who may be rationally selected for further reduction of therapy in future trials. Overall, our study is the first to identify subgroup-specific outcomes for irradiated children with medulloblastoma, who form the majority of patients currently being targeted in cooperative studies.
Supplementary Material
Funding
Funding for this work was provided by funds from Pediatric Brain Tumor Foundation; funds from the Garron Family Chair in Childhood Cancer Research at the Hospital for Sick Children and the University of Toronto; the National Institutes of Health (R01CA159859 and R01CA148699) to M.D.T, a Canadian Institutes of Health Research Fellowship; and Alberta Innovates-Health Solutions Clinical Fellowship; a Young Investigator Award from Alex's Lemonade Stand Foundation to V.R.; and a Mildred Scheel Cancer Foundation fellowship to M.R. The authors acknowledge support from American Lebanese Syrian Associated Charities (ALSAC).
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Ramaswamy V, Northcott PA, Taylor MD. FISH and chips: the recipe for improved prognostication and outcomes for children with medulloblastoma. Cancer genetics. 2011;204(11):577–588. [DOI] [PubMed] [Google Scholar]
- 2.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. [DOI] [PubMed] [Google Scholar]
- 3.Tarbell NJ, Friedman H, Polkinghorn WR, et al. High-risk medulloblastoma: a pediatric oncology group randomized trial of chemotherapy before or after radiation therapy (POG 9031). J Clin Oncol. 2013;31(23):2936–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakacki RI, Burger PC, Zhou T, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a Children's Oncology Group phase I/II study. J Clin Oncol. 2012;30(21):2648–2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 6.Ramaswamy V, Remke M, Bouffet E, et al. Recurrence patterns across medulloblastoma subgroups: an integrated clinical and molecular analysis. Lancet Oncol. 2013;14(12):1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramaswamy V, Remke M, Shih D, et al. Duration of the pre-diagnostic interval in medulloblastoma is subgroup dependent. Pediatr Blood Cancer. 2014;61(7):1190–1194. [DOI] [PubMed] [Google Scholar]
- 8.Taylor MD, Northcott PA, Korshunov A, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123(4):465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubuc AM, Morrissy AS, Kloosterhof NK, et al. Subgroup-specific alternative splicing in medulloblastoma. Acta Neuropathol. 2012;123(4):485–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kool M, Korshunov A, Remke M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Northcott PA, Korshunov A, Witt H, et al. Medulloblastoma comprises four distinct molecular variants. J Clin Oncol. 2011;29(11):1408–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Remke M, Hielscher T, Korshunov A, et al. FSTL5 is a marker of poor prognosis in non-WNT/non-SHH medulloblastoma. J Clin Oncol. 2011;29(29):3852–3861. [DOI] [PubMed] [Google Scholar]
- 13.Remke M, Hielscher T, Northcott PA, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29(19):2717–2723. [DOI] [PubMed] [Google Scholar]
- 14.Shih DJ, Northcott PA, Remke M, et al. Cytogenetic prognostication within medulloblastoma subgroups. J Clin Oncol. 2014;32(9):886–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Akker M, Northcott P, Taylor MD, et al. Anaplastic medulloblastoma in a child with Duchenne muscular dystrophy. J Neurosurg Pediatr. 2012;10(1):21–24. [DOI] [PubMed] [Google Scholar]
- 16.Northcott PA, Shih DJ, Remke M, et al. Rapid, reliable, and reproducible molecular sub-grouping of clinical medulloblastoma samples. Acta Neuropathol. 2012;123(4):615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol. 2013;31(23):2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tabori U, Baskin B, Shago M, et al. Universal poor survival in children with medulloblastoma harboring somatic TP53 mutations. J Clin Oncol. 2010;28(8):1345–1350. [DOI] [PubMed] [Google Scholar]
- 19.Pietsch T, Schmidt R, Remke M, et al. Prognostic significance of clinical, histopathological, and molecular characteristics of medulloblastomas in the prospective HIT2000 multicenter clinical trial cohort. Acta Neuropathol. 2014;128(1):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwalbe EC, Williamson D, Lindsey JC, et al. DNA methylation profiling of medulloblastoma allows robust subclassification and improved outcome prediction using formalin-fixed biopsies. Acta Neuropathol. 2013;125(3):359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kool M, Jones DT, Jager N, et al. Genome sequencing of SHH medulloblastoma predicts genotype-related response to smoothened inhibition. Cancer Cell. 2014;25(3):393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moxon-Emre I, Bouffet E, Taylor MD, et al. Impact of craniospinal dose, boost volume, and neurologic complications on intellectual outcome in patients with medulloblastoma. J Clin Oncol. 2014;32(17):1760–1768. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.